The aim of this study is to identify factors associated to recurrence and survival in primary retroperitoneal liposarcomas.
MethodsProspective database of 35 patients with primary retroperitoneal liposarcoma treated 2004–2015 were retrospectively analyzed. Exclusion criteria were recurrent and metastatic tumors. Overall survival (OS) and disease-free survival were reviewed. Patient data were compared between patients with or without recurrence within 12 months after surgery. Risk factors were determined using logistic regression analysis.
ResultsFive-year OS was 61.1%. One and three-year disease-free survival were 68.6% and 17.1% respectively. OS in the early recurrence group was 36.4 months compared with 43.2 months in the group without early recurrence (P=.011). Early recurrence was associated with a reduction in OS (HR=4.05; CI95%: 1.27–12.96; P=.018). Multifocality and microscopic positive margins R1 were associated with early recurrence. Histologic subtype, margin of resection, histologic grade and multifocality were factors associated with recurrence. Contiguously involved organ resection had a beneficial effect on early recurrence and was associated with an increase in disease-free survival and OS. Adjuvant treatments had no protective effect on recurrence.
ConclusionsThis study underlines the crucial role aggressive surgical approach in retroperitoneal Liposarcoma treatment, especially in those patients with histological characteristics that adversely the prognosis.
El objetivo de este estudio consistió en identificar los factores asociados a la recurrencia y supervivencia del liposarcoma retroperitoneal primario.
MétodosSe analizó retrospectivamente una base de datos prospectiva de 35 pacientes con liposarcoma retroperitoneal primario tratados quirúrgicamente entre 2004-2015. Los criterios de exclusión fueron tumores recurrentes y metastásicos. Se analizó la supervivencia global (SG) y la supervivencia libre de enfermedad. Los datos de los pacientes se compararon entre los pacientes con o sin recurrencia dentro de los 12 meses posteriores a la cirugía. Los factores de riesgo se determinaron mediante análisis de regresión logística.
ResultadosLa SG a los 5 años fue del 61,1%. La supervivencia libre de enfermedad al año y a los 3 años fue del 68,6% al año y del 17,1% respectivamente. La SG en el grupo con recurrencia precoz fue del 36,4% a los 5 años frente al 71,3% en el grupo sin recurrencia precoz (p=0,011). La recurrencia precoz se asoció a una disminución de la SG (HR=4,05; IC95%: 1,27-12,96; p=0,018). La multifocalidad y márgenes quirúrgicos R1 estuvieron asociados a la recurrencia precoz. Los factores asociados a la recurrencia fueron el subtipo histológico, multifocalidad, grado histológico y márgenes quirúrgicos. La cirugía en bloque presentó un efecto protector frente a la recurrencia precoz y estuvo asociada a una mayor supervivencia libre de enfermedad y SG.
ConclusionesEste estudio pone de manifiesto la importancia del abordaje quirúrgico agresivo en el tratamiento del liposarcoma retroperitoneal, especialmente en aquellos pacientes con características histopatológicas que empobrecen el pronóstico.
Sarcomatous tumors represent 1% of the tumors found in adults. Only 12% to 20% are located in the retroperitoneal space.1–3 These tumors have an incidence rate of 1–5 cases per million.3,4
Liposarcoma (LPS) is the most common histologic tumor type, representing nearly 50% of all retroperitoneal sarcomas (RPSs).1 The LPSs are divided into various subtypes: well-differentiated (WD LPS), myxoid/round cell (MIX LPS), pleomorphic (PL LPS), dedifferentiated (DD LPS), and mixed type.5
From a clinical point of view, these tumors are characteristically difficult to detect because of their silent growth; therefore, they can reach a disproportionate size, compressing the surrounding vital structures.6
Local recurrence is the primary cause of mortality.4,7 Distant metastases, which are more often located in the lung or liver and have been found to occur around 10% of the time, are rarely diagnosed.8
Surgery is the most effective treatment of RPSs.9 The primary objective of this process is to ensure that affected tissues surrounding a tumor are removed. To that end, extensive multivisceral resections are used.4,10,11 Tumor size and the extent to which it affects other abdominal structures are the primary limitations to excision without affecting tumor margins.10
Use of radiotherapy has not been free of controversy. Traditionally, it has been considered helpful with the local control associated with surgery; however, prospective studies that support this statement must be developed.12 Chemotherapy has been limited to recurrent and metastatic tumors because it lacks effectiveness.13
The behavior of retroperitoneal LPSs is poorly studied. They are viewed as part of the incidence of LPSs in other locations, or they are included as minor offshoots of RPSs. This study was based on the results obtained from a regional reference center for this pathology.
