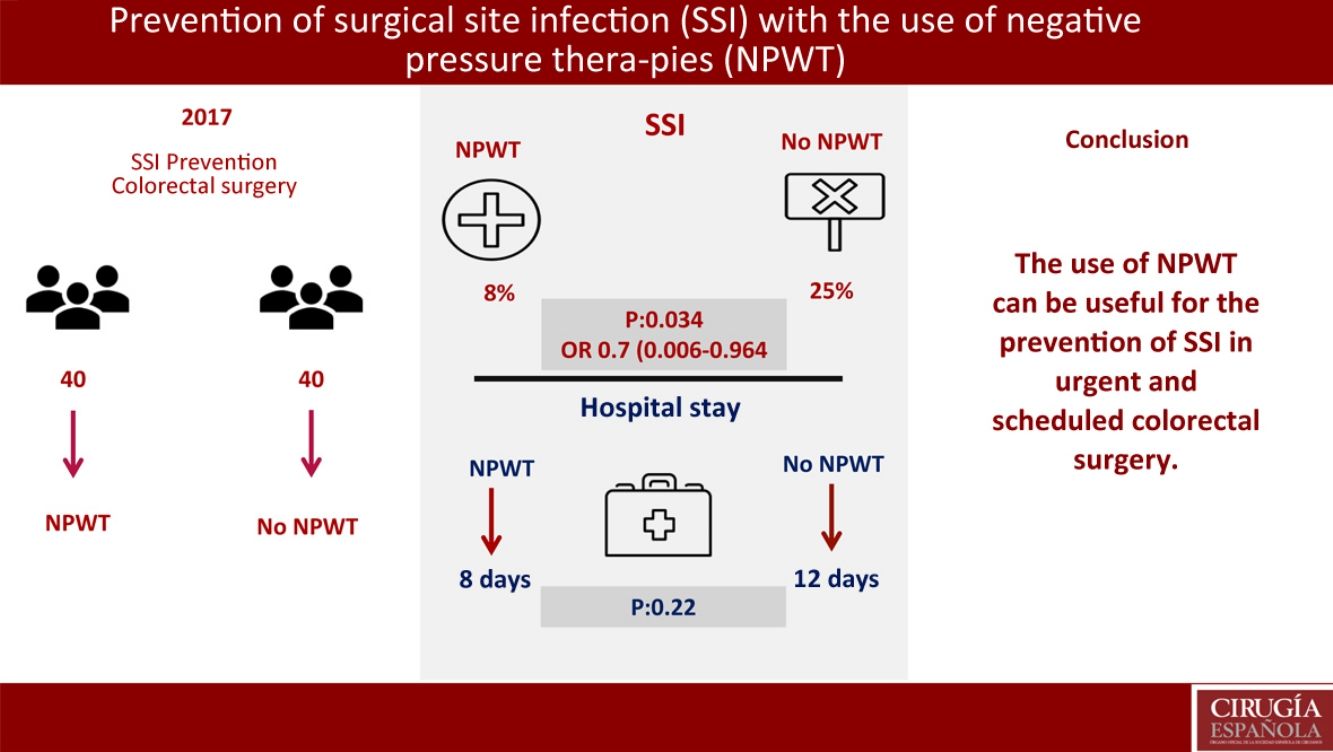
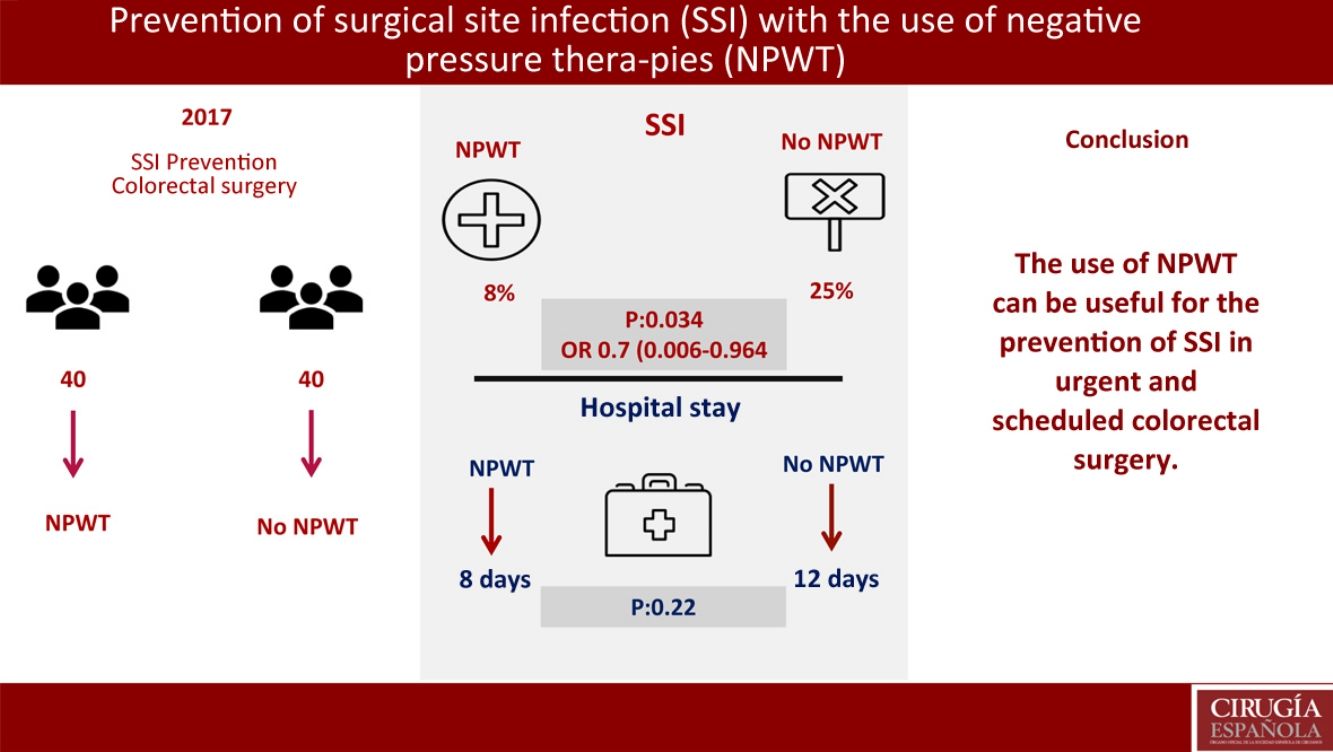
Surgical site infection (SSI) is one of the most frequent complications in colorectal surgery. It is diagnosed in 10%–20% of colorectal procedures. Negative Pressure Wound Therapy (NPWT) has shown efficacy in the treatment of chronic and traumatic wounds, wound dehiscence, flaps and grafts. The main objective of this study is to assess NPWT in the prevention of SSI in colorectal surgery. Hospital stay reduction and SSI risk factors are secondary objectives.
MethodsWe present a prospective case–control study including 80 patients after a colorectal diagnosis and surgical procedure (elective and non-elective) in 2017. Forty patients were treated with prevention NPWT for one week. Forty patients were treated according to the standard postoperative surgical wound care protocol.
ResultsNo significant differences were found in demographic variables, comorbidities, surgical approach, elective or non-elective surgery, mechanical bowel preparation and surgical procedure. Three patients has SSI in the NPWT group (8%) (95%CI 0–17.5). Ten patients presented SSI in the control group (25%) (95%CI 12.5–37.5) (P=.034); OR 0.7 (95%CI 0.006–0.964). Hospital stay in the NPWT group was 8 days versus 12 days in the non-NPWT group (P=.22). In the multivariate analysis, mechanical bowel preparation was found to be the only risk factor for SSI (P=.047; OR: 0.8, CI 0.45–0.93).
ConclusionsNPWT is a useful SSI prevention treatment in colorectal surgery.
