Testicular germ cell tumor metastases have experienced a dramatic improvement in survival with chemotherapy. Retroperitoneal masses are common and are initially treated with chemotherapy; however, if the residual mass remains stable, surgery is considered. Occasionally, these masses can differentiate into mature teratomas. In this case, surgical treatment is the most appropriate option, due to the problems that they can cause in the long-term resulting from compression and dissemination.
We present the case of a 27-year-old male patient with a diagnosis of embryonal carcinoma of the right testicle at 20 years of age, which required right orchiectomy. The pathology results showed embryonal carcinoma with no areas of teratoma. Afterwards, due to recurrence in the form of retroperitoneal dissemination, he received 5 cycles of chemotherapy with cisplatin + etoposide + bleomycin, the last of which was in 2013. During follow-up, a retroduodenal para-aortic mass was detected in 2015; an exploratory laparoscopy was performed, and biopsied samples were compatible with sclerosing fibrous tissue. The patient had no further follow-up visits due to social problems.
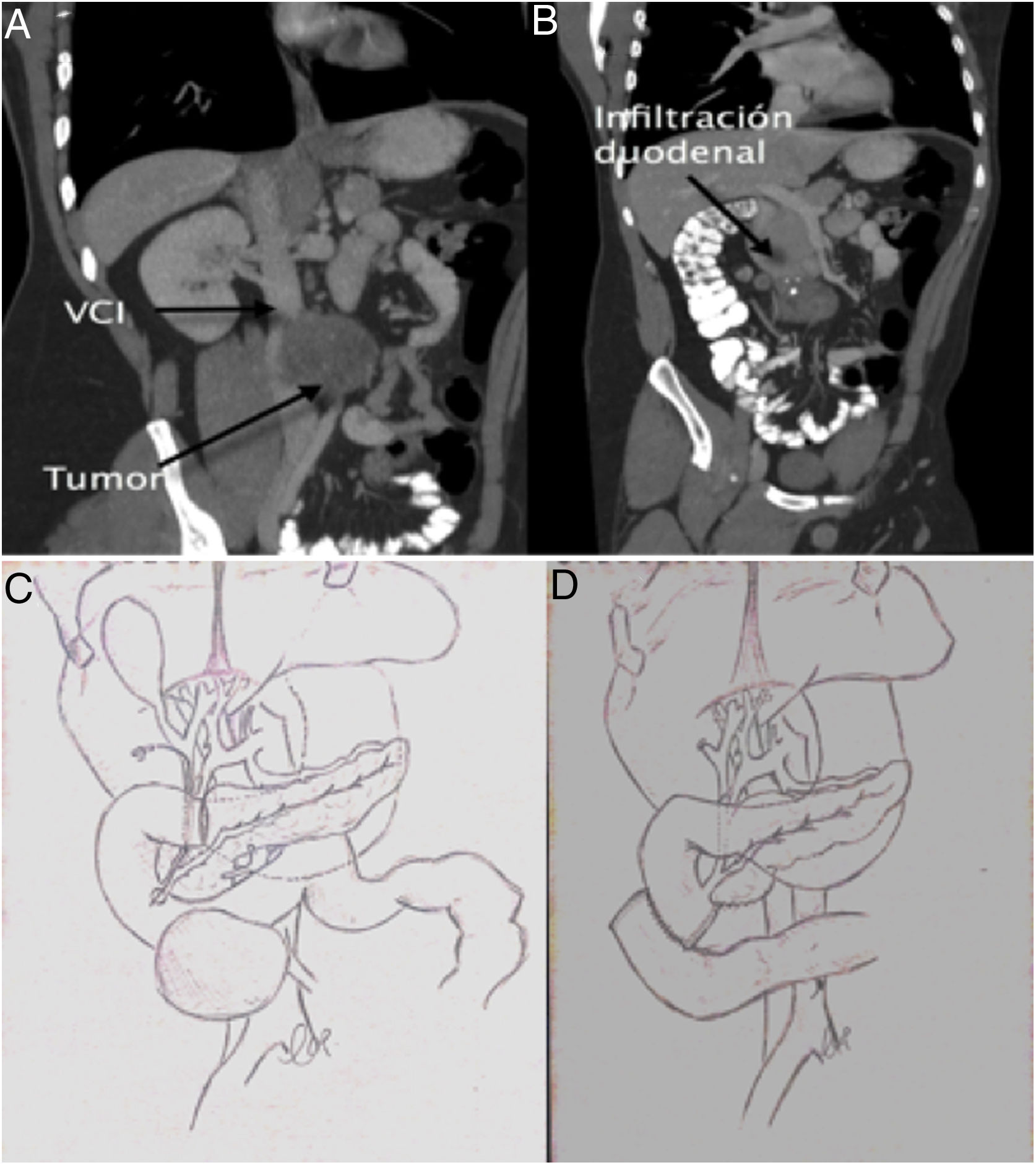
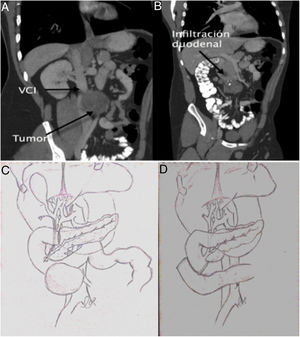
At our hospital, the patient presented with upper gastrointestinal bleeding. He presented anemia of 7 hemoglobin points that required the transfusion of 4 units of packed red blood cells. Gastroscopy identified an ulcerated lesion in the 2nd–3rd parts of the duodenum that was infiltrative in appearance and close to the duodenal papilla. The biopsy was not conclusive for malignancy. A computed tomography scan showed a 10-cm aorto-cava retroperitoneal mass, with invasion of the duodenum and no plane of separation with the cava or aorta, as well as invasion of the inferior mesenteric artery (Fig. 1). Tumor marker levels were normal. Surgery was considered necessary due to a high risk of bleeding recurrence.