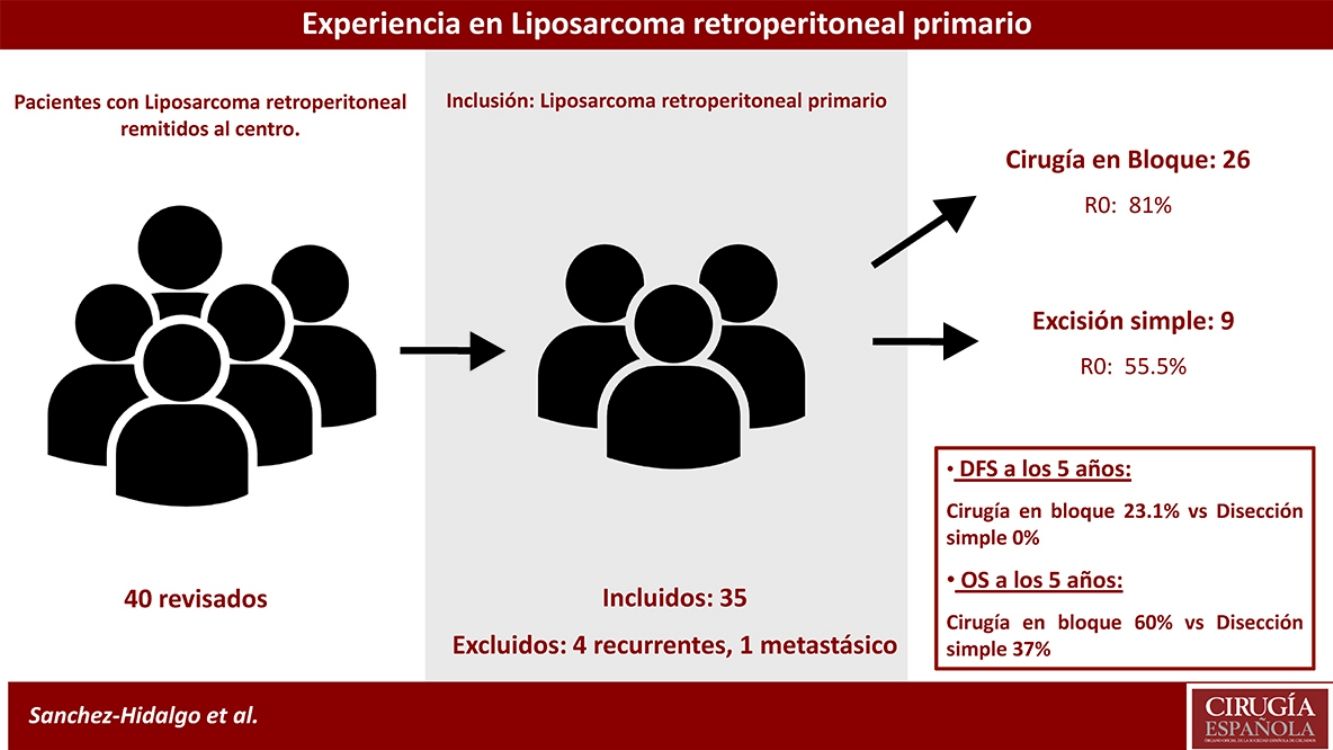
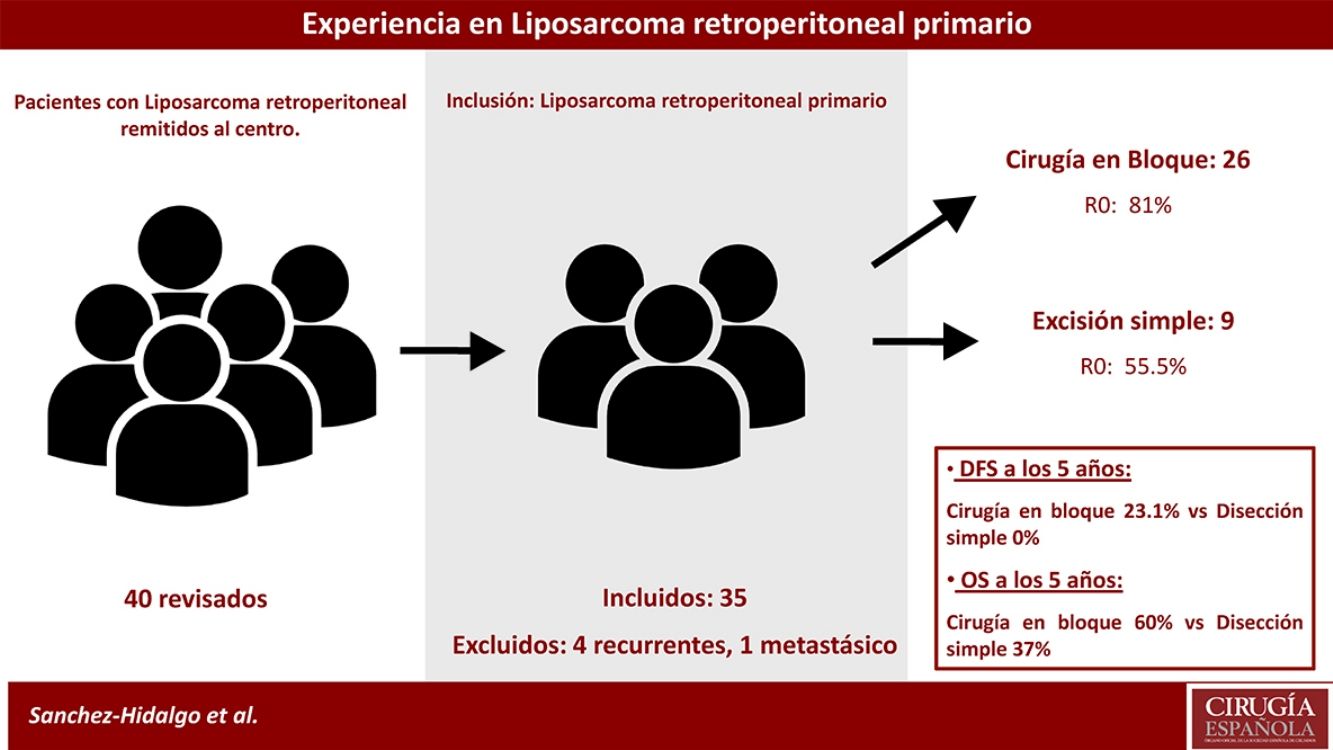
MethodsA prospective database of 40 patients with retroperitoneal LPS between 2004 and 2015 were retrospectively analyzed.
Inclusion criteria were primary retroperitoneal LPS, aged more than 18 years, Karnofsky Index greater than 70%, and ASA I, II, or III.
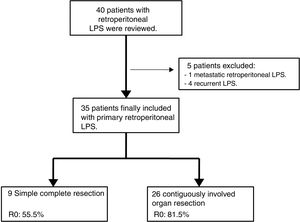
Exclusion criteria were metastatic or recurrent LPS. Five patients were excluded: one primary metastatic retroperitoneal LPS and four patients affected with recurrent LPS and treated in other centers (n=35). See Fig. 1.
Patient characteristics variables were age, sex, body mass index, and ASA score.
Tumor-related variables included were: tumor size (diameter), multifocality, histologic subtype assigned by the criteria published by the World Health Organization5 (WD LPS, Mix LPS, PL LPS, or DD LPS), tumor grade (high, Grade 2 or 3 according to the French FNCLCC system or low, Grade 1 according to FNCLCC),14 and stage assigned by the AJCC Staging System, 7th edition.15
The entire treatment procedure was devised by the multidisciplinary Sarcoma Committee of the Hospital.