La infección del sitio quirúrgico (SSI) es una de las principales complicaciones quirúrgicas, con una incidencia del 10-20% en cirugía colorrectal. Las terapias basadas en presión negativa (NPWT) han mostrado su eficacia en el tratamiento de heridas crónicas, traumáticas, en las dehiscencias de piel, en el uso de colgajos o injertos. El objetivo principal del estudio es valorar la eficacia de NPWT en la prevención de SSI en cirugía colorrectal. Los objetivos secundarios son valorar la reducción del ingreso hospitalario y analizar los factores de riesgo de SSI.
MétodosEstudio casos y controles prospectivo. Ochenta pacientes intervenidos tras diagnóstico de enfermedad colorrectal, de forma programada o urgente durante el año 2017. Cuarenta pacientes fueron tratados con NPWT preventivo durante una semana. Cuarenta pacientes fueron tratados según protocolo habitual postoperatorio de cuidado de herida quirúrgica.
ResultadosNo se encontraron diferencias entre NPWT y grupo control en variables demográficas, comorbilidades, abordaje quirúrgico, indicación urgente o programada, preparación colónica o procedimiento quirúrgico. Se objetivó SSI con el empleo de NPWT en 3 (8%) pacientes (IC del 95%, 0-17,5). SSI en 10 (25%) pacientes (IC del 95%, 12,5-37,5) (p=0,034); OR 0,7 (IC del 95% 0,006-0,964). La estancia hospitalaria en el grupo NPWT fue de 8 días vs 12 días en el grupo control (p=0,22). En el análisis multivariante se encontró como único factor relacionado con SSI la preparación colónica (p=0,047; OR: 0,8, IC 0,45-0,93).
ConclusionesEl uso de dispositivos NPWT para la cobertura de incisiones cerradas tras cirugía colorrectal puede disminuir la incidencia de SSI.
Surgical site infection (SSI) is one of the main medical complications associated with surgery. Although it is preventable in most cases, it continues to be an important problem requiring our attention.1,2 This problem causes a significant burden on public healthcare systems that bear the costs resulting from the greater number of hospitalization days and higher cost of patient treatment.3–6
The CDC's Healthcare-Associated Infection (HAI) prevalence survey has estimated a total of 157500 wound infections in 2011.7 NHSN data reported 16147 SSI after 849659 surgical procedures in all groups reported in the study, for an average SSI rate of 1.9% between 2006 and 2008.8 A reduction in the SSI rate of 19% in 10 selected procedures was reported between 2008 and 2013, with an SSI rate of 2% in colon surgery.9 In Europe, a wound infection rate of 9.5% was found in the period from 2010 to 2011.10
SSI rates are directly related to surgery type. Surgical wounds are classified as clean, clean-contaminated, contaminated and dirty, with an associated SSI risk of 1%–5%, 3%–11%, 10%–17% and 27%, respectively.11 In scheduled colorectal surgery, the SSI rate in some series reaches 20%.12
Negative pressure wound therapies (NPWT) were introduced in 1997 as a possible method to reduce SSI rates.13 Classically, the indications for the use of NPWT have been chronic wounds, trauma wounds, wounds with sub-acute evolution, and wounds associated with skin dehiscence, burns, ulcers (especially vascular and diabetic in origin), flaps and grafts.14,15 However, there is no strong evidence regarding the prophylactic use of NPWT in closed laparotomy wounds.
The main objective of this study was to try to evaluate the clinical effectiveness of NPWT in the primary prevention of SSI in colorectal surgery. The secondary objectives of the study were to analyze hospital stay with the use of these measures and to analyze risk factors for the appearance of SSI.
MethodsA prospective case–control study was carried out with patients undergoing colorectal surgery (scheduled and urgent) at the same tertiary hospital in 2017. In all cases, the surgical interventions were performed by members of the colorectal surgery team. The procedures analyzed were left colectomies, right colectomies, anterior resections, abdominoperineal resections and reconstruction after a temporary stoma. The study included both open surgeries and laparoscopic surgeries with assistance incisions to create the anastomoses. The patients included in the control group were patients treated surgically in the same period, with the same diagnoses and undergoing the same procedures, without the application of NPWT.
The criteria for inclusion in the study were: patients who underwent colorectal surgery in the period described (both scheduled and urgent surgery) that had been performed by a surgeon from the colorectal surgery unit. Patients who had undergone colorectal surgery outside the unit were not included in the study.
