Pericardial effusion is a clinical condition requiring multidisciplinary management. There are several surgical techniques for its diagnosis and treatment. In the present study we report our experience in performing a pericardial window (PW) by videothorascopy.
Material and methodsWe performed surgery on 56 patients (20 females and 36 males), with a mean age of 56±1.22 years, and diagnosed with moderate to severe chronic pericardial effusion. The side chosen for the approach depended on whether there was an associated pleural effusion or lung lesion, and if not the left side was chosen.
ResultsThe mean duration of the surgery was 37.6±16min. The definitive diagnoses were malignant processes in 23% of cases, including bronchogenic carcinoma and breast cancer. The intraoperative mortality was 0%.
ConclusionsVideothorascopic pericardial window is an effective and safe technique for the diagnosis and treatment of chronic pericardial effusion, and which enables it to be drained and perform a pleuropulmonary and/or mediastinal biopsy during the same surgical act.
El derrame pericárdico (DP) es una entidad clínica de manejo multidisciplinar. Existen varias técnicas quirúrgicas para su diagnóstico y tratamiento. En el presente estudio aportamos nuestra experiencia en la realización de ventana pericárdica (VP) por videotoracoscopia.
Material y métodosHemos intervenido a 56 pacientes (20 mujeres y 36 hombres) con el diagnóstico de DP crónico moderado-severo. La edad media fue de 56±1,22 años. El lado elegido para el abordaje dependía de la existencia del derrame pleural o lesión pulmonar asociada, en su defecto por el lado izquierdo.
ResultadosLa duración media de la intervención fue de 37,6±16 minutos. Los diagnósticos definitivos fueron en el 23% de los casos por procesos malignos, destacando el carcinoma broncogénico y el cáncer de mama. La mortalidad intraoperatoria fue del 0%.
ConclusionesLa VP videotoracoscópica es una técnica efectiva y segura para el diagnóstico y tratamiento del DP crónico, que permite en un mismo acto quirúrgico su drenaje y la biopsia pleuropulmonar y/o mediastínica.
The pericardium is a serous membrane composed of two layers (parietal and visceral) containing a small amount of fluid. The inflammatory response in the pericardium is accompanied by a varying accumulation of fluid (>50ml).1
The pericardium can be directly affected by infectious, inflammatory, physical, or traumatic agents, and secondarily by metabolic disorders and systemic diseases. The reaction of the pericardium to this type of aggression can involve, among other things, accumulated fluid in the pericardial space (pericardial effusion [PE], and on occasion, cardiac tamponade).2
Several different classification systems have been elaborated in the attempt to quantify pericardial effusion, considering that pericardial fluid is not usually directly measured, and effusion is usually diagnosed indirectly, through several imaging techniques, generally echocardiogram. In a practical sense, the separation of the visceral and parietal pericardium can be very indicative of the level of effusion. According to the Weitzman criteria,1 a separation between pericardial sheets less than 0.5cm is considered to be mild PE, 0.5–1.5cm: moderate, 1.5–2.5cm: moderate–severe, and >2.5cm: severe.
PE is frequently observed as a casual finding, since it does not cause symptoms in many cases. In spite of this, its diagnosis can have important consequences for the prognosis and treatment of patients. Approximately half of the patients with PE have cardiac tamponade,3–5 which is a medical emergency requiring drainage through pericardiocentesis, regardless of the aetiology.
The treatment of PE depends fundamentally on the underlying disease and haemodynamic state.2 If the PE is mild, there is no need for further examination in the absence of symptoms. In moderate PE, a general blood analysis is necessary, along with a study of thyroid function. In this case, the treatment will depend on the aetiology of the condition, and if this is unknown, a yearly follow-up of the patient is needed. In massive effusion, the same analyses are performed as in moderate effusion in addition to pericardiocentesis, with an analysis of the pericardial fluid. If the effusion is repeated with a massive amount of fluid, pericardiocentesis is repeated as well, except for those patients who have already undergone this procedure several times. After the new session of pericardiocentesis, the patient must be monitored, and if massive effusion continues, a pericardiectomy is indicated.2,6
In recent years, percutaneous balloon pericardiotomy has become an important tool in the diagnosis and treatment of PE. However, in certain cases, such as recurrent or loculated effusion, a pericardial window (PW) is indicated.7 In this study, we present our experience and the results from performing PW using video-assisted thoracoscopic surgery (VATS).
Material and MethodWe performed a retrospective study during the period of July 1996 to December 2009 with 56 patients with moderate–severe PE (36 men and 20 women) undergoing PW using video-assisted thoracoscopic surgery. The mean patient age was 56±1.22 years. Central chest pain and dyspnoea were present in 82% of the patients. We requested a CAT scan for determining the PE location, as well as the presence of associated pleuropulmonary disease. In the case of radiological or echocardiographic signs of constrictive pericarditis or unstable haemodynamic state, the patient was excluded from VATS. All patients were evaluated in the cardiology department, with the primary indications being:
- –
chronic moderate–severe PE,
- –
recurrent PE,
- –
PE of unknown aetiology,
- –
PE associated with pleuropulmonary disease,
- –
PE resistant to medical treatment, and
- –
loculated PE.
