Bariatric surgery has proven to be highly effective in controlling obesity and metabolic syndrome; the results of this surgery are not only expressed in terms of weight loss, but also in terms of resolution of comorbidities, improved quality of life and complications. The different parameters used to measure these outcomes require uniformity and reference patterns. Therefore, it is essential to identify those indicators and quality criteria that are helpful in defining the “best practice” principles in bariatric surgery. In this regard, the Section of Obesity of the Spanish Association of Surgeons, in collaboration with the Spanish Society for Bariatric Surgery (SECO), present as an objective to identify the key points that define “quality” in this type of surgery. We describe the main indicators based on the published literature as well as the criteria for referral of the main comorbidities according to the evidence found and grades of recommendation.
La cirugía bariátrica ha demostrado ser muy eficaz en el control de la obesidad y el síndrome metabólico. Sus resultados no solo se expresan en términos de pérdida de peso, sino también en la resolución de comorbilidades, mejoría de la calidad de vida y de las complicaciones derivadas. Los diferentes parámetros utilizados para medir estos resultados requieren de una uniformidad y de unos patrones de referencia. Por ello, es fundamental establecer cuáles son los indicadores y los criterios de calidad que definen las «buenas prácticas» en cirugía bariátrica. En este sentido, la Sección de Obesidad de la Asociación Española de Cirujanos (AEC), en colaboración con la Sociedad Española de Cirugía de la Obesidad (SECO), se plantea como objetivo identificar los puntos clave que definen la calidad en este tipo de cirugía. Para ello se describen los principales indicadores basados en la literatura publicada, así como los criterios de remisión de las principales comorbilidades según las evidencias encontradas y sus grados de recomendación.
Bariatric surgery has been shown to be effective for controlling morbid obesity and metabolic syndrome, with a clear superiority over medical treatments.1,2 It is essential to establish quality criteria to define “good practice” in bariatric surgery in order to be able to compare results and know if we are offering effective surgical treatment. Taking into account that the loss of patient follow-up is the main limiting factor in the evaluation of results, there should be a minimum follow-up of 60% for at least 5 years according to the International Bariatric Surgery Registry and the Standards Committee.3 The Obesity Division of the Asociación Española de Cirujanos (Spanish Association of Surgeons, AEC) in collaboration with the Sociedad Española de Cirugía de la Obesidad (Spanish Society of Obesity Surgery, SECO) has proposed identifying the key points that define the quality of bariatric surgery.
Based on the published literature, the objective of this article is to describe current quality indicators and a minimum threshold required in clinical practice, as well as criteria for remission of the main comorbidities.
MethodsSearchA bibliographic search was conducted by groups of 2 members of the AEC Morbid Obesity Division, using 3 bibliographic databases (Pubmed, Scopus and Web of Science) with the keywords “bariatric surgery and weight loss results/excess weight loss”, “bariatric surgery and comorbidities/diabetes mellitus/cholesterol/dyslipidaemia/obstructive apnea/hypertension”, “bariatric surgery and morbidity/complications”, “bariatric surgery and mortality”, “bariatric surgery and revisional surgery”, “bariatric surgery and standards” and “bariatric surgery and quality of life”. The members considered articles in both English and Spanish that had been published between 2005 and 2015. Articles were classified by subject areas and reviewed by division members, who initially decided to include or exclude them according to the information that answered the key questions raised. Subsequently, two supervisory members assessed the included articles and confirmed their inclusion/exclusion. Articles were excluded if they provided no specific numerical data or clinical cases, and type 2++ studies were accepted as a minimum level of evidence (cohort studies or well-conducted case–control studies with low risk of bias according to the Scottish Intercollegiate Guidelines Network4 [SIGN] scale). Quality standards were then defined based on the evidence found, as well as their levels of evidence and grades of recommendation.
