Simultaneous pancreas–kidney transplantation (SPKT) constitutes the therapy of choice for diabetes type 1 or type 2 associated with end-stage renal disease, because is the only proven method to restore normo-glicemic control in the diabetic patient.
MethodsRetrospective and descriptive study of a series of 175 patients who underwent SPKT from March 1995 to April 2016. We analyze donor and recipient characteristics, perioperative variables and immunosuppression, post-transplant morbi-mortality, patient and graft survival, and risk factors related with patient and graft survival.
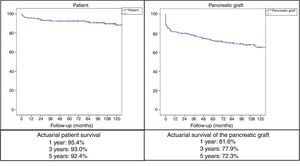
ResultsMedian age of the donors was 28years and mean age of recipients was 38.8±7.3years, being 103 males and 72 females. Enteric drainage of the exocrine pancreas was performed in 113 patients and bladder drainage in 62. Regarding post-transplant complications, the overall rate of infections was 70.3%; graft pancreatitis 26.3%; intraabdominal bleeding 17.7%; graft thrombosis 12.6%; and overall pancreas graft rejection 10.9%. The causes of mortality were mainly cardiovascular and infectious complications. Patient survival at 1, 3 and 5-year were 95.4%, 93% and 92.4%, respectively, and pancreas graft survival at 1, 3 and 5-year were 81.6%, 77.9% and 72.3%, respectively.
ConclusionsIn our 20-year experience of simultaneous pancreas–kidney transplantation, the morbidity rate, and 5-year patient and pancreas graft survivals were similar to those previously reported from the international pancreas transplant registries.
El trasplante de páncreas-riñón simultáneo constituye el tratamiento de elección en la diabetes tipo 1 o tipo 2 con fallo renal terminal o preterminal (diálisis o prediálisis), por ser la única terapia que consigue el estado euglucémico (insulino-independiente) en el paciente diabético.
MétodosEstudio retrospectivo y descriptivo de una serie de 175 pacientes trasplantados de páncreas-riñón simultáneo entre marzo de 1995 y abril de 2016. Se analizan las características de los donantes y receptores, variables perioperatorias e inmunosupresión, morbimortalidad postrasplante, supervivencia del paciente e injerto y factores de riesgo de supervivencia del paciente e injerto.
ResultadosLa mediana de edad de los donantes fue de 28 años y la media de los receptores, de 38,8±7,3años, siendo 103 hombres y 72 mujeres. La derivación duodeno-entérica se realizó en 113 casos y la duodeno-vesical, en 62. Las tasas de complicaciones postrasplante fueron las siguientes: infección global (70,3%), pancreatitis del injerto (26,3%), hemorragia intraabdominal (17,7%), trombosis del injerto (12,6%) y rechazo pancreático global (10,9%). Las causas de mortalidad fueron fundamentalmente cardiovasculares e infecciosas. La supervivencia del paciente a 1, 3 y 5años fue del 95,4, del 93 y del 92,4%, respectivamente, mientras que la del injerto correspondió al 81,6, al 77,9 y al 72,3%, respectivamente, durante el mismo periodo.
ConclusionesEn nuestra experiencia de 20 años de trasplante pancreático-renal simultáneo las tasas de morbilidad y supervivencia del paciente y del injerto a 5 años son similares a las referidas en los registros internacionales de trasplante pancreático.
Simultaneous pancreas–kidney transplantation (SPKT) is the treatment of choice for type 1 diabetes in end-stage renal failure, as it is the only therapy that achieves an insulin-independent euglycemic state with normal glucose homeostasis.1,2 In type 2 diabetes, pancreas transplantation is recommended in patients with unstable glycemia who have been insulin dependent for at least 5 years with a body mass index (BMI) less than 32kg/m2 and no cardiovascular disease.3,4 In terms of patient and graft survival, the results of SPKT are similar in type 1 and type 2 diabetics.5 Among the advantages of pancreas transplantation are the improvement of diabetes-related complications: incipient diabetic nephropathy of the native kidneys, sensory and autonomic peripheral neuropathy, gastroparesis, retinopathy, microvascular and macrovascular disease, cardiac and sexual function6–16 and quality of life.17
From 1966, when Lillehei and Kelly18 carried out the first pancreas transplantation in Minneapolis, until December 2014, more than 48000 pancreas transplantations were registered worldwide (more than 29000 in the United States and more than 19000 in other countries), including the different transplant modalities of simultaneous pancreas–kidney (SPKT), pancreas after kidney (PAKT) or pancreas transplant alone (PTA).19 In this study, we will analyze our results obtained with SPKT.
