A 62-year-old woman, who had a history of uterine fibroids requiring hysterectomy and double adnexectomy at age 44, came to the emergency department of our hospital due to a 3-day history of emetic syndrome associated with altered bowel habits over the previous 2 months and weight loss of 12kg.
On examination, the patient presented a slightly distended, soft abdomen with discomfort in the right iliac fossa but no peritonism. Bowel sounds were increased, and the digital rectal examination was normal.
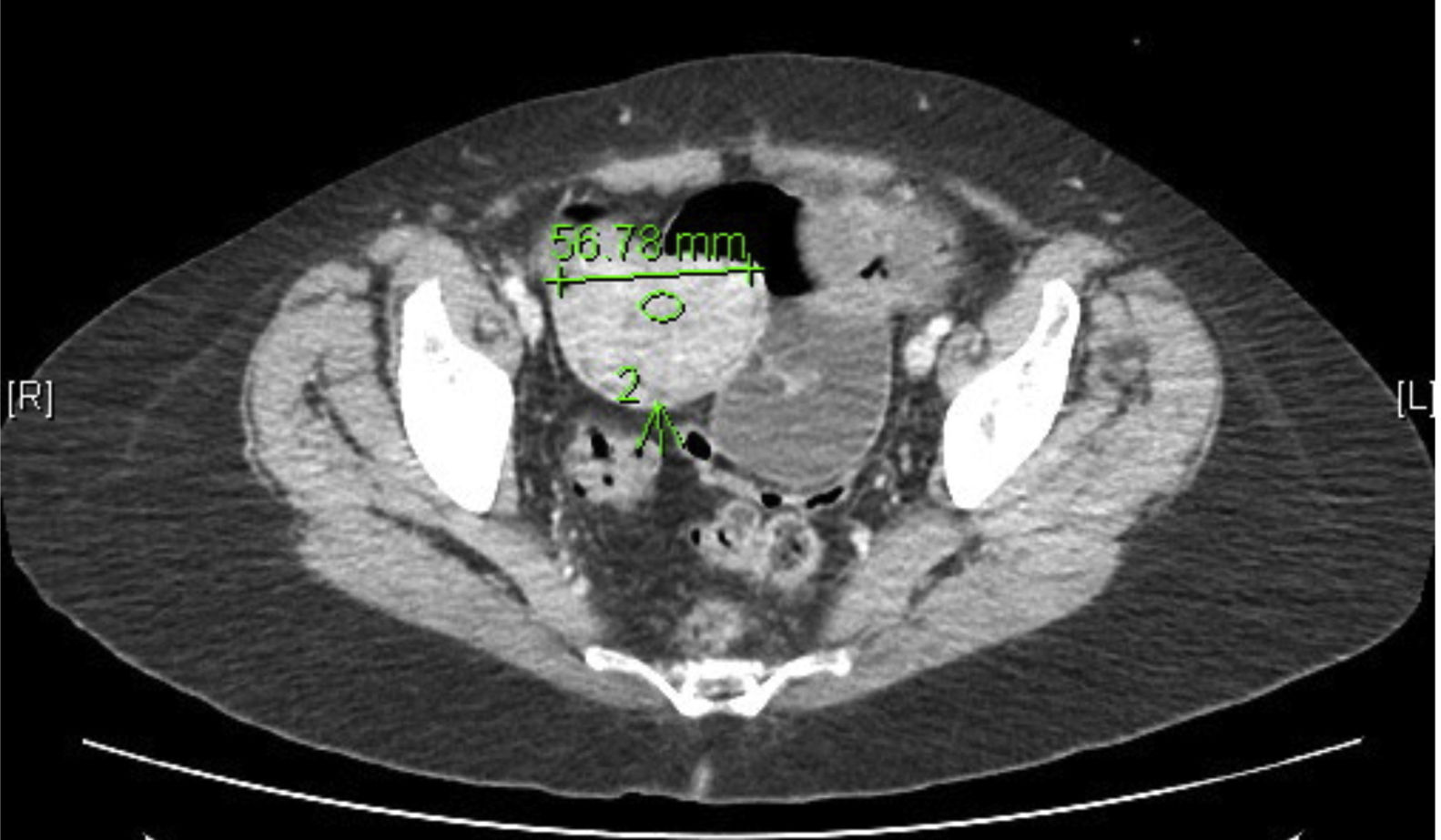
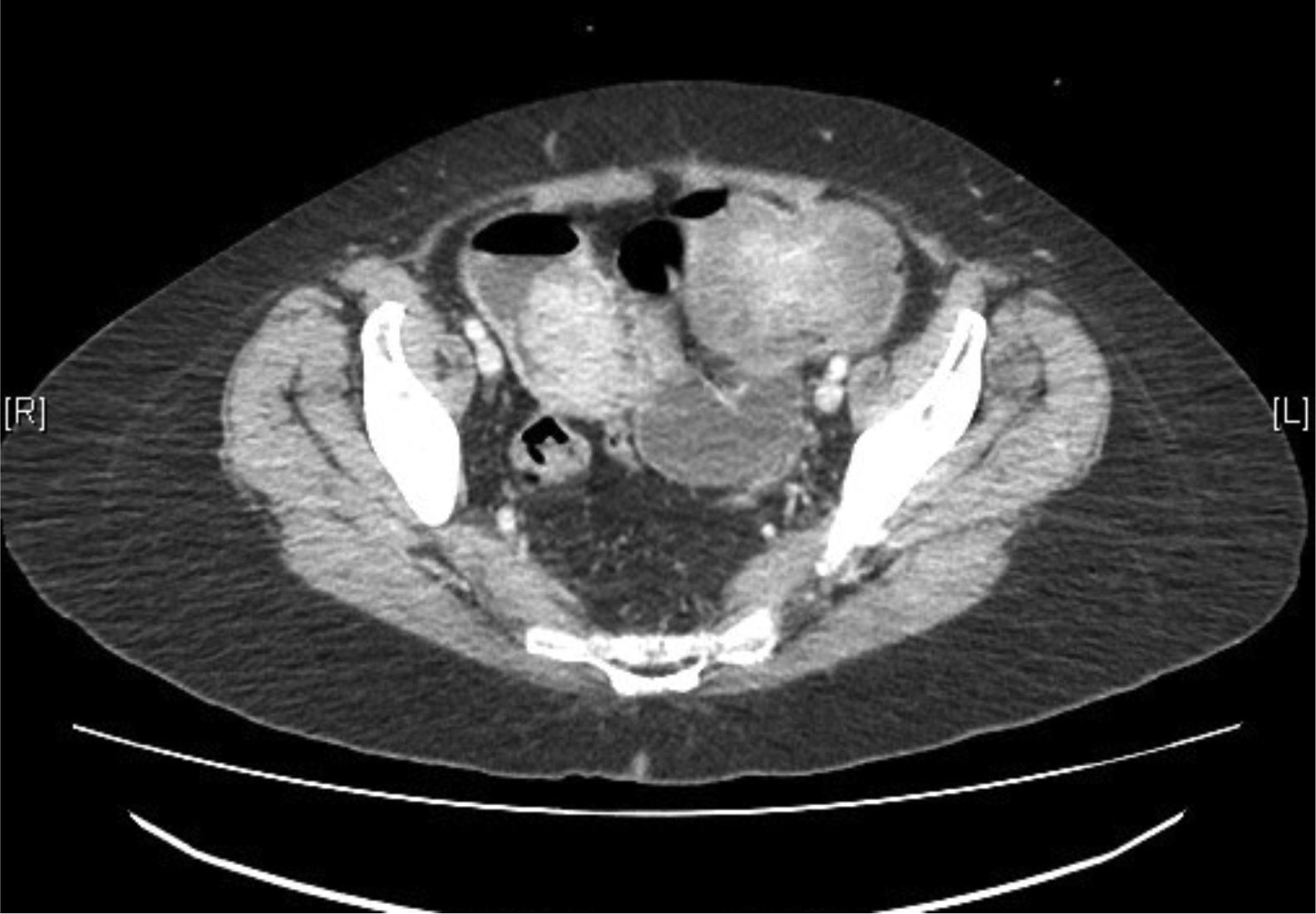
Lab work showed hemoglobin levels of 9.2g/dL, 15,000 leukocytes and a prothrombin activity of 73%; the remaining parameters were normal. Abdominal radiography demonstrated distended intestinal loops and the absence of gas in the colon and rectum. Abdominal CT scan identified 2 intestinal masses that were causing intestinal intussusception and mechanical obstruction (Figs. 1 and 2).