Surgery type was either simple complete dissection or contiguously-involved organ resection.4 The former was when removal of the tumor alone was achieved simply and with safe margins. The latter was when resection of macroscopically-involved adjacent organs (frequently kidney or colon) was required to obtain a margin of normal tissue around the tumor. Histologic margins were defined as R2 (macroscopic tumor at the margins), R1 (microscopic tumor infiltration at the margins, <1mm from tumor), or R0 (no tumor at the margins).
Adjuvant treatments were applied for high grade tumors that were dedifferentiated and/or had R1 histologic margins. Treatment involved chemotherapy using ifosfamide or adriamicine regimens and radiotherapy delivered through external beams (mean doses of 45Gy administrated at the surgical site previously marked with surgical clips).
Patients had not received previous treatments.
Postoperative complications were staged according to the Dindo-Clavien classification: Grades 3 or higher indicated a complication requiring intervention.16
Data for overall survival (OS) and disease-free survival (DFS) were collected. Early recurrence was defined as disease reappearance within 12 months of surgery, diagnosed using radiological assessment.
Statistical AnalysesStandard descriptive analyses were performed to define the primary characteristics of the study group.
Curves for OS and DFS were constructed using the Kaplan–Meier method, and the log-rank test was used for comparison. Univariate analyses were performed for OS and DFS using the Cox proportional hazards regression model, yielding hazard ratios (HRs).
All relevant parameters were compared between the group that experienced postsurgical recurrence within 12 months and the group that did not using the Fisher exact test according to sample size. For continuous variables, the Student t test or the Mann–Whitney test were used, depending on distribution as determined by the Shapiro–Wilk test. Factors showing P≤.05 were included in a binary logistic regression analysis. Also, it was made a comparative study between Simple complete dissection group and contiguously involved organ resection group.
Statistical significance was recognized when P<.05.
All participants gave consent to be included in the study, and the study protocol was approved by the Research Ethics Committee.
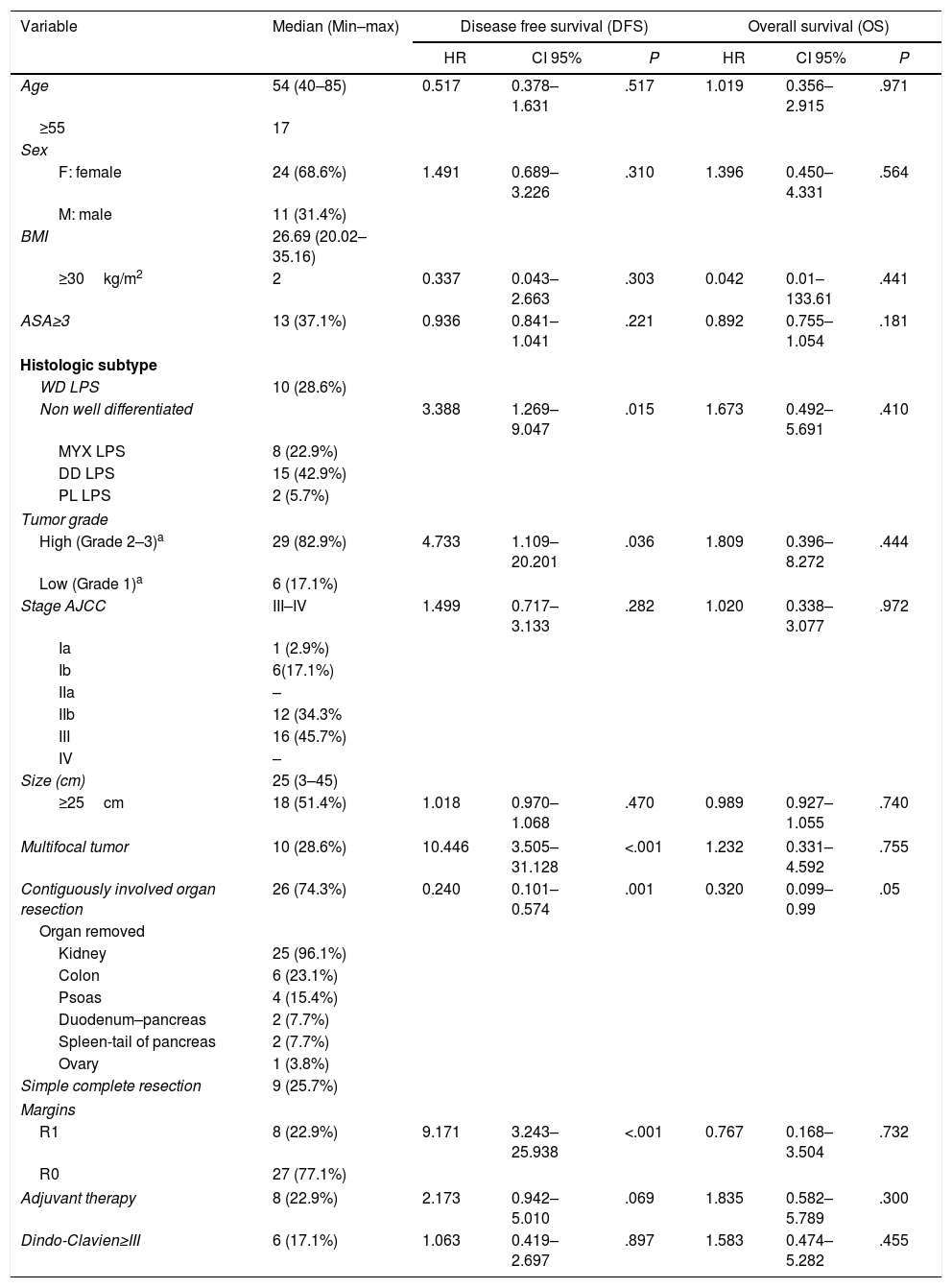
ResultsThe primary characteristics of our cohort are summarized in Table 1 (n=35).
