Within the levels of scientific evidence, the randomised clinical trial (RCT) is the methodological design that provides us with the highest quality information.1
The RCT is a study in which participants are randomly divided into intervention groups. It is an analytical, experimental, prospective (i.e. forward looking) and controlled study, as the researcher is present at the time of exposure and effect (concurrent temporality). The purpose of randomising a clinical study is to balance and homogenise the groups participating in it and thus reduce selection bias. Randomisation causes the groups generated to be similar and comparable in all but the intervention, so that if statistical differences in response are detected between the groups generated, they are likely to be due to the study intervention.2
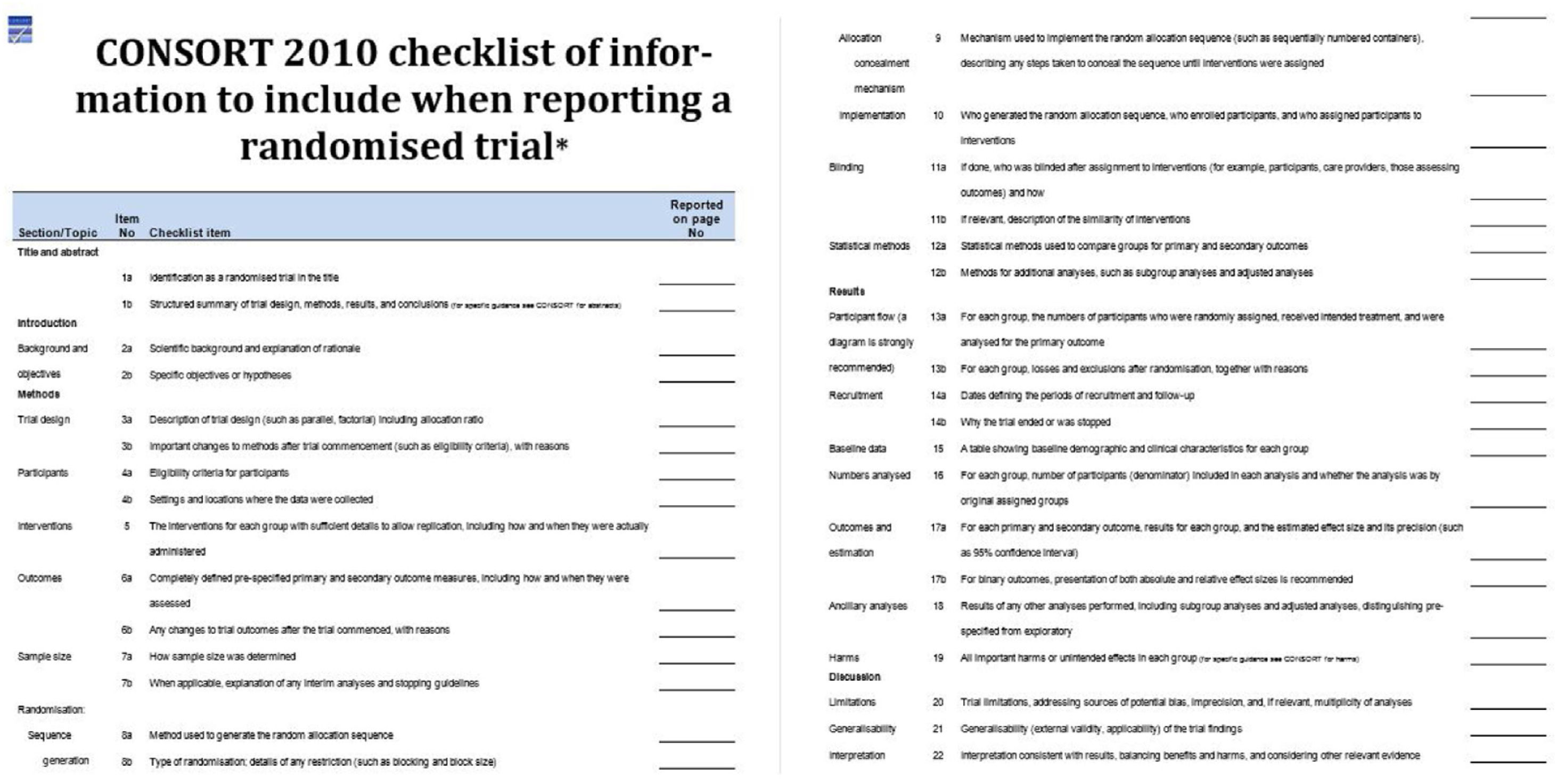
In order to be able to assess the methodology of a RCT, its design, execution, analyses and results must be accurately and transparently detailed. For this purpose, we will use the CONSORT (CONsolidated Standards Of Reporting Trials) statement (Fig. 1).3
Research hypothesisThe RCT must answer a clearly defined and structured question, such that it must clearly state the working hypothesis with its corresponding null and alternative hypotheses on a clinically relevant intervention. In addition, it must have the basic variables defined, such as the sample to be studied, the intervention, or how the analysis will be carried out.