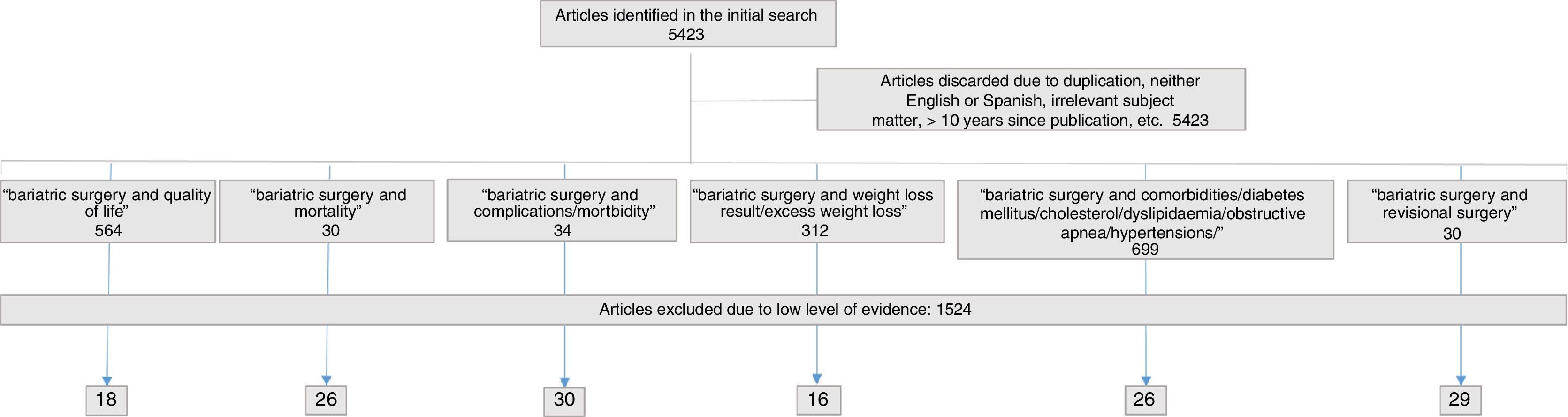
SelectionInitially, 5423 references were identified, from which 312 articles were selected related to weight, 699 articles about comorbidities, 564 on quality of life, 30 on revision surgery, 30 on mortality and 34 about postoperative complications. The final selection of articles and their distribution are shown in Fig. 1. The figures provided in the standards or indicators are the same as those found during the review process.
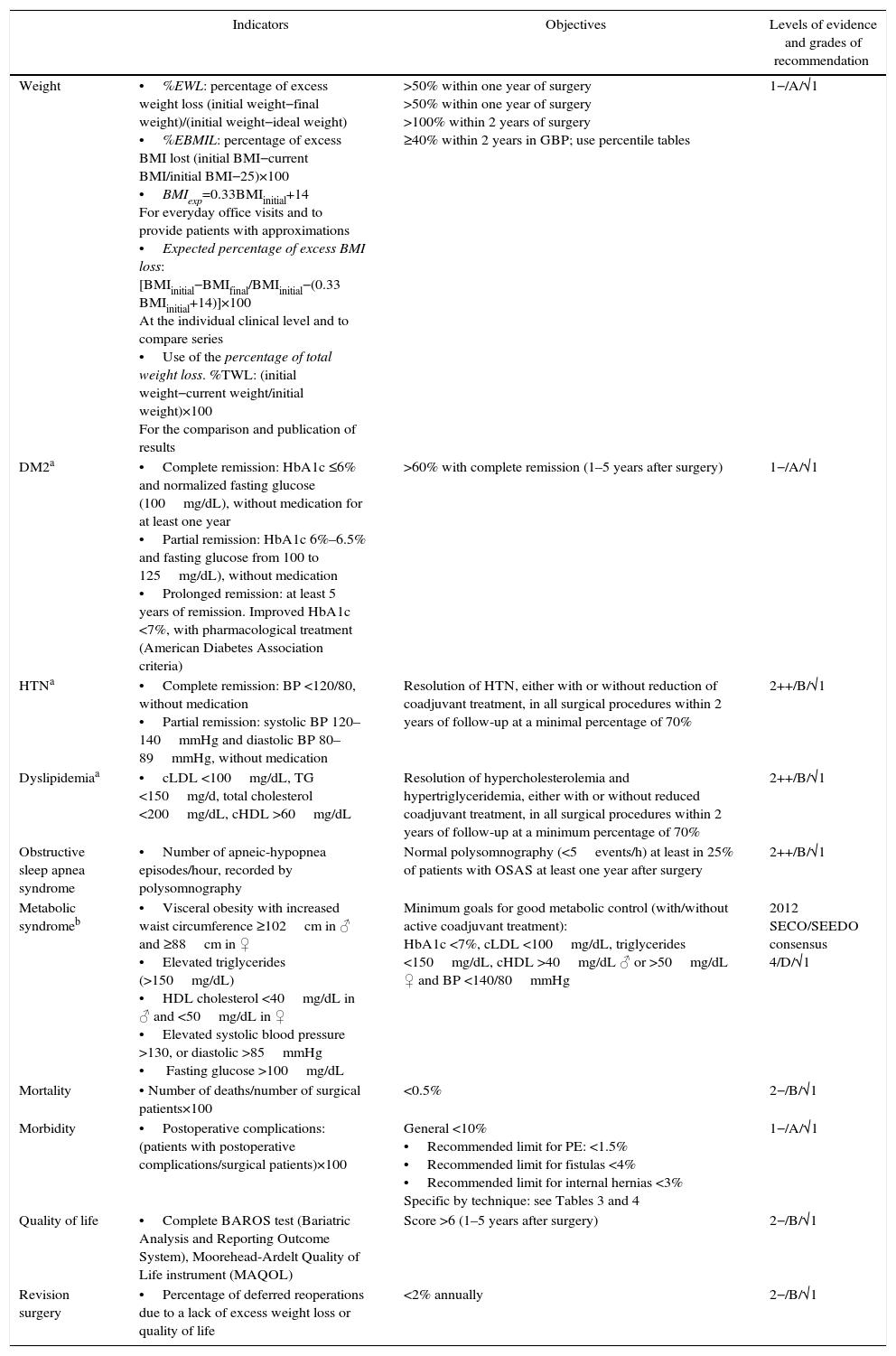
ResultsTable 1 summarizes the main quality indicators, objectives and grades of recommendation. In the case of comorbidities, the indicators are substituted by the remission criteria. Table 2 compiles the summary of the recommendations.