We performed the procedure under general anaesthesia and selective intubation, placing the patient in lateral decubitus position for lateral thoracotomy. Five patients with signs of compromised haemodynamic state and tamponade required pericardiocentesis prior to anaesthesia.
We chose the side to operate upon based on the presence of associated pulmonary lesions or pleural effusion, choosing the left side in their absence. The left approach was used in 45 cases.
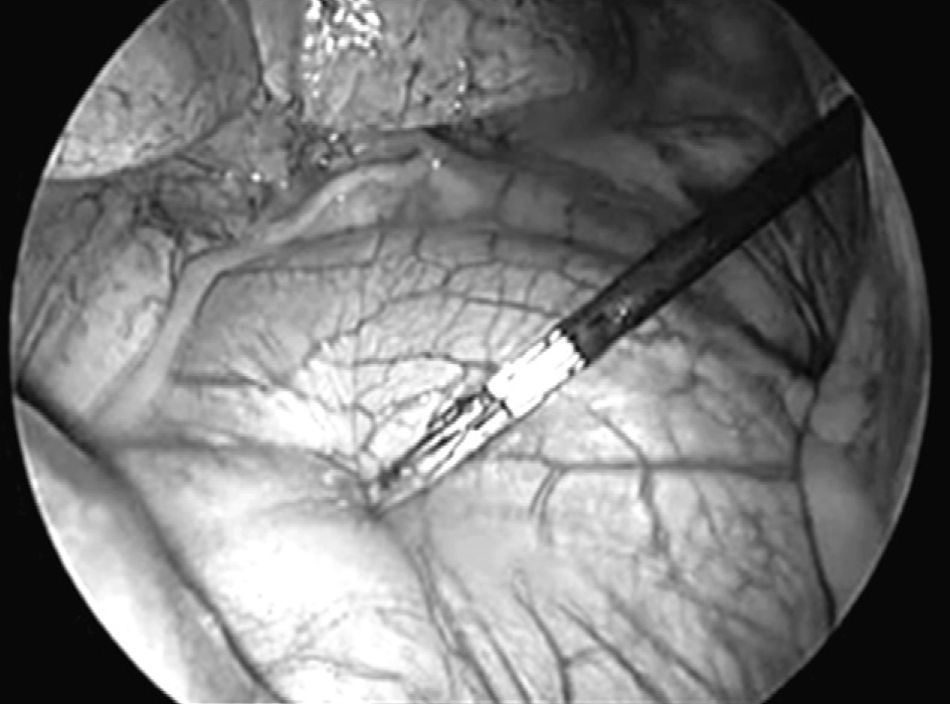
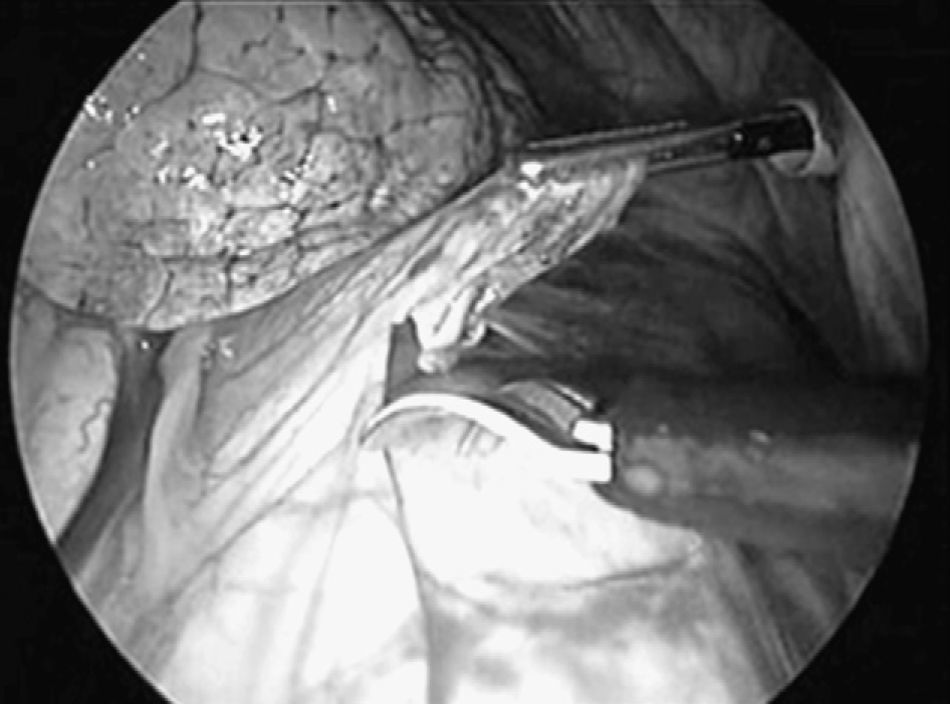
We used three different access ports in 45 cases and four in the rest due to problems with collapsed lungs and adherences. The first access port (11.5mm trocar) was at the seventh to the eighth intercostal space on the midaxillary line, for inserting the optics trocar. After exploring the pleural cavity, pleural effusion was aspirated when encountered and sent for a smear test. The second and third access ports (5mm trocars) were at the fifth and the third intercostal spaces on the anterior axillary line. Once the phrenic nerve was identified, traction of the pericardium was performed using an endoscopic clamp (Fig. 1). We then followed with a dissection of the pericardium using endoscissors (Fig. 2) with electrocoagulation (42 cases) and ultrasonic scalpel (12 cases). In two cases the pericardium was thickened and heavily vascularised, requiring three 2.5mm EndoGIA staples, with no intraoperative complications.