In all cases, preoperative antibiotic treatment was administered as a prophylactic measure.
All patients were followed up for one month after surgery. SSI was considered to exist when alterations were observed in the normal wound evolution or when signs appeared in the clinical situation of the patient, such as fever, purulent discharge, extensive erythema or any alteration requiring therapeutic measures, such as wound drainage, administration of antibiotics or a pharmacological preparation for the resolution of SSI.
The risk factors studied were age, sex, body mass index (BMI), ASA scale, comorbidities, open versus laparoscopic surgery, urgent versus scheduled surgery, preoperative bowel preparation, presence of stoma, surgical time and wound location.
The hospital stay was measured in days of admission after surgery.
The statistical study was performed with SPSS Statistics® v20 (SPSS, Inc., Chicago, IL). The chi-squared test and Fisher's test were used for categorical variable analyses. The Student's t test was used to compare means in variables with normal distribution, while the Wilcoxon test was used to compare means of non-normal distribution. The multivariate analysis was used in the study of risk factors. A P value<.05 was considered statistically significant.
ResultsThe study included 80 patients in total. Forty had NPWT dressings applied at the end of the surgery while in the operating room. The vacuum dressings were maintained for a week without being changed, unless there were medical complications that advised against their continued use. Another 40 patients were selected within the same period who underwent the same surgical interventions. The follow-up of these patients was conducted in accordance with the standard care of the hospital ward. Their dressings were carefully changed after 48h, and their surgical wounds were cleaned by nursing staff.
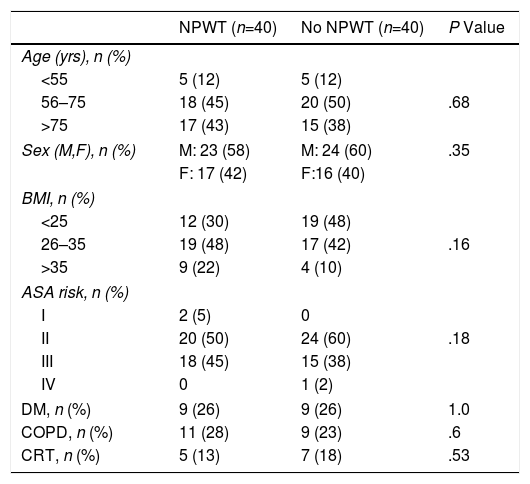
In both study groups, we found similar demographic characteristics and no significant differences between the different factors analyzed. Three age groups were established in the patients treated with NPWT: <55 years old, 5 (12%); 56–75 years, 18 (45%); and >75 years, 17 (43%). The distribution by age groups in non-NPWT patients was: <55 years old, 5 (12%); 56–75 years, 20 (50%); and >75 years, 15 (38%). No differences were found between the 2 treatment groups in the distribution by age (P=.66).
There were 23 (58%) male patients treated with NPWT and 24 (60%) in the non-NPWT group. In the treatment group, there were 17 (42%) women and 16 (40%) in the control group (P=.35). The distribution according to the body mass index was: BMI<25, 12 (30%) subjects in the NPWT group and 19 (48%) in the non-NPWT group; BMI 26–35, 19 (48%) patients in the NPWT group and 17 (42%) in the control group; BMI>35, 9 (22%) subjects in the NPWT group and 4 (10%) in the control group (P=.1464).
The distribution and presence of comorbidities in the two groups was homogeneous: no differences were observed in the different ASA grades in the 2 groups (P=.187). In the treatment group, 9 (26%) patients with diabetic mellitus were found, and there were also 9 (26%) in the control group (P=1.0) The presence of chronic obstructive pulmonary disease in the treatment group was 11 (28%) patients versus 9 (23%) in the control group (P=.60). Five (13%) patients in the treatment group received chemoradiotherapy prior to the surgical intervention, while in the control group 7 (18%) patients were administered this treatment (P=.53) (Table 1).