Patients Characteristics and Their Influence in Survival.
| Variable | Median (Min–max) | Disease free survival (DFS) | Overall survival (OS) | ||||
|---|---|---|---|---|---|---|---|
| HR | CI 95% | P | HR | CI 95% | P | ||
| Age | 54 (40–85) | 0.517 | 0.378–1.631 | .517 | 1.019 | 0.356–2.915 | .971 |
| ≥55 | 17 | ||||||
| Sex | |||||||
| F: female | 24 (68.6%) | 1.491 | 0.689–3.226 | .310 | 1.396 | 0.450–4.331 | .564 |
| M: male | 11 (31.4%) | ||||||
| BMI | 26.69 (20.02–35.16) | ||||||
| ≥30kg/m2 | 2 | 0.337 | 0.043–2.663 | .303 | 0.042 | 0.01–133.61 | .441 |
| ASA≥3 | 13 (37.1%) | 0.936 | 0.841–1.041 | .221 | 0.892 | 0.755–1.054 | .181 |
| Histologic subtype | |||||||
| WD LPS | 10 (28.6%) | ||||||
| Non well differentiated | 3.388 | 1.269–9.047 | .015 | 1.673 | 0.492–5.691 | .410 | |
| MYX LPS | 8 (22.9%) | ||||||
| DD LPS | 15 (42.9%) | ||||||
| PL LPS | 2 (5.7%) | ||||||
| Tumor grade | |||||||
| High (Grade 2–3)a | 29 (82.9%) | 4.733 | 1.109–20.201 | .036 | 1.809 | 0.396–8.272 | .444 |
| Low (Grade 1)a | 6 (17.1%) | ||||||
| Stage AJCC | III–IV | 1.499 | 0.717–3.133 | .282 | 1.020 | 0.338–3.077 | .972 |
| Ia | 1 (2.9%) | ||||||
| Ib | 6(17.1%) | ||||||
| IIa | – | ||||||
| IIb | 12 (34.3% | ||||||
| III | 16 (45.7%) | ||||||
| IV | – | ||||||
| Size (cm) | 25 (3–45) | ||||||
| ≥25cm | 18 (51.4%) | 1.018 | 0.970–1.068 | .470 | 0.989 | 0.927–1.055 | .740 |
| Multifocal tumor | 10 (28.6%) | 10.446 | 3.505–31.128 | <.001 | 1.232 | 0.331–4.592 | .755 |
| Contiguously involved organ resection | 26 (74.3%) | 0.240 | 0.101–0.574 | .001 | 0.320 | 0.099–0.99 | .05 |
| Organ removed | |||||||
| Kidney | 25 (96.1%) | ||||||
| Colon | 6 (23.1%) | ||||||
| Psoas | 4 (15.4%) | ||||||
| Duodenum–pancreas | 2 (7.7%) | ||||||
| Spleen-tail of pancreas | 2 (7.7%) | ||||||
| Ovary | 1 (3.8%) | ||||||
| Simple complete resection | 9 (25.7%) | ||||||
| Margins | |||||||
| R1 | 8 (22.9%) | 9.171 | 3.243–25.938 | <.001 | 0.767 | 0.168–3.504 | .732 |
| R0 | 27 (77.1%) | ||||||
| Adjuvant therapy | 8 (22.9%) | 2.173 | 0.942–5.010 | .069 | 1.835 | 0.582–5.789 | .300 |
| Dindo-Clavien≥III | 6 (17.1%) | 1.063 | 0.419–2.697 | .897 | 1.583 | 0.474–5.282 | .455 |
Univariate analysis performed by Cox regression test. Statistical significance was recognized when P<.05.
LPS: liposarcoma; WD: well differentiated; MYX: myxoid; DD: dedifferentiated; PL: pleomorphic; AJCC: American Joint Committee on Cancer.
R0: microscopic negative margins; R1: microscopic positive margins R1.
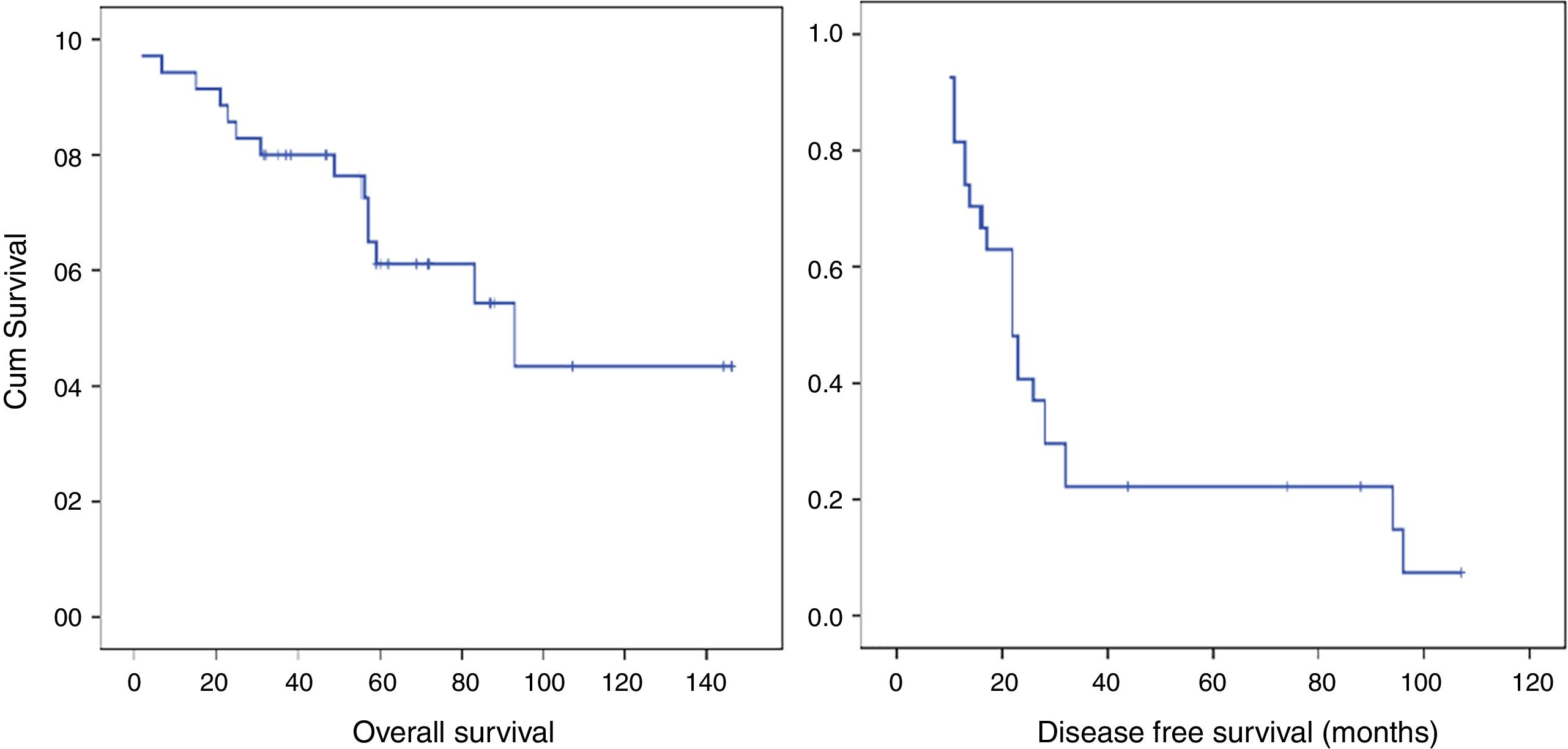
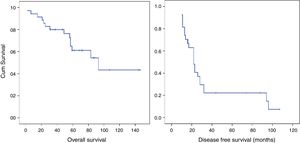
Median OS of the group was 93.00 months (95% confidence interval [CI], 44.90–141.02), and 5-year OS was 61.1%. Median DFS was 22.0 months, and 1- and 3-year DFSs were 8.5.6% and 22.2%, respectively (patients with microscopically affected margins R1 were excluded). The early recurrence rate was 31.4% (Fig. 2).