Preoperatively, an introducer was placed in the right femoral artery due to the possibility of hemorrhage during dissection, especially of the vena cava.
Using a midline approach, we conducted cholecystectomy and transcystic revision of the bile duct, observing the duodenal papilla at about 9 mm from the tumor. We decided to perform a pancreas-preserving duodenectomy and side-to-side duodenal-jejunal reconstruction. The inferior mesenteric artery was ligated and the right mesocolon was mobilized, which required resection of its parietal peritoneum. Using blunt dissection, the tumor was separated from the wall of the aorta. The same procedure was done with the cava, without requiring vascular resection as the tumor was partially encapsulated. The intervention was completed with a lymph node dissection of the aorto-cava space.
The patient’s progress was satisfactory, with no incidents, and he was discharged on the 8th postoperative day. The 6-month follow-up showed no recurrence of the disease.
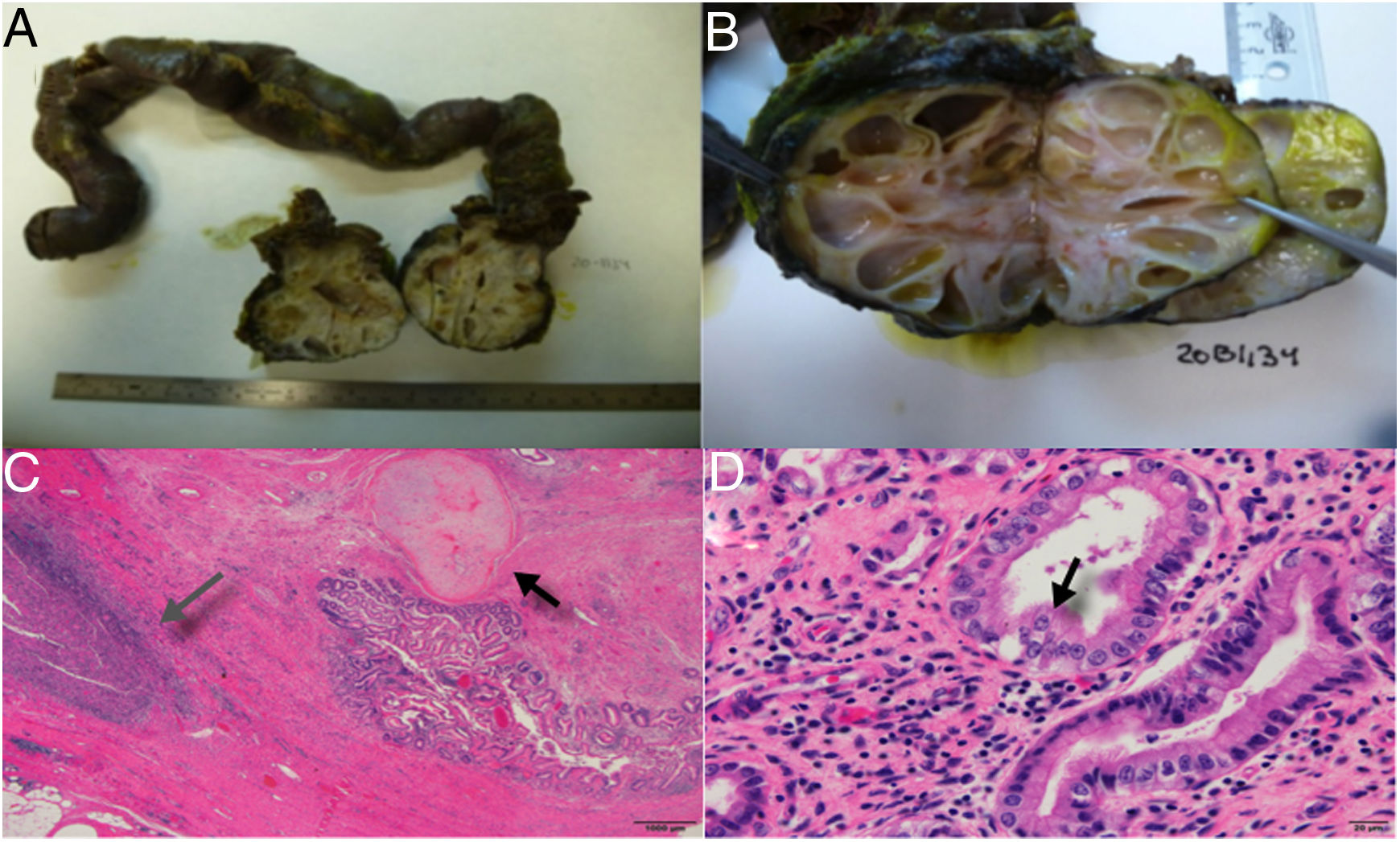
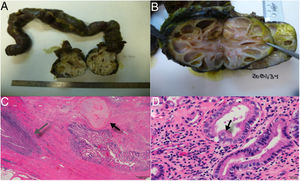
The definitive histological study confirmed metastasis of the embryonal carcinoma after treatment, with differentiation into a mature teratoma in its entirety, invasion of the third part of the duodenum. Therefore, the mass was considered a visceral lesion with only 5% necrosis and free tumor margins (R0) (Fig. 2).
A) Duodenal invasion by a multicystic nodular mass, B) Image of the tumor with cysts of liquid content, and mucoid consistency in some, C) Presence of a fibrous infiltrating mass with areas that are mesenchymal in appearance with differentiation into cartilage (black arrow) and areas of inflammation and necrosis (grey arrow), D) Gland structures covered in epithelium, in some areas immature and in others showing differentiation into respiratory-like ciliated epithelium (black arrow), dissimilar from the mature intestinal epithelium covering the lumen.