Demographic Characteristics and Risk Factors by Groups According to NPWT Use.
| NPWT (n=40) | No NPWT (n=40) | P Value | |
|---|---|---|---|
| Age (yrs), n (%) | |||
| <55 | 5 (12) | 5 (12) | |
| 56–75 | 18 (45) | 20 (50) | .68 |
| >75 | 17 (43) | 15 (38) | |
| Sex (M,F), n (%) | M: 23 (58) | M: 24 (60) | .35 |
| F: 17 (42) | F:16 (40) | ||
| BMI, n (%) | |||
| <25 | 12 (30) | 19 (48) | |
| 26–35 | 19 (48) | 17 (42) | .16 |
| >35 | 9 (22) | 4 (10) | |
| ASA risk, n (%) | |||
| I | 2 (5) | 0 | |
| II | 20 (50) | 24 (60) | .18 |
| III | 18 (45) | 15 (38) | |
| IV | 0 | 1 (2) | |
| DM, n (%) | 9 (26) | 9 (26) | 1.0 |
| COPD, n (%) | 11 (28) | 9 (23) | .6 |
| CRT, n (%) | 5 (13) | 7 (18) | .53 |
ASA: American Society of Anesthesiology; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; M: male; BMI: body mass index; F: female; NPWT: negative pressure wound therapy; CRT: chemoradiotherapy.
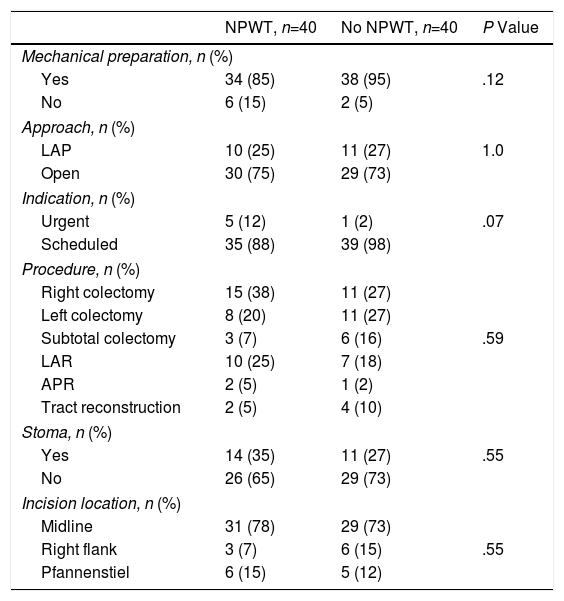
Mechanical bowel preparation prior to surgery was carried out in 34 (85%) patients with NPWT therapy and in 38 (95%) non-NPWT patients (P=.12). In 30 (75%) patients with NPWT and 29 (73%) patients in the control group, the initial surgical approach used was open surgery (P=1.0). The remaining surgeries are included in the laparoscopic approach group and correspond with surgeries with assistance incisions through which the anastomosis was performed, which was necessary in each case. The surgery had been scheduled in 35 (88%) patients of the treatment group and in 39 (98%) of the control group (P=.07). The main procedures performed were: right colectomy, left colectomy, subtotal colectomy, low anterior resection, abdominoperineal excision and reconstruction of the tract after a previous temporary stoma. No differences were found between the groups in terms of the procedure performed (P=.59). Midline incisions were used in 31 (78%) patients in the treatment group and in 29 (73%) in the control group; other incisions used were the right flank and Pfannenstiel (P=.55) (Table 2).
Factors Related With Surgery; Distribution by Groups According to Use of NPWT.
| NPWT, n=40 | No NPWT, n=40 | P Value | |
|---|---|---|---|
| Mechanical preparation, n (%) | |||
| Yes | 34 (85) | 38 (95) | .12 |
| No | 6 (15) | 2 (5) | |
| Approach, n (%) | |||
| LAP | 10 (25) | 11 (27) | 1.0 |
| Open | 30 (75) | 29 (73) | |
| Indication, n (%) | |||
| Urgent | 5 (12) | 1 (2) | .07 |
| Scheduled | 35 (88) | 39 (98) | |
| Procedure, n (%) | |||
| Right colectomy | 15 (38) | 11 (27) | |
| Left colectomy | 8 (20) | 11 (27) | |
| Subtotal colectomy | 3 (7) | 6 (16) | .59 |
| LAR | 10 (25) | 7 (18) | |
| APR | 2 (5) | 1 (2) | |
| Tract reconstruction | 2 (5) | 4 (10) | |
| Stoma, n (%) | |||
| Yes | 14 (35) | 11 (27) | .55 |
| No | 26 (65) | 29 (73) | |
| Incision location, n (%) | |||
| Midline | 31 (78) | 29 (73) | |
| Right flank | 3 (7) | 6 (15) | .55 |
| Pfannenstiel | 6 (15) | 5 (12) | |
APR: abdominoperineal resection; LAP: laparoscopic; NPWT: negative pressure wound therapy; LAR: low anterior resection.
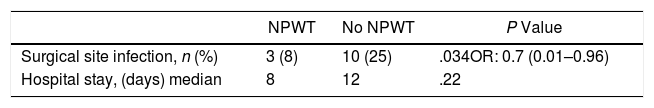
The primary objective of the study was the SSI analysis in the treatment group with NPWT therapy compared with a control group where surgical wound care was performed without NPWT. In the treatment group with NPWT, SSI was found in 3 (8%) patients (95% CI 0–18). In the control group (no NPWT), SSI was observed in 10 (25%) patients (95% CI, 13–38) (P=.034). OR was 0.7 (95% CI 0.006–0.964). In cases where SSI developed with the use of NPWT, this device was removed prematurely and the wound was drained, after which antibiotic treatment was initiated (Table 3).
A secondary objective of the study was the analysis of the hospital stay, finding a median stay of 8 days in the treatment group vs 12 days in the control group (P=.22).
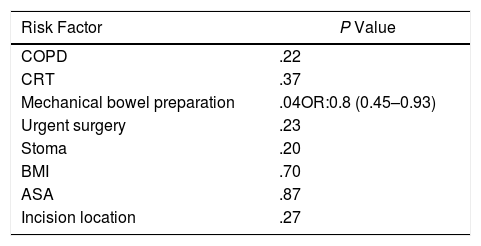
Another of the secondary objectives of the study was the global estimation of risk factors for surgical site infection, including the 80 patients who were part of the study, regardless of the assigned group. In the multivariate analysis, colon preparation was found to be the only factor related with SSI (P=.047; OR: 0.8; CI 0.45–0.93) (Table 4).
Risk Factors for Surgical Site Infection; Multivariate Analysis.
| Risk Factor | P Value |
|---|---|
| COPD | .22 |
| CRT | .37 |
| Mechanical bowel preparation | .04OR:0.8 (0.45–0.93) |
| Urgent surgery | .23 |
| Stoma | .20 |
| BMI | .70 |
| ASA | .87 |
| Incision location | .27 |
ASA: American Society of Anesthesiology; COPD: chronic obstructive pulmonary disease; BMI: body mass index; CRT: chemoradiotherapy.
SSI is one of the most frequent complications after open colorectal surgery, with a significant morbidity rate.16–20 Regardless of the strict follow-up and the best practices of antisepsis and prophylactic antibiotics, SSI rates remain high in this type of surgical interventions, reaching 30% in different series.16,17 The increasing use of laparoscopic approaches in colorectal surgery has significantly reduced SSI rates, but these advantages of laparoscopy are not applicable to all patients. Some are not candidates for this approach, while in others the conversion rate to open procedures can reach 15%.21
The main effects caused by NPWT devices on the wound have to do with the application of negative pressure at −80mmHg in a hypoxic environment,22 producing macrodeformation and approximation of tissues (depending on their mobility), microdeformation with approximation at the cellular level favoring division and proliferation, elimination of fluids (corresponding to the edema of the extracellular space to a greater extent) and favoring a microclimate in the wound that hinders bacterial overgrowth, with probable repercussions in the second and third phases of wound healing.23–26
Certain studies have not found significant differences in SSI rates when comparing the use of NPWT with standard surgical wound care (gauze dressings), both in intestinal surgery procedures (gastrointestinal, pancreatic and colorectal) as well as vascular surgery,27–29 among them a previous Cochrane review.30 In our series, however, the hypothesis of our main objective was proven to be true, and a significant decrease in the SSI rate was observed in the NPWT group (8 vs 25%, P=.034). Although it is true that this study is a prospective series that is neither randomized nor blinded, the absence of heterogeneity between both groups has been thoroughly established. The rate of wound infection in the non-NPWT group of our series is relatively high, although within the ranges described in other published series,16–20 especially taking into account that both elective and urgent procedures were included. Nonetheless, given the homogeneity of the groups in preoperative and surgical parameters, we cannot attribute the difference in the SSI rate of this group versus the NPWT group to a factor other than the use of negative pressure devices. The postoperative management of the both patient groups followed the same protocol, and the only difference was the use of NPWT.