In univariate analysis, those factors with negative effects on DFS were histologic subtype with any grade of dedifferentiation, high grade, presence of multifocal tumors during surgery, and R1 margins (Table 1). There weren’t patients with R2 margins.
Application of chemotherapy with or without radiotherapy in those patients with poor prognoses (dedifferentiated tumor with our without R1 surgery margins) did not result in improved outcomes (DFS or OS) and had no protective effect from early recurrence (Table 1).
Contiguously-involved organ resection showed a preventive effect in DFS (HR=0.240; 95% CI, 0.101–0.574; P=.001) and was the only factor positively influencing OS (HR=0.320; 95% CI, 0.099–0.99; P=.05; Table 1).
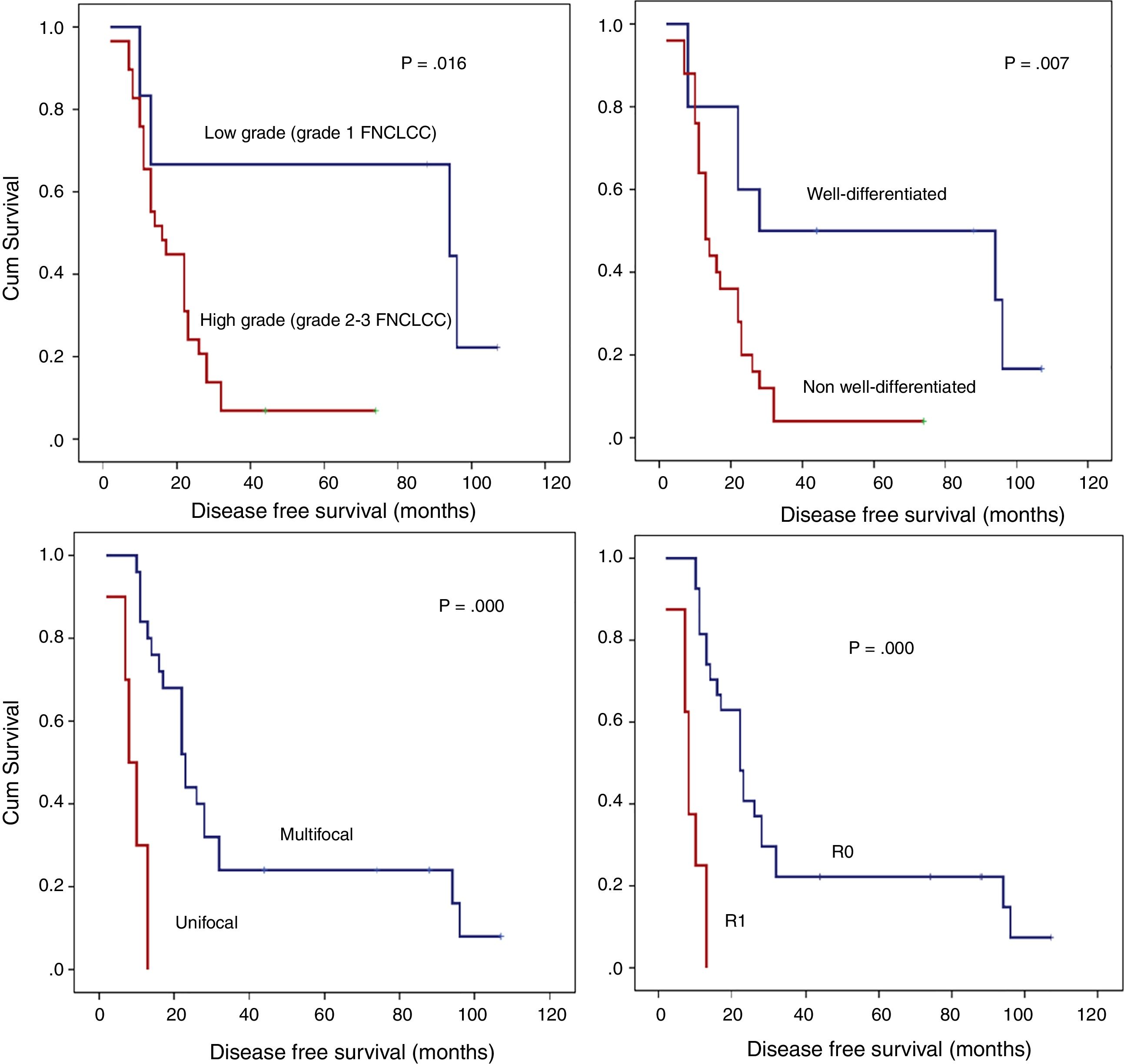
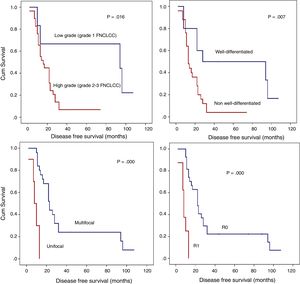
Detailed DFS analysis (Fig. 3):
- A)
Patients with low grade retroperitoneal LPSs had a 5-year-DFS of 66.7%; however, for those with high grade LPSs, it was lower than 10% (P=.016).