In all cases, the PW was larger than 3×5cm. The pericardial fluid and tissue were sent for microbiological and histological analyses. In all cases, we left a Blake® pleural drainage tube that was removed when the output was less than 250cm3.
ResultsOf the 56 patients, 18 had macroscopically blood-stained fluid (32.2%), 16 had serosanguineous fluid (28.5%), and 22 had serous fluid (39.3%). The mean volume drained was 430.6±120cm3. The results of the microbiological fluid analysis were negative. The smear test revealed the presence of atypical cells in the pericardial fluid in five cases.
The definitive diagnosis was benign processes in 77.3% of cases: idiopathic (12), tuberculosis (9), non-tuberculosis infection (7), post-cardiovascular surgery (5), uraemic (3), amyloidosis (2), lupus (2), liver disease (1), mononucleosis (1), and sarcoidosis (1); and the other 23.7% were due to malignant processes: bronchogenic carcinoma (6), breast carcinoma (3), lymphoma (2), sarcoma (1), and mesothelioma (1). Intraoperative mortality was 0%. The mean duration of the procedure was 37.6±16min.
During the postoperative period, we registered one case of air leak and two cases of prolonged output, which were treated with pleural drainage and respiratory physical therapy. The mean hospital stay was 3.7 days (range: 2–9 days). We found two cases of recurrence: one occurred 1 week after the initial operation, requiring subxiphoid drainage, and one after 7 months, requiring a second PW in the contralateral hemithorax.
Mean patient survival in cases of PE due to malignant processes was 5.6 months (range: 2–18 months). The non-neoplastic cases included two deaths due to stroke and one from acute pulmonary oedema.
DiscussionThe most common aetiology of PE is malignant, with lung and breast cancer as the most frequent causes. Other causes that have been described include lymphoma, infectious diseases, metabolic disorders, post-thoracic surgery, trauma, mesenchymopathy, and even idiopathic causes.8 In our cohort, 23% of cases were due to malignant processes, and bronchogenic carcinoma and breast cancer were the most common neoplastic diseases.PE is a clinical entity requiring multidisciplinary management. Pericardiocentesis is the technique of choice in acute PE, since it is a quick method that does not require general anaesthesia.9 However, for chronic PE, the optimal course of action for diagnosis and drainage is highly debated, and varies according to the needs and circumstances of each patient.9–13Several different approaches have been described for the diagnosis and treatment of PE: pericardiocentesis, percutaneous balloon pericardiotomy, subxiphoid pericardial drainage, pericardial–peritoneal shunt, subxiphoid PW, and PW through anterior thoracotomy/sternotomy or VATS.9–13
The primary advantage of VATS compared to the subxiphoid approach or percutaneous balloon pericardiotomy is the use of a wide resection of the pericardium under visual control, as well as the availability of a pleuropulmonary and/or mediastinal biopsy in the case of associated infection.13 In our cohort, in addition to the PW, VATS also allowed for performing a pleural biopsy in five cases (mesothelioma [1], lung cancer [2], and fibrosis [2]), and a mediastinal biopsy in seven cases (sarcoidosis [2], tuberculosis [4], and lymphoma [1]). Another advantage is the low rates of morbidity and mortality as compared to other approaches.9–13 In our study, intraoperative mortality was 0%, and the rate of complications was 5%.
In our study, following radiological (echocardiographic) and clinical follow-up for 2 years, two cases of recurrence were found. Piehler et al.14 suggest the existence of a relationship between the window dimensions and the incidence of recurrence and development of constriction. The subxiphoid window, in spite of being a relatively quick procedure, with low costs and morbidity/mortality rates, has limited exposure and only allows for the resection of a small section of the pericardium, with a recurrence rate of 2.6%–20%.12
In recent years, percutaneous balloon pericardiotomy has undergone major development.10 This technique has been used on many patients with symptomatic PE secondary to pericardial metastases from extra-cardiac tumours. However, as other authors, we believe that this technique is useful in cases with malignant aetiology, mid-term prognosis, and general poor state of health.11
In conclusion, surgery is indicated when medical treatment fails or when it is necessary for establishing a diagnosis.13 Although PW requires general anaesthesia and selective intubation, it is a minimally invasive technique that facilitates effective drainage and biopsies, especially in loculated effusion and in cases of associated pleural disease, thus avoiding complications from classically used surgical procedures. Given the results and experience from our study, we believe that VATS using video-assisted thoracoscopic surgery is a safe and effective technique for this type of PE.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Triviño A, et al. Ventana pericárdica por videotoracoscopia. Cir Esp. 2011;89:677–80.