These data are consistent with many other controlled and randomized studies that include clean, clean-contaminated and contaminated wounds.22,31 In addition, the recent meta-analysis by Strugala and Martin, which includes 16 studies (10 randomized) with a total of 1863 patients (2202 incisions), has shown an overall reduction in the SSI rate from 13 to 5.2% with the use of NPWT (RR 0.43 [95% CI 0.32–0.57], P<.001).32 Although this meta-analysis included trauma, gastrointestinal, colorectal and cesarean procedures, no differences were observed in the SSI rate reduction regarding the type of surgery in the multivariate analysis.
Some studies have found no significant differences in SSI rates regarding the use or not of NPWT. However, the devices were maintained for a short time after surgery, with treatment durations of 48h.26 In contrast, we have advocated longer-term treatments (7 days, except for signs of SSI before scheduled removal), which is more in line with most authors.
Postoperative hospital stays were also shorter in the NPWT group, although this difference was not statistically significant (8 vs 12 days, P=.22). However, this trend is also supported by other studies with similar results,27,33 in which statistically significant differences were observed of up to −0.47 days (95% CI −0.71 to −0.23; P<.001), reaching differences of −5.1 days when analyzing specifically the subgroup of patients with colorectal surgery.32 In our series, the lack of significance is probably due to the fact that we do not have an enhanced recovery after surgery program (ERAS, fast-track), which makes the magnitude of the difference less striking and requires a larger number of patients to reach statistical significance. Given the absence of significant differences in the composition of both groups in the aspects analyzed, it is worthwhile to clarify that the increased length of hospital stay in the control group is a direct consequence of surgical wound complications. Moreover, it has been widely demonstrated that reductions in hospital stay associated with a decrease in the SSI rate entail a significant savings in healthcare resources and patient suffering.3,22,31–37
This study has some limitations, such as not being blinded, which may have introduced biases. It is also non-randomized, although we believe that this could have been partially corrected by the strict verification of the absence of preoperative and surgical heterogeneity between groups. In any case, biases related to these factors should be non-directional. Another limiting factor is that the SSI were not classified as superficial or deep, so differences were not evaluated between the two types of infection. This study has also not evaluated whether the improved SSI rate occurs in all wounds, or in which type of wounds specifically this improvement is most significant (clean, clean-contaminated or contaminated). The effect is probably greater in the latter, but this has not yet been proven, and more studies are needed.
Given these limitations, we believe it is necessary to conduct prospective, randomized studies to redefine the effectiveness of the use of this type of therapies.
In short, the use of NPWT devices to cover closed incisions after colorectal surgery (both elective and urgent) is recommended in the context of other factors, including correct antisepsis, careful surgical technique and colon preparation to reduce the risk of SSI. An associated decrease in postoperative hospital stay has also been observed. It remains unclear whether the use of these devices is more beneficial in certain types of wounds (contaminated). The widespread use of NPWT devices should be determined by future comparative randomized studies.
Conflict of InterestsNone of the authors have conflicts of interests to declare.
Please cite this article as: Ocaña Jiménez J, Abadía Barno P, Ramos Rubio D, Pina Hernández JD, García Pérez JC, Moreno Montes I, et al. Papel de la terapia presión negativa en la prevención de infección del sitio quirúrgico en cirugía colorrectal. Cir Esp. 2019;97:268–274.