- B)
Patients with non-well-differentiated retroperitoneal LPSs (MIX LPSs, DD LPSs, or PL LPSs) have poor prognoses in terms of the 5-year DFS (<10%) compared to those with well-differentiated retroperitoneal LPSs (50%; P=.007).
- C)
Another histopathologic point of interest in terms of DFS is multifocality. The patient group without multifocal tumors had a 1-year DFS of 84% and a 5-year DFS of 24%. However, the group with multifocal retroperitoneal LPSs had a 1-year DFS of 30% and a 5-year DFS of 0% (P=.001).
- D)
When an R0 margin was achieved, 1-year DFS was nearly 81%, but when the margin was R1, it was approximately 25% (P=.001).
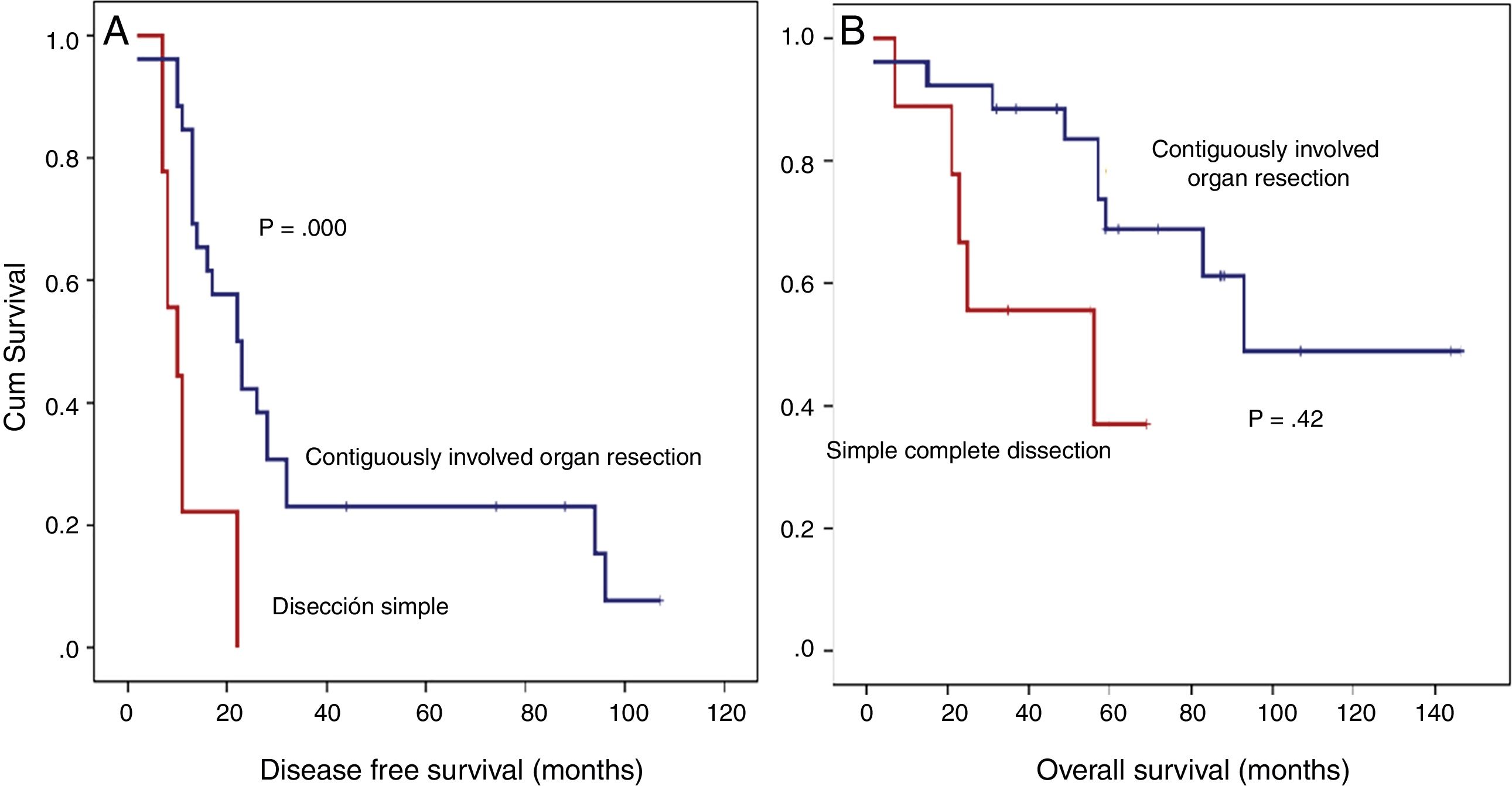
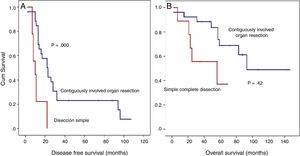
Detailed DFS analysis (Fig. 4):
Patients treated with simple complete dissection had a 5-year DFS of 0%, while the group treated with contiguously-involved organ resection, it was 23.1% (P=.001). Group treated with simple complete dissection had a 5-year OS near 37%, while group treated with contiguously-involved organ resection presented a 5-year OS near 70% (P=.042). See Fig. 4. No differences were observed when comparing both groups. R0 margins in the group treated with contiguously-involved organ resection were obtained in 21 patients (81%) and in 5 patients (55.5%) when a simple complete dissection was performed.