MethodsAt the Hospital Universitario Doce de Octubre (Madrid, Spain), from March 1995 to April 2016 we performed 206 pancreas transplantations, using the following methods: 175 simultaneous pancreas–kidney transplantations (SPKT), 15 pancreas after kidney (PAKT) and 16 re-transplantations. In this observational and retrospective study, we analyze our experience with SPKT carried out during the reference period, as well as a minimum post-transplantation follow-up of 6 months. Donor, recipient and perioperative variables were analyzed, as well as post-transplantation complications and patient/graft survival and risk factors for recipient/graft survival.
Donor Selection CriteriaIn our protocol, pancreatic grafts were accepted from donors between 10 and 50 years of age who were hemodynamically stable, weighed more than 30kg, with normal color and consistency and absence of: type 1 diabetes in the donor or first-degree relatives, cardiac arrest or prolonged stay in the ICU, alcoholism, calcifications, steatosis, major pancreatic edema, chronic graft pancreatitis or trauma, abdominal bacterial contamination, infection (viral, bacterial or fungal), tumors (except skin and brain) and iv drug addiction. Hyperamylasemia more than double the normal level and glycemia more than 200ng/mL were considered relative contraindications, especially when traumatic brain injury was the cause of death of the donor.
Recipient Selection CriteriaCandidates were evaluated by the abdominal organ transplant, kidney transplant and anesthesiology teams, following our testing protocol. SPKT was indicated in type 1 or 2 diabetic patients, aged between 25 and 66 years, presenting terminal nephropathy in the dialysis or pre-terminal phases (creatinine clearance less than 40/mL/min) with neuropathy and/or retinopathy. Likewise, these patients should not present absolute contraindications for transplantation (peripheral gangrene, active infection, tumor treated in a period less than 5 years or recurrence, severe neuropsychiatric tumor, HIV+, iv drug addiction, untreatable coronary artery disease, terminal heart or respiratory failure). We consider hemorrhagic retinopathy, advanced iliofemoral atherosclerosis and disabling peripheral neuropathy to be relative contraindications. We routinely performed aortoiliac CT angiography to rule out iliac atherosclerosis and a thallium stress test or dobutamine echocardiography to rule out coronary disease, which was complemented with coronary angiography in case of suspected coronary artery disease.20
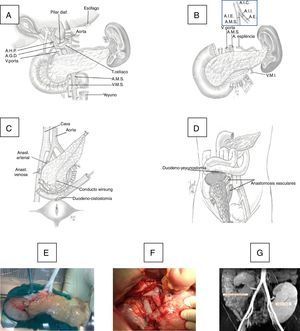
Surgical TechniqueThe pancreaticoduodenal graft was extracted en bloc and then perfused with Belzer's or Celsior's solution, depending on the time of the transplantation. On the bench, the graft was prepared with closure of the proximal and distal ends of the duodenum in 3 planes (linear stapling and 2 continuous 4/0 polypropylene sutures) and interposing an inverted Y artery graft of the common iliac artery, anastomosing the external branch with the superior mesenteric artery (SMA) of the graft and the internal artery with the splenic artery, using continuous or interrupted 6–7/0 polypropylene suture. The common iliac artery of the interposed Y-graft was anastomosed with the common iliac artery (CIA) or external iliac artery (EIA) of the recipient.