Retroperitoneal masses are common findings after chemotherapy of embryonal metastatic tumors. When a retroperitoneal mass grows, it can be due to failure of chemotherapy or transformation to a teratoma. Some 48% of patients with residual masses after chemotherapy end up developing teratoma, especially when areas of teratoma were already present in the histological study of the orchiectomy.2 For these cases of ‘growing teratoma syndrome’, radical resection is the only universally accepted therapy3 since teratomas are refractory to chemotherapy and radiotherapy.1
Generally, the growth of tumor masses is associated with elevated tumor markers, but 1.9% of patients with normal markers have a mature teratoma,4 and it is important not to confuse the diagnosis of teratoma with recurrence/persistence of the germ cell tumor.
A greater percentage of necrosis is associated with benign disease.5 In our case, the percentage of necrosis was very low, which is consistent with greater aggressiveness. The rarity of a teratoma invading the duodenum and causing hemorrhage is a presentation that has not been previously reported in the literature.
The first duodenectomy without pancreatectomy was described in the 1990s,6 but no indication was found in the literature of duodenectomy due to infiltration of a mature teratoma originating from a germ cell tumor. The aim of the procedure is to try to preserve the head of the pancreas in cases of duodenal involvement but no contact with the pancreatic parenchyma. This is essential in cases such as the one described to avoid the morbidity and mortality associated with pancreaticoduodenectomy, provided that a sufficient oncological margin can be ensured.7 In our case, there were doubts about the position of the papilla with respect to the tumor due to the discrepancy between gastroscopy and tomography, so we decided to explore the papilla via the transcystic duct after cholecystectomy for better localization of the lesion.
The differentiation into teratoma of germ cell tumors continues to be a diagnostic and surgical challenge that requires a multidisciplinary approach for the greatest benefit of the patient, which generally involves the participation of several specialties.
The authors would like to thank Dr del Pozo for her fantastic illustrations.
Please cite this article as: Justo I, Rodríguez-Gil Y, Villar R, Kadaoui S-D, Rodríguez de la Calle J. Pancreas sparing duodenectomy como tratamiento de tumor germinal con infiltración duodenal. Cir Esp. 2021;99:392–394.