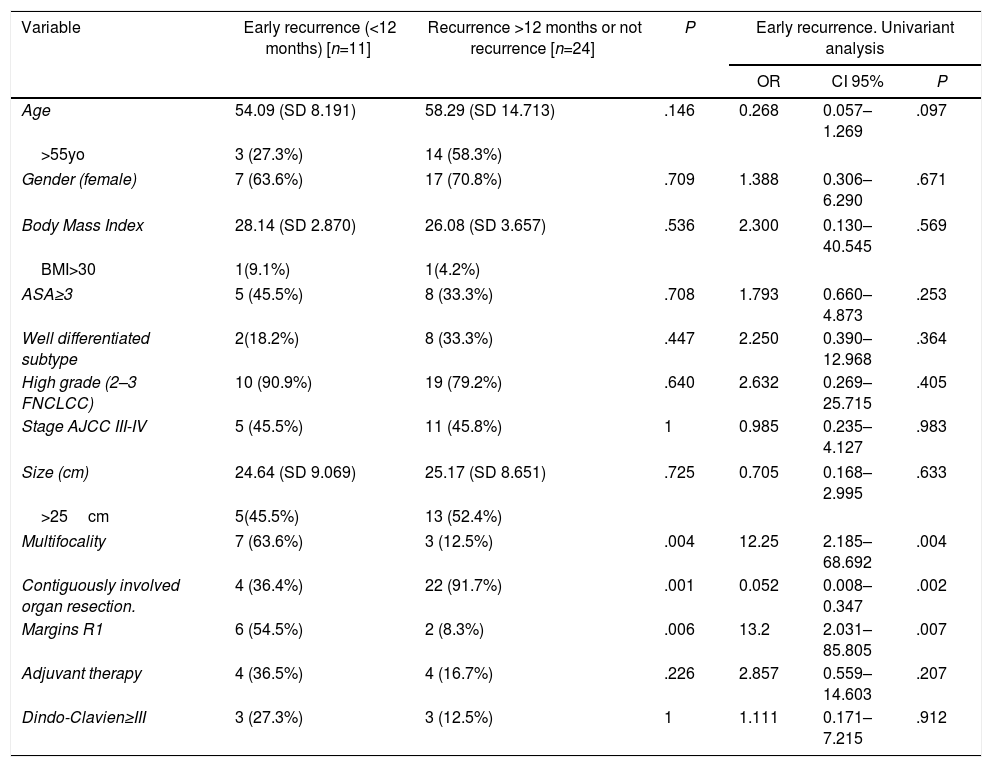
Another point of interest was the influence of different clinical factors in early recurrence (Table 2).
Clinical Factors Which Impact in Early Recurrence of Retroperitoneal Liposarcomas.
| Variable | Early recurrence (<12 months) [n=11] | Recurrence >12 months or not recurrence [n=24] | P | Early recurrence. Univariant analysis | ||
|---|---|---|---|---|---|---|
| OR | CI 95% | P | ||||
| Age | 54.09 (SD 8.191) | 58.29 (SD 14.713) | .146 | 0.268 | 0.057–1.269 | .097 |
| >55yo | 3 (27.3%) | 14 (58.3%) | ||||
| Gender (female) | 7 (63.6%) | 17 (70.8%) | .709 | 1.388 | 0.306–6.290 | .671 |
| Body Mass Index | 28.14 (SD 2.870) | 26.08 (SD 3.657) | .536 | 2.300 | 0.130–40.545 | .569 |
| BMI>30 | 1(9.1%) | 1(4.2%) | ||||
| ASA≥3 | 5 (45.5%) | 8 (33.3%) | .708 | 1.793 | 0.660–4.873 | .253 |
| Well differentiated subtype | 2(18.2%) | 8 (33.3%) | .447 | 2.250 | 0.390–12.968 | .364 |
| High grade (2–3 FNCLCC) | 10 (90.9%) | 19 (79.2%) | .640 | 2.632 | 0.269–25.715 | .405 |
| Stage AJCC III-IV | 5 (45.5%) | 11 (45.8%) | 1 | 0.985 | 0.235–4.127 | .983 |
| Size (cm) | 24.64 (SD 9.069) | 25.17 (SD 8.651) | .725 | 0.705 | 0.168–2.995 | .633 |
| >25cm | 5(45.5%) | 13 (52.4%) | ||||
| Multifocality | 7 (63.6%) | 3 (12.5%) | .004 | 12.25 | 2.185–68.692 | .004 |
| Contiguously involved organ resection. | 4 (36.4%) | 22 (91.7%) | .001 | 0.052 | 0.008–0.347 | .002 |
| Margins R1 | 6 (54.5%) | 2 (8.3%) | .006 | 13.2 | 2.031–85.805 | .007 |
| Adjuvant therapy | 4 (36.5%) | 4 (16.7%) | .226 | 2.857 | 0.559–14.603 | .207 |
| Dindo-Clavien≥III | 3 (27.3%) | 3 (12.5%) | 1 | 1.111 | 0.171–7.215 | .912 |
Continues variables comparison by T-student and U Mann–Whitney test. Qualitative variables comparison by Fisher test. Univariant analysis performed by binary logistic regression analysis.
In the group affected by early recurrence, multifocality was more frequent (63.6%) than in the opposing group, where multifocal tumors were present only 12.5% of the time (P=.004). Other tumor characteristics such as size, histological subtype, and grade were not statistically different between these two groups.
The group treated with contiguously-involved organ resection showed a smaller early recurrence index than the other treatment group (36.4% vs 91.7%; P=.001). The early recurrence rate was higher in the group with R1 margins than in R0 group (91.7% vs 45.5%; P=.006). Great morbidity associated with surgery (Dindo-Clavien>3) did not influence the recurrence rate (P>.05).
Neither chemotherapy nor radiotherapy improved this rate.
In univariate analysis (Table 2), the presence of multifocal tumors and R1 margins were associated with early recurrence. On the other hand, contiguously-involved organ resection proved to be effective against early recurrence (OR=0.052; 95% CI, 0.008–0.347; P=.002).
Median survival in the early recurrence group was 42.2 months (95% CI, 26.6–57.8 months) compared to 105.7 months (95% CI, 84.1–127.3 months) in the group without early recurrence (P=.011; Fig. 3). The overall 3- and 5-year survival rates in the early recurrence group was 54.5% and 36.4%, respectively, compared to 91.7% and 71.3% in the group without early recurrence.
Early recurrence was associated with a reduction in OS (HR=4.05; 95% CI, 1.27–12.96; P=.018).
DiscussionThe demographic features in this cohort of participants were similar to those of a group previously studied.4,7,17–20
Early recurrence appeared to be a risk factor in the overall survival of patients (HR=4.05; 95% CI, 1.27–12.96; P=.018). The patient group affected by early recurrence had a 5-year OS of 36.4%, as opposed to 71.3% for the non-affected group. This difference shows the impact of early recurrence on patients’ vital prognoses (P=.011).
The DDLPS histologic subtype was the most common (≈43%) in this sample of patients, differing from that reported by Singer7 and Bonvalot4 in which WDLPS was the most common. Our data have special relevance because it is commonly known that the probability of non-recurrence is higher with the WDLPS subtype (69%) than with Mix LPS.7 This plays an important role when evaluating OS and DFS due to the fact that aggressive histologic subtypes confer a worse prognosis.
The primary problem with these tumors is their high frequency of locoregional recurrence plus their impact on the patient's survival prognosis.7
Greater dedifferentiation has previously been shown to coincide with a decrease in DFS and OS in RPSs.4,8,21,22 Our work shows that 90% of patients affected by early recurrence had FNCLCC Grade 2 or 3 tumors, in contrast to the 79.2% among those patients not affected by early recurrence; However, this difference is not statistically significant. It has previously been observed that FNCLCC Grade 3 LPSs were associated with a local recurrence rate of 20% during the 12 months following surgery.13 The presence of FNCLCC Grade 2 or 3 tumors proved to be related to a decrease in DFS in similar studies of RPSs.7,17,23 However, an association between dedifferentiation and decreased OS was not proven in this study. This relationship was found in other studies of RPSs.7,23–26 In the work of Gronchi et al.,3 OS among those with Grade 1 WDLPSs was 92.9%, whereas it was 56.5% among those with Grade 2 DDLPSs and 21.2% among those with Grade 3 DDLPSs. Similarly, we found that the 5-year OS was 100% for Grade 1 LPSs and 53.7% for Grades 2 and 3 LPSs. Nevertheless, these differences were not statistically significant, likely because of the small sample size.
The presence of multifocality in the diagnosis was associated with early recurrence (P=.004). Probably, this relation between early recurrence and multifocality is justified due to de high difficulty to obtain a complete removal with R0 margins in these cases; when studying this phenomenon, in the case of unifocal tumor R0 was obtained in 96% while in the group with multifocal tumor, R0 margins supposed 30% (P=.001). Additionally, multifocality was established as a statistically significant risk factor related to DFS. This was also found in a study performed by the Multi-Institutional Collaborative RPS Working Group in 2015.13 Even though we did not find multifocality to be related to OS, other studies have proven the relationship with a decrease in RPSs survival.13,17,23 Furthermore, multifocality was included in the Multi-Institutional Retroperitoneal Sarcoma Nomogram.27
In the multivariate analysis performed by Gronchi et al.,13 histologic R0/R1 margins lead to better prognoses in RPSs (OS and DFS) compared to R2 margins. In our study, R1 margins were associated with a decrease in DFS and early recurrence. When there were R1 margins, 1 year-DFS was near 25% while in case of R0 margins, it was near 81%; it should be considered that this 75% of relapse at 1st year is more likely to be a persistence of the disease and highlights the implication of R1 margins in early recurrence. Our data support the multivariate analysis performed by Bonvalot et al.4 (HR=1.88; 95% CI, 1.18–2.98; P=.008), who found a relationship between affected margins and OS (HR=1.70; 95% CI, 1.07–2.72; P=.03). The presence of R1 (microscopic positive) or R2 (gross residual disease) margins and surgical technique have been found to be important factors for recurrence.4,13
Even though surgery is established as the primary treatment for these tumors, the extent of the tissue to be excised has yet to be determined. Consequently, significant differences between surgical centers, even those with international standing, are found in the way RPSs are treated.13 The essence of extensive resections that include non-affected organs is to obtain margins without microscopic residual tumor cells, and consequently improve DFS. However, the retroperitoneal anatomy complicates the use of this extensive surgical procedure. In a considerable number of centers, a highly-aggressive approach called complete compartmental resection is performed. This technique used in the treatment of RPSs commonly includes colon resection in the front, psoas muscle in the back, and kidney within,4,7,23 achieving a 3-year local recurrence rate of 10% without improvement in OS. In our study, contiguously-involved organ resection was a protective factor for DFS and OS, and it decreased early recurrence (OR=0.052; 95% CI, 0.008–0.347; P=.002) while yielding morbidity rates similar to those of others,4 supporting an increased overall survival after complete dissection, against previous studies.4,10,11
In Miura et al.,28 chemotherapy proved have no significant effect on OS in RPSs, as seen in other studies.13,19 In our study, adjuvant therapy was indicated in specific patients whose prognoses are negatively affected by factors such as unsuccessful surgery from the continuous presence of tumor material in the marginal tissue (R1 margins) or because of aggressive histologic subtypes without any improvement in early recurrence, DFS and OS.
A substantial number of studies show that adjuvant radiotherapy has a positive impact in controlling local recurrence in RPSs.4,13,28 Currently, the EORTC multicenter clinical trial, now in Phase III, is being developed, consisting of comparisons between the effect of preoperative radiotherapy plus surgery versus surgery alone. The conclusions of this study would be especially valuable in determining the effectiveness of each of these strategies for treating this type of tumor. On the other hand, intraoperative radiotherapy has demonstrated time-tested effectiveness, reducing local relapse.29
Our results should be interpreted in view of the small sample size. The small number of patients included in this study did not allow multivariate analyses.
In conclusion, early recurrence is fundamentally associated with suboptimal surgery with margins affected by tumor. The relationship between multifocality and early recurrence probably is due to the difficulty to obtain wide margins free of tumor cells.
In retroperitoneal sarcoma, tumor characteristics such as histological subtype, dedifferentiation grade, and multifocality have proven to be important indicators in DFS. R1 margins have been associated with decreased DFS.
This study underlies the crucial role of an aggressive surgical approach to retroperitoneal LPSs due to the importance of obtaining R0 margins. Contiguously-involved organ resection has been proven to be an effective preventive measure in early recurrence, and it increases DFS and OS.
FundingThe authors declare that they did not receive any funding to complete this study.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Sánchez-Hidalgo JM, Rufián-Peña S, Durán-Martínez M, Arjona-Sánchez Á, Salcedo-Leal I, Lopez-Cillero P, et al. Factores de riesgo implicados en la recurrencia precoz del liposarcoma retroperitoneal. Cir Esp. 2018;96:568–576.