Main Indicators With Objectives and Recommendations.
| Indicators | Objectives | Levels of evidence and grades of recommendation | |
|---|---|---|---|
| Weight | •%EWL: percentage of excess weight loss (initial weight−final weight)/(initial weight−ideal weight) •%EBMIL: percentage of excess BMI lost (initial BMI−current BMI/initial BMI−25)×100 •BMIexp=0.33BMIinitial+14 For everyday office visits and to provide patients with approximations •Expected percentage of excess BMI loss: [BMIinitial−BMIfinal/BMIinitial−(0.33 BMIinitial+14)]×100 At the individual clinical level and to compare series •Use of the percentage of total weight loss. %TWL: (initial weight−current weight/initial weight)×100 For the comparison and publication of results | >50% within one year of surgery >50% within one year of surgery >100% within 2 years of surgery ≥40% within 2 years in GBP; use percentile tables | 1−/A/√1 |
| DM2a | •Complete remission: HbA1c ≤6% and normalized fasting glucose (100mg/dL), without medication for at least one year •Partial remission: HbA1c 6%–6.5% and fasting glucose from 100 to 125mg/dL), without medication •Prolonged remission: at least 5 years of remission. Improved HbA1c <7%, with pharmacological treatment (American Diabetes Association criteria) | >60% with complete remission (1–5 years after surgery) | 1−/A/√1 |
| HTNa | •Complete remission: BP <120/80, without medication •Partial remission: systolic BP 120–140mmHg and diastolic BP 80–89mmHg, without medication | Resolution of HTN, either with or without reduction of coadjuvant treatment, in all surgical procedures within 2 years of follow-up at a minimal percentage of 70% | 2++/B/√1 |
| Dyslipidemiaa | •cLDL <100mg/dL, TG <150mg/d, total cholesterol <200mg/dL, cHDL >60mg/dL | Resolution of hypercholesterolemia and hypertriglyceridemia, either with or without reduced coadjuvant treatment, in all surgical procedures within 2 years of follow-up at a minimum percentage of 70% | 2++/B/√1 |
| Obstructive sleep apnea syndrome | •Number of apneic-hypopnea episodes/hour, recorded by polysomnography | Normal polysomnography (<5events/h) at least in 25% of patients with OSAS at least one year after surgery | 2++/B/√1 |
| Metabolic syndromeb | •Visceral obesity with increased waist circumference ≥102cm in ♂ and ≥88cm in ♀ •Elevated triglycerides (>150mg/dL) •HDL cholesterol <40mg/dL in ♂ and <50mg/dL in ♀ •Elevated systolic blood pressure >130, or diastolic >85mmHg • Fasting glucose >100mg/dL | Minimum goals for good metabolic control (with/without active coadjuvant treatment): HbA1c <7%, cLDL <100mg/dL, triglycerides <150mg/dL, cHDL >40mg/dL ♂ or >50mg/dL ♀ and BP <140/80mmHg | 2012 SECO/SEEDO consensus 4/D/√1 |
| Mortality | • Number of deaths/number of surgical patients×100 | <0.5% | 2−/B/√1 |
| Morbidity | •Postoperative complications: (patients with postoperative complications/surgical patients)×100 | General <10% •Recommended limit for PE: <1.5% •Recommended limit for fistulas <4% •Recommended limit for internal hernias <3% Specific by technique: see Tables 3 and 4 | 1−/A/√1 |
| Quality of life | •Complete BAROS test (Bariatric Analysis and Reporting Outcome System), Moorehead-Ardelt Quality of Life instrument (MAQOL) | Score >6 (1–5 years after surgery) | 2−/B/√1 |
| Revision surgery | •Percentage of deferred reoperations due to a lack of excess weight loss or quality of life | <2% annually | 2−/B/√1 |
Summary of the Recommendations by the Morbid Obesity Division of the AEC and SECO.
| -It is recommended that bariatric surgeons complete the specific training programs promoted and supervised by the Spanish Association of Surgeons (Asociación Española de Cirujanos, AEC) and the Spanish Society of Obesity Surgery (Sociedad Española de Cirugía de la Obesidad, SECO) or other duly certified organisms. |
| -A multidisciplinary approach to patient treatment is necessary: surgeon, anesthesiologist, endocrinologist, nutritionist, psychologist, etc. |
| -The use of specific scales to predict risk of mortality is recommended before surgery. |
| -It is necessary to incorporate the systematic use of the percentage of total weight loss (%TWL) to express the results of the weight loss, in addition to traditional indicators. These values should be included in the percentile tables for reference values. The use of expected BMI may be useful in everyday clinical practice. |
| -The use of official resolution criteria (ADA, ASBMS and 2012 SECO-SEEDO Consensus) is recommended for expressing and publishing results in terms of comorbidities. |
| -It is recommended to systematically record and analyze data on early- and late-onset complications, as well as mortality. |
| -Specific quality-of-life tests are recommended, to be used before and after bariatric surgery. |
| -Systematic, detailed follow-up is necessary for all comorbidities, as well as polysomnography follow-up studies after surgery in patients who are also affected by OSAS. |
| -Bariatric revision surgery should be performed by experienced bariatric surgeons and at expert or certified institutions (with a recommended minimum of 50 cases per year, in accordance with criteria of the European Accreditation Council for Bariatric Surgery). |
Complete weight normalization is not an essential condition for achieving a significant improvement in health.5 Some authors argue that the improvement of comorbidities and social repercussions are more important than the quantification of weight loss.6 In 1981, it was proposed to use the percentage of excess weight loss (%EWL) to quantify the outcome of bariatric surgery, putting the success limit at 50% EWL.7,8 In 1997, Baltasar et al.9 proposed considering body mass index (BMI) associated with the %EWL to classify the results. In 2004, the concept of percentage of excess BMI lost (%EBMIL)10 was introduced. In 2013, the meta-analysis by Courcoulas et al.11 analyzed the results of 161756 patients, in terms of %EWL, for up to 5 years of follow-up, with an initial mean BMI of 45.5kg/m2. One year after surgery, the estimated %EWL for gastric bypass (GBP) ranged from 63% to 72% and from 51% to 69% for vertical sleeve gastrectomy (VSG). After 2 years, the %EWL for GBP was 74%–80% vs 42%–50% for VSG. After the fourth and fifth years, the variability in the GBP increased (59%–93% and 44%–85%, respectively), with insufficient data for VSG. The Spanish VSG registry12 obtained, one year after surgery, a %EBMIL of 78% for a BMI <40kg/m2, 75% for a BMI between 40 and 49kg/m2, 55% for a BMI between 50 and 59kg/m2 and 67% for a BMI >60kg/m2. Three years after surgery, %EBMIL results were close to 100% in BMI <40kg/m2, while for BMI >40kg/m2 the range was between 60% and 78%.
Regarding biliopancreatic diversion (BPD) with duodenal switch (DS), the results of Nelson et al.13 reported a %EWL of 79% 2 years after surgery in patients with a BMI >50kg/m2, which is comparable to the results published by Buchwald et al.14 Also, VSG with duodenoileal bypass (SADIs) offered a %EWL of 94.7% one year after surgery.15
However, there are other ways to express weight loss. Along these lines, the Bariatric Outcomes Longitudinal Database (BOLD)16 in 2012 postulated that the most homogeneous value with the least variability is the percentage of total weight loss in kg (%TWL)=(initial weight−current weight/initial weight)×100.17 Several studies have already published their results in this manner, with values of −44% for BPD-DS and −34% for GBP at the 2-year follow-up,18 or −33.5% after 5 years.19 The %TWL allows for comparisons between series, while avoiding the bias of the initial BMI, and can be represented graphically in tables of percentiles created from the data of different series. It is difficult for a super-obese individual (BMI >50kg/m2) to reach a BMI of 25kg/m2 after surgery: it seems reasonable to set a more realistic limit to rationalize their expectations. In this context, Baltasar et al.20 propose the term of expected BMI (BMIexp=0.33BMIinitial+14), calculated by linear regression and eliminating the cut-off point of 25 as a constant. The formula applied for the EBMI is: [BMIinitial−BMIfinal/BMIinitial−(0.33BMIinitial+14)]×100. The result is classified as excellent if ≥100% and improvable if ≤100%. Subsequently, Baltasar has adjusted the constants for each surgical technique. Thus, there is a different formula for each, so series and surgical techniques can be compared more precisely.21
Quality Standards for the Resolution of Comorbidities: the Resolution of Comorbidities Should Be Collected, Analyzed and Reported According to the Official Resolution Criteria of the Scientific Societies InvolvedBariatric surgery significantly resolves obesity-related comorbidities and improves long-term morbidity and mortality.22,23 As early as 2004, Buchwald et al.24 described the resolution of comorbidities according to the different techniques in their meta-analysis that has been widely discussed in the scientific community.