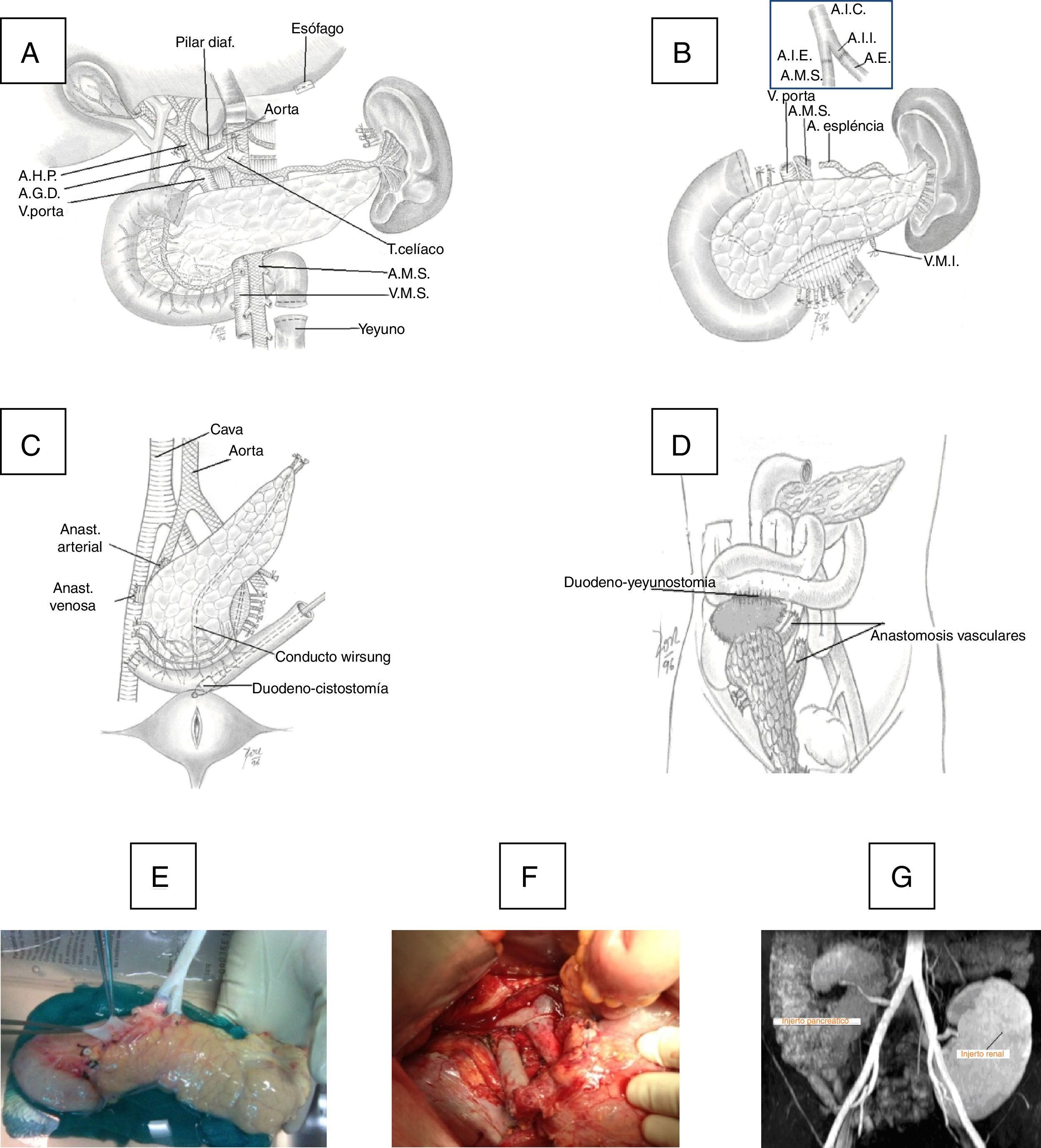
The duodenum-pancreas graft was implanted in the right iliac fossa. Initially, the portal vein of the graft was anastomosed (end-to-side) with the external iliac vein of the recipient with 6/0 polypropylene in continuous suture, and in a second phase an end-to-side porto-caval anastomosis was created. At the beginning of the program, the exocrine secretion of the graft was transferred to the bladder through a duodeno-cystostomy using circular instrumental suture, reinforced internally with 4/0 polyglyconate. More recently, we changed this technique for a duodeno-enterostomy, either with the jejunum when the head of the graft was placed in the cephalic position or with the terminal ileum (100cm from the ileocecal valve) when the head of the pancreas was situated caudally (Fig. 1).
(A) Donor organ procurement technique; (B) bench: iliac artery Y anastomosis of the graft with the superior mesenteric artery (SMA) and splenic artery (SA); (C) implantation in the right iliac fossa: arterial anastomosis between the Y end of the graft with the common iliac or external artery of the recipient and venous anastomosis between the portal vein of the graft with the common iliac vein or vena cava of the recipient and duodenal-vesical anastomosis with a mechanical stapler; (D) implantation in the right iliac fossa with side-to-side duodenal-jejunal anastomosis with the head of the pancreas in cranial position; (E) graft on the bench; (F) vascular anastomoses and reperfused graft; G) