Breast-conserving surgery is the most widely used technique in breast cancer treatment. Although it is responsible for the decline in surgical morbidity, its indication in association with radiotherapy can lead to other complications. Radiation pneumonitis is a rare complication (<1% of cases), and its presentation in a non-irradiated area is even rarer. We report the case of a patient with this complication and we comment on its most relevant aspects.
The patient is a 60-year-old woman with no history of interest who underwent quadrantectomy for a tumour in the left breast, followed by radiotherapy. The pathology study of the specimen demonstrated an in situ carcinoma with positive hormone receptors. For this reason, prophylactic hormone therapy (exemestane) was indicated. Radiotherapy was applied with a linear accelerator, using a global dose of 50grays (Gy) and a daily fraction of 200cGy. The irradiated area was exclusively the left breast, with no axillary or internal mammary chain irradiation.
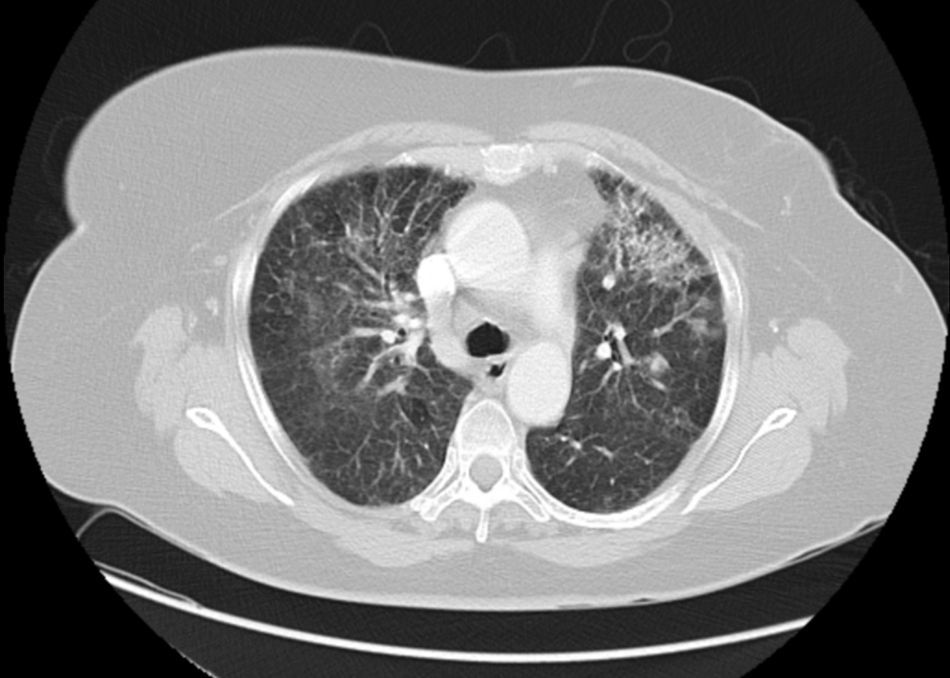
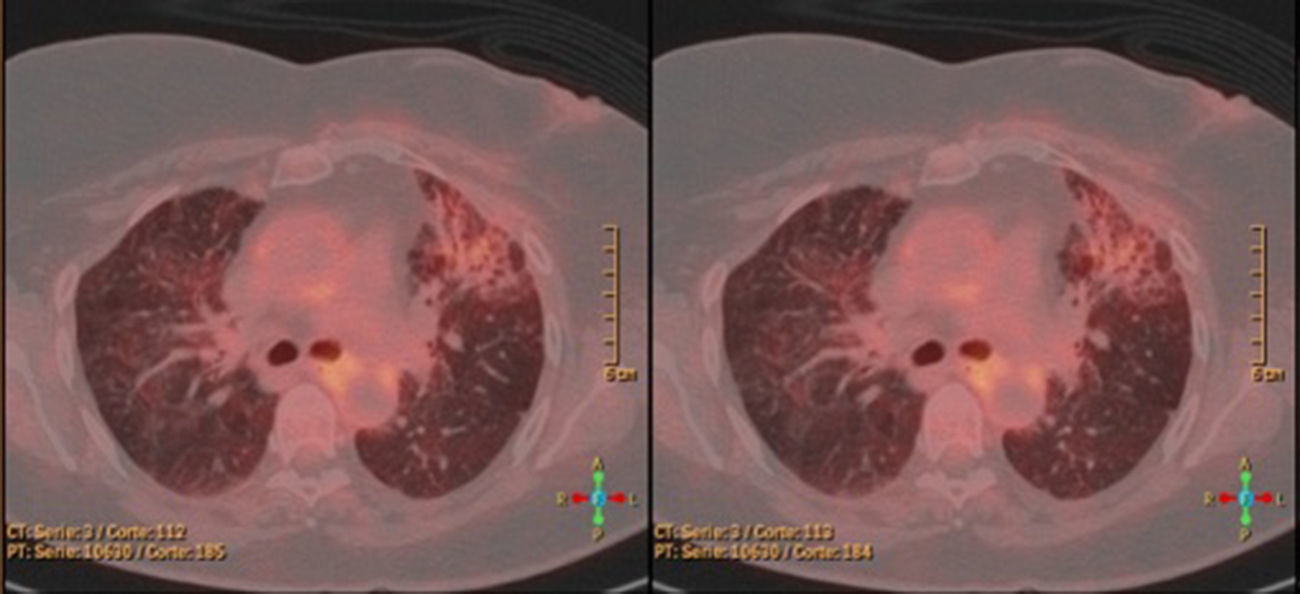
Two months after radiotherapy, the patient came to the Emergency Department due to cough associated with dyspnoea on moderate exertion that had been progressing over the previous two weeks and had not improved with antibiotics or bronchodilators. Upon physical examination, the patient was afebrile, hemodynamically stable and had baseline oxygen saturations of 99%. On auscultation, bilateral (predominantly right) crackles were detected. Chest radiograph revealed a bilateral mid-lower interstitial pattern and bilateral hilar increase (mainly right). Blood work detected no alterations, except for elevated CA 15.3 (131IU/mL). The study was completed with computed tomography (CT) of the lungs, which showed an area with ground glass pattern located in both hilar regions and in the lingular segment of the left lung, with fibrous tracts (Fig. 1). PET/CT was used to rule out distant disease that might have justified the elevated tumour markers, although no lung uptake images were seen (Fig. 2). The patient had a severe restrictive pattern on spirometry. Bronchoscopy with transbronchial biopsy and bronchoalveolar lavage (BAL) cytology showed fibrosis and inflammatory changes with desquamative pneumonitis, but no observed malignant lesions. The BAL cytology demonstrated elevated neutrophils (38%) and the immunophenotype showed evidence of a high CD4/CD8 ratio (8.3). Given the findings of the biopsies (no lymphangitis carcinomatosa), BAL and PET/CT, we decided to initiate treatment for radiation pneumonitis with steroids at high doses (prednisone 60mg/day), which obtained a favourable response and improved symptoms. After 4 weeks of treatment, the patient was asymptomatic and the interstitial pattern in the right lung had disappeared on radiology studies.
Symptoms of radiation pneumonitis include cough and dyspnoea. These signs usually appear 12 weeks after radiotherapy, although their onset may be earlier, as in our patient. Radiological characteristics include images with increased hilar density or alveolar opacities on simple radiography and ground glass pattern on CT. It is also associated with a restrictive pattern on spirometry and a reduction in compliance.
Although radiation-induced lesions are usually located in irradiated areas and are dose-dependent, there are reports of the appearance of lung lesions outside the area of irradiation, as in the case of our patient. These cases are considered rare and unpredictable and do not seem to be predictive of progression towards fibrosis. Several researchers have described alveolitis attributed to increased CD4+ lymphocytes, consistent with a pneumonitis-like hypersensitivity reaction.1 It is accepted that the risk for pneumonitis is directly proportional to the irradiated lung volume. It is known that approximately 5% of irradiated patients after breast-conserving surgery for breast cancer will have an inflammatory reaction after radiotherapy. In cases where lymph node chains are irradiated, the risk increases by 8%.2 In our patient, radiotherapy doses were standard. The concomitance of radiotherapy and chemotherapy has also appeared as an independent risk factor in the development of radiation pneumonitis that is 9 times greater compared with patients who only received radiotherapy or received it sequentially.3 Furthermore, the association with hormone therapy (tamoxifen) seems to be related with increased lung fibrosis,4 although the use of exemestane, as in our case,5 showed no increase. Last of all, the finding of elevated CA 15.3 markers after curative breast surgery usually occurs in 4% of patients during follow-up,6 but in our case they may also have been high due to their relationship with pulmonary interstitial diseases and pulmonary fibrosis.7 Treatment of radiation pneumonitis is based on the use of corticoids; good response is obtained in most cases and symptoms disappear a few weeks after their introduction,8 although small flare-ups of the condition are not uncommon as the corticoid dose is reduced.
In conclusion, it is important to identify the appearance of radiation pneumonitis. This diagnosis should not be ruled out when lesions are bilateral.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Martínez Pérez C, Gumbau Puchol V, Basés Valenzuela C, Villalba Ferrer F, Fuster Diana C. Neumonitis rádica bilateral tras cirugía conservadora del cáncer de mama. Cir Esp. 2015;93:e91–e93.
This manuscript was presented in poster format at the 31st Congress of the Spanish Society of Senology and Mammary Pathology in Barcelona (18–20 October, 2013).