Type 2 Diabetes MellitusAccording to a review published by the Cochrane Library,25 the remission rate of type 2 diabetes mellitus (DM2) depends on the type of surgery. For BPD, it is about 95% and 57% for gastric banding (GB), without sufficient long-term data for VSG. In 2009, Buchwald14 focused on the resolution of DM2 with an overall remission rate of 78%, which remained stable after 2 years at 62%. The highest resolution was obtained with BPD (95.1%), followed by GBP (80.3%), vertical banded gastroplasty (79.7%) and GB (56.7%). A recent meta-analysis published remission rates of 60.8% for VSG.26 With the SADIs technique, complete resolution is described in 75% of patients 3 years after surgery in patients treated with oral antidiabetics and 38.4% in patients receiving insulin.27 The most recently reported global remission rates are lower than previously reported in the literature: it is estimated that 60% of patients achieve remission within the first 5 years after surgery, as indicated by several clinical trials and meta-analyses.28–33 There are differences depending on the criteria used to consider diabetes resolved or not. Because of this variability, which has been recently demonstrated,34 it is recommended to use the criteria of the American Diabetes Association (ADA) exclusively (Table 1).35
Arterial Hypertension and Cardiovascular RiskA good scale for measuring cardiovascular risk in the Western world is the Framingham risk score,36 although in Spain there are other scales adapted to our population, such as REGICOR, which is based on a lower cardiovascular risk than the Anglo-Saxon world.37 The estimated risk of coronary heart disease after GBP drops from 11% to 5% in men and 6% to 3% in women for both the diabetic and non-diabetic populations.38,39 After GBP, the resolution or improvement of arterial hypertension (HTN) ranges between 61% and 78.5% of patients at the 2-year follow-up, even in patients ≥55 years of age.40 In the SOS study,41 however, during the early years of follow-up there is a rebound of this disease, with 13.2% of patients relapsing into hypertension. Subsequently, the same study shows that restrictive techniques exert a transient effect on hypertension, whereas GBP is associated with more sustained reductions and even with increased daily diuresis.42 There are studies that indicate that vitamin D deficiency after surgery plays an important role in the development of hypertension: patients with vitamin supplementation resolve HTN more effectively than non-supplemented patients.43,44 Remission criteria are defined according to recently published standards by Brethauer et al.45 of the American Society for Bariatric and Metabolic Surgery (ASBMS) (Table 1).
DyslipidemiaSeveral studies analyze the improvement of dyslipidemia after surgery.46 One year after GBP, antidiabetic, antihypertensive and hypolipidemic medication was decreased by 76%, 51% and 59%, respectively.47 These results contrast with initial data from the SOS study,41 in which no differences were found in total cholesterol levels between the control patients and surgically treated patients after 10 years (except for the group treated with GBP). Afterwards, dyslipidemia was observed to normalize and continue to be stable after 10 years in 69.7% of the patients treated surgically, compared to 22% of the non-surgical patients.48 In the Buchwald meta-analysis,24 the lipid profile significantly improved in all the surgical procedures at the 2-year follow-up in a minimum percentage of 70%, with maximum improvements for BPD-DS (99.1%) and GBP (96.9%). The resolution criteria were those defined by Brethauer et al.45 (Table 1).
Metabolic SyndromeMetabolic syndrome encompasses a set of risk factors that relate to cardiovascular disease and diabetes. For its definition, the criteria published by Alberti et al.49 are used (1: visceral obesity with increased waist circumference ≥102cm in ♂ and ≥88cm in ♀; 2: elevated triglycerides [>150mg/dL]; 3: HDL cholesterol <40mg/dL in ♂ and <50mg/dL in ♀; 4: elevated systolic arterial pressure >130 or diastolic >85mmHg; 5: fasting glucose >100mg/dL). The minimum follow-up goal after surgery is defined by the approved criteria in the SECO/SEEDO 2012 Consensus50 (Table 1).
Obstructive Sleep Apnea SyndromeSeveral studies have demonstrated that weight loss due to bariatric surgery improves obstructive sleep apnea syndrome (OSAS), even in the long term.51 The diagnosis is made with ≥5 apneas or hypopneas/hour.52 The prevalence ranges in obese individuals vary from 55% to 100%, depending on whether all patients are evaluated or exclusively those with symptoms.24 Buchwald et al.24 show a significant improvement in the series analyzed of 85.7%. However, Greenburg et al.53 determine that residual disease continues to exist in the majority of older and more obese patients and only 23% meet resolution criteria. The lack of “daytime sleepiness” does not indicate OSAS resolution.54 Therefore, routine diagnostic tests including polysomnography should be carried out when a stable weight is reached (after the first year, at least).
Mortality Standards in Bariatric Surgery: Currently Mortality Should Be Less Than 0.5%In 1991, the accepted mortality rate ranged from 0.5% to 1.5%.55 Currently, it is close to 0% thanks to laparoscopic procedures, training programs and multidisciplinary patient management.56 Recent publications of the Longitudinal Assessment of Bariatric Surgery Consortium data or the Bariatric Outcome Longitudinal Database (BOLD), among others, confirm that the mortality rate is below 0.5%.57–63 This is an acceptable rate, considering that long-term mortality in non-operated morbid obese patients is greater than 6%.64 In 2011, mortality from this cause was analyzed specifically, and the overall 30-day mortality rate was 0.3%.65 The most frequent cause of death was multiple organ failure due to sepsis (33%), followed by cardiac pathologies (28%) and pulmonary embolism (17%). Abdominal sepsis, especially when associated with anastomotic leakage, continues to be a challenge in this type of patients.66,67 Mortality is variable and depends on the experience of the surgical group, which reinforces the importance of the learning curve.68 Mortality during the learning curve of training programs is 0.57% for surgeons with no specific training and 0% for those who are trained, with a reduction in complications from 18% to 7.7% in surgeons who are well trained.69 A mortality rate of 5% has been recorded in groups that perform less than 10 procedures/year and 0.2% in groups with large patient volumes.70,71 Mortality is also influenced by the approach used and patient sex (0.30% in open surgery vs 0.07% in laparoscopic surgery; 4.74% ♂ vs 0.13% ♀).72 The surgical technique itself is an independent risk factor: mortality rates are reported at less than 0.3% for GB73 0.4% for GBP.74–76 Similarly, the Spanish VSG registry12 situates the rate at 0.36%, which is in line with other groups with extensive experience.
There are several predictive scales for risk of mortality that stratify patients into subgroups. Such tools have shown increased rates of complications, reoperations and mortality when certain factors are present.77 These scales are the Obesity Surgery Mortality Risk Score (which contemplates BMI >50kg/m2, age >45 years, male sex, HTN and risk of pulmonary embolism [PE]),78 the Longitudinal Assessment of Bariatric Surgery (LABS) Consortium Study (which contemplates extreme obesity, history of thromboembolism, presence of OSAS and inability to walk 60m)79 and the Metabolic Acuity Score (which adds DM and psychological factors to the previous scores).80
Morbidity Standards in Bariatric Surgery: Recording and Analyzing Complications Is Mandatory at Hospitals Where This Type of Surgery is PerformedIn the postoperative period, we refer to early morbidity (<30 days: PE, leaks and hemorrhages) or late morbidity (>30 days: marginal ulcers, stenosis and internal hernias). Currently, the overall early morbidity rate is below 7% in centers with more extensive experience.81,82 The complication rate was influenced by the surgical technique, with a higher rate of major complications in GBP (2.5%–3.6%) compared to the VSG (2.2%–2.4%) and, finally, compared to GB (0.9%–1%).82 Morbidity is also related to the volume of procedures performed, both by the hospital in general as well as by the specific surgeon. The performance of fewer procedures has been identified as a risk factor (morbidity at hospitals with <150 patients: 4.1%; 150–300 patients: 2.7%; >300 patients: 2.3%), although no differences were found depending on whether the hospital was a certified “center of excellence” in bariatric surgery.82,83
As a general complication, the incidence of thromboembolic disease varies widely, ranging from 0%84 to 3.5%.85 An incidence of 0.9% has been reported for PE, 1.3% for deep vein thrombosis and 2.2% for thrombosis associated with PE.86 As for surgical wounds, the open approach has an associated risk of seromas of approximately 40%, with a risk for incisional hernias of 32%.87,88 The percentage of incisional hernias through trocar wounds is 0.57%.89 With regard to the risk of developing internal hernias, there is consensus on its reduction if an antecolic gastric bypass is created, and it is recommended to close all defects with non-absorbable sutures.90–92 Its late diagnosis can lead to perforation of the herniated loop and, consequently, the death of the patient.93 The incidence of internal hernias published in cases of laparoscopic GBP had reached 10%,94 although currently the incidence has been reduced to 0.2%, which has probably been influenced by the non-division of the small bowel mesentery.95–97 The specific morbidity according to the surgical technique is summarized in Tables 3 and 4. The overall risk of bleeding after GBP ranges from 0.94% to 4.4%, which usually occurs in most cases in the immediate postoperative period as a consequence of hemorrhage at the anastomoses, staple lines, dissection of the mesentery or visceral lesions.98,99
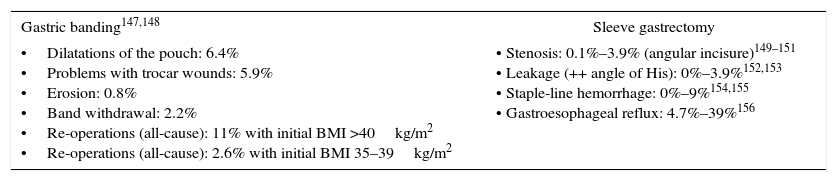
Specific Morbidity for Gastric Banding and Vertical Sleeve Gastrectomy Procedures.
| Gastric banding147,148 | Sleeve gastrectomy |
|---|---|
| •Dilatations of the pouch: 6.4% •Problems with trocar wounds: 5.9% •Erosion: 0.8% •Band withdrawal: 2.2% •Re-operations (all-cause): 11% with initial BMI >40kg/m2 •Re-operations (all-cause): 2.6% with initial BMI 35–39kg/m2 | • Stenosis: 0.1%–3.9% (angular incisure)149–151 • Leakage (++ angle of His): 0%–3.9%152,153 • Staple-line hemorrhage: 0%–9%154,155 • Gastroesophageal reflux: 4.7%–39%156 |
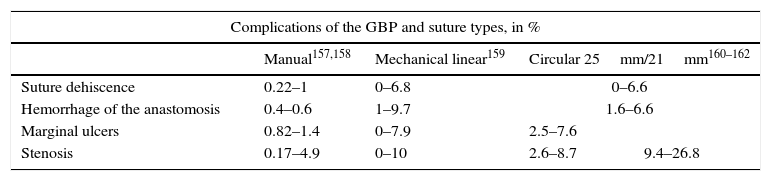
Specific Morbidity for Roux-en-Y Gastric Bypass According to the Type of Suture Used.
| Complications of the GBP and suture types, in % | ||||
|---|---|---|---|---|
| Manual157,158 | Mechanical linear159 | Circular 25mm/21mm160–162 | ||
| Suture dehiscence | 0.22–1 | 0–6.8 | 0–6.6 | |
| Hemorrhage of the anastomosis | 0.4–0.6 | 1–9.7 | 1.6–6.6 | |
| Marginal ulcers | 0.82–1.4 | 0–7.9 | 2.5–7.6 | |
| Stenosis | 0.17–4.9 | 0–10 | 2.6–8.7 | 9.4–26.8 |
GBP: Roux-en-Y gastric bypass.
Bariatric surgery improves quality of life, with changes that are maintained in the long term.100,101 Several instruments are available to assess quality of life102; the most widely used are the Bariatric Analysis and Reporting Outcome System (BAROS),103 Moorehead-Ardelt Quality of Life instrument (MAQOL),104 Impact of Weight on Quality of Life (IWQOL),105 36-Short Form Health Survey (SF-36)106 and the Nottingham Health Profile (NHP).105 Several studies have confirmed a very significant improvement in quality of life after GBP, both one and 5 years after surgery and at different BMI categories.107–109 Patients with a lower BMI prior to surgery correlate with better scores in BAROS and MAQOL-II.110 With regard to long-term follow-up (>6 years), Himpens et al.111,112 demonstrate high satisfaction levels after GBP and VSG (despite the latter having an incidence of 23% of gastroesophageal reflux). Other studies show that, after VSG, a PSP >50% correlates with better scores in areas related to physical function and general health perception within the SF-36 test.113 There are comparative studies on the quality of life for VSG vs GBP and BPD vs GB, with similar results.114,115 Differences have been found between the VSG technique vs GB (in favor of VSG) in the Bariatric Quality of Life (BQL) telephone survey.116 Often the results do not correlate with what is medically desirable and patient expectations: in the case of weight, there are publications that situate the ideal weight desired by patients at a weight equivalent to an EWL of 90%, which makes one realize that their expectations are an important factor to take into account in the overall score of these tests.117
Quality Indicators in Bariatric Revision Surgery: Revision Surgery Should Be Performed at Hospitals With Extensive Experience in Bariatric SurgeryRevision surgery is performed when an initial bariatric procedure has failed or has caused intolerable sequelae. Over the years, several failure criteria have been defined based on final weight, but we can also affirm that there is a failure when the comorbidities related to early mortality cannot be controlled.10 The causes of failure are related to the surgical technique selected, multidisciplinary bariatric team, correct patient selection and follow-up, and the patient's inability to follow a proper diet.118–123 The standards published by Baltasar et al.9 contemplate an annual percentage below 2%; however, as the volume of primary surgeries increases, this percentage increases. There have been reports of series with reoperation rates from 5% to 56%.124–126 Revision surgery is technically complex and is generally associated with higher risk than primary procedures,127,128 and there appears to be no standardized surgical approach.129 Although traditionally performed by laparotomy, today there is a growing tendency to use a laparoscopic approach.130,131 Laparoscopic revision surgery can be conducted safely if performed by experienced bariatric surgeons at hospitals with a high volume of bariatric and laparoscopic surgeries.129,132–135 The restrictive procedures that most frequently require revision surgery due to insufficient weight loss are vertical banded gastroplasty (25%–54%) and GB (40%–50%), most of which are converted to GBP.136,137 Despite the success of primary GBP, between 10% and 20% of patients have a lack of adequate weight loss or regain weight.138–140 The latest published series describe 5- and 13-year reoperation rates between 8.1% and 9%, respectively.141,142 In VSG, published results are based on complications such as gastroesophageal reflux or stenosis, and rates between 2% and 10% are described.143 Conversion from VSG to BPD-DS is not considered revision surgery in the publications found.144 Furthermore, in cases of insufficient weight loss, many authors perform the second surgery with GBP and not the theoretical secondary procedure (DS), which presents more complications than GBP.145 Revision surgery can present complications of up to 14% and a mortality rate of 0.86%.146 Follow-up periods of more than 5 years are required to evaluate its efficacy.
LimitationsThis study is the result of joint collaboration between different members of the scientific societies represented. The main aim was to identify objective indicators and minimum quality criteria in the overall context of bariatric surgery. Our intention was not to make a systematic comparison between the different surgical techniques, so that specific recommendations for each cannot be extrapolated with sufficient scientific analysis and rigor. Undoubtedly, this represents a future challenge for our work within the Spanish scientific societies.
ConclusionsAdvances in technology, better training of multidisciplinary teams and simplification of laparoscopic surgical techniques have made bariatric surgery one of the safest and most effective procedures, but this efficacy and safety must be verified based on minimum required results. Existing clinical practice guidelines do not always include strict criteria, hence the establishment of quality standards and recommendations is very useful to serve as a basis for the continuous improvement of care provided by all professionals dedicated to the treatment of morbid obesity. Determining how the results fit within a defined framework also undoubtedly benefits patients, and the use of objective tools in daily clinical practice will also help us rationalize expectations in this type of surgery.
Authorship/collaborationsDesign, data collection and composition of the article: Fátima Sabench Pereferrer, Eduardo Domínguez-Adame Lanuza, Ainitze Ibarzabal Olano, María Socas Macias and Víctor Valentí Azcárate
Review of the results: Amador García Ruiz de Gordejuela and Francisca García-Moreno Nisa, Jesús González Fernández, Ramón Vilallonga Puy and Nuria Vilarrasa García
Critical review of the manuscript: Raquel Sánchez Santos and Fátima Sabench Pereferrer
Institutional results and review: Asociación Española de Cirujanos (Spanish Association of Surgeons, AEC) and Sociedad Española de Cirugía de la Obesidad (Spanish Society of Obesity Surgery, SECO).
Conflict of InterestsThe authors have no conflicts of interests to declare.
Please cite this article as: Sabench Pereferrer F, Domínguez-Adame Lanuza E, Ibarzabal A, Socas Macias M, Valentí Azcárate V, García Ruiz de Gordejuela A, et al. Criterios de calidad en cirugía bariátrica: revisión de conjunto y recomendaciones de la Asociación Española de Cirujanos y de la Sociedad Española de Cirugía de la Obesidad. Cir Esp. 2017;95:4–16.