Urgent surgery was indicated, and exploratory laparoscopy revealed 2 intestinal loops with stenosis caused by tumors and retrograde dilation. No free fluid or space-occupying lesion in the liver were observed. Using an assistance incision, the intestines were eviscerated, an 80-cm section of the jejunum-ileum was resected and a side-to-side reinforced anastomosis was created mechanically.
The postoperative period was uneventful, and the patient was discharged on the sixth day.
The pathology study reported the presence of 2 lesions, both compatible with malignant melanoma of the synchronous intestinal type, with ulceration present. These lesions affected the mucosa and muscular layers. The proliferative index was high; maximum Ki-67 was 63%. Lymphovascular invasion was detected. The immunophenotype of the cells was vimentin, HMB-45 and INI1, positive diffuse; S-100 and SOX-10, positive focal; CKAE1/AE3, negative. No involvement was identified in the 12 lymph nodes studied.
The patient was evaluated by the Dermatology Unit, which ruled out malignant melanoma of cutaneous origin.
An extension study was carried out one month after surgery, where a 1-cm pulmonary nodule suggestive of metastasis and a subcutaneous nodule compatible with skin metastasis were observed. The tumor committee indicated systemic treatment with ipilimumab+nivolumab. After 4 cycles of treatment, a partial response was seen on CT scan, so maintenance therapy was continued with nivolumab.
Mucosal melanoma is a rare entity that represents 1% of all melanomas.1 The most frequently affected locations are the mouth, neck, anus and vagina, but the small intestine is rarely affected.2 It is more frequent in women and patients of advanced ages,3 as well as in blacks and Asians. The risk factors that favor its onset are unknown.1
20% of mucosal melanomas are multifocal, unlike cutaneous melanoma, which is usually unifocal.4
A generalized staging system has been established for all mucosal melanomas, which classifies them as localized disease (stage I), local lymph node involvement (stage II) and metastatic disease (stage III).5 Metastatic involvement is very frequent in these patients, and 5-year survival is 25%.1
The treatment of choice is complete resection,5 which can sometimes be difficult due to local tumor conditions. The use of adjuvant chemotherapy has been shown to be beneficial in randomized studies, increasing the rates of disease-free survival and overall survival; however, it has not yet been established as the standard to be followed.6
10% of these tumors show mutation in BRAF and 25% in KIT,7 which is important because the latter present a response to imatinib, sorafenib and sunitinib.8
Immunotherapy with anti-CTLA4 (ipilimumab), which is clearly useful in cutaneous melanoma, has not yet shown its usefulness in the mucosa.9 However, the analysis of studies comparing the response to ipilimumab+nivolumab in cutaneous melanoma and metastatic mucosal melanoma shows, in the latter, response rates around 37%10 and 6-month disease-free survival.
Please cite this article as: García-Romera Á, Morales Hernández A, Medina-Arana V, Bravo Gutiérrez A, Alarcó A. Melanoma mucoso intestinal: una rara causa de obstrucción. Cir Esp. 2019;97:295–296.