Most RCTs are superiority trials, which hypothesise that one intervention is superior to another in a statistically significant way. Some RCTs are equivalence trials in which the hypothesis is that two interventions are indistinguishable from each other. Finally, non-inferiority RCTs are those that determine whether a new intervention is no worse than a reference treatment.4
Classification according to design- -
Parallel design. This is the most commonly used design. In this design, we have an initial sample that is randomly divided into two groups: one group that receives the intervention under study and another group that is the control, which serves as a comparison and which is usually subjected to a placebo, to the absence of intervention or to an alternative treatment.
- -
- Crossover design. The initial population is randomised into two groups. Each group receives one of the two interventions and, after a window period, receives the other intervention. That is, in this type of design, each group receives the two interventions (study and control) at different times, so each group will be its own control.
- -
Factorial design. This type of design allows the evaluation of two or more interventions in the same study, as long as the treatments or interventions studied have independent mechanisms of action and effects. The most basic form would be as follows: the sample is randomly divided into four groups; the first group receives the two interventions, the second group receives one intervention, the third group receives the other intervention under study, and the fourth group receives the placebo.5
- -
Cluster allocation design. This is a trial in which the allocation of the intervention to be studied is done by previously established groups of individuals (clusters) in a randomised manner, such as health areas or hospitals.
- -
- Sequential design. In this type of clinical trial, observations are assessed as they occur; the total number of participants is not predetermined, but depends on the cumulative results.6,7
Internal validity is directly related to the methodology used for its design, execution, data collection and interpretation of results. External validity, on the other hand, refers to the applicability of the results obtained in our routine clinical practice and the reproducibility of the results.
The two main threats to internal validity are random error and bias.
Random error can be divided into two types. Type I error, significance risk or risk, refers to the probability of defining a false positive conclusion by incorrectly rejecting a true null hypothesis (.05–.025). Type II error, risk or power of the test, refers to the probability that the researcher does not reject the null hypothesis as false (90%–80%).8,9
Among the biases that can affect RCTs is selection bias, which is controlled by randomisation. Randomisation is the non-predictable assignment of trial participants to one of the intervention alternatives. The fundamental objective of randomisation is to balance the groups involved in the trial so that they are homogeneous in the distribution of all those factors, known or unknown, that may bias the study results.10
The most common randomisation techniques are:
- -
Simple randomisation. This technique randomly assigns each participant to an intervention group regardless of the assignment of previous participants.
- -
- Block randomisation. In this case the randomisation sequence is divided into blocks and the assignment of each participant is randomised but ensuring a periodic balance in the number of subjects assigned to each group.
- -
- Stratified randomisation. This model is similar to the block model, but divides the groups into different subgroups or strata taking into account an important factor that is thought to influence the final results and is divided according to cut-off points usually based on knowledge from previous studies.
- -
- Randomisation by minimisation. Also called adaptive randomisation, this attempts to minimise the differences between the different groups as much as possible. The process starts with a simple randomisation up to a previously agreed number and then adjusts the probability of assignment to each group based on any imbalances that may have arisen between the different intervention groups or between prognostic factors that may influence outcomes.11,12
To avoid selection bias or classification bias we will use the randomisation process and the randomisation sequence concealment process. These two processes are complemented by masking, by which we avoid information bias in the measurement of the outcome variable and possible co-interventions throughout the trial. While sequence concealment is performed prior to randomisation, masking is performed after randomisation. There are four types of blinding: open or unblinded trial, single-blind, double-blind, triple-blind.
Follow-up and loss of informationDuring this period there may be a loss of information or participants. It is important to consider the timing of losses. Pre-randomisation losses primarily affect the generalisability of the study's findings, compromising external validity. Post-randomisation losses, on the other hand, may compromise the internal validity of the study by reducing the number of subjects, thus reducing the effective sample size. It is generally considered that more than 10% of losses may compromise the validity of the results.
Outcome analysisSubjects should preferably be analysed according to the group to which they were initially assigned (intention-to-treat analysis) and not according to the group in which they finally participated (per-protocol analysis). Adequate outcome analysis requires determining which variables have been measured and adequately expressing the magnitude and precision of the results. An outcome variable in an RCT is any characteristic measured in the study subjects that allows us to differentiate the effect found in the compared groups and to test the hypothesis. Typically, the null hypothesis of a clinical trial states that there is no difference in effect between the compared interventions with respect to the chosen outcome variable.13
Outcome variables are classified as primary and secondary. Primary variables are those that help answer the main research question and condition the sample size of the clinical trial. In cases of continuous outcome variables, it is usual to express the magnitude of the results as mean or median differences, depending on the measure of centralisation most appropriate to the distribution of the variable. On the other hand, in cases of dichotomous variables, the relative risk, the absolute and relative differences in risk and the number needed to treat will be used. The precision of the results is expressed by the confidence intervals of the calculated estimators.14,15
Conflict of interestsThe authors declare there are no potential conflict of interests relating to this article.
Please cite this article as: Bollo J, Fernández-Ananin S, Targarona E. Ensayo clínico aleatorizado. Cir Esp. 2022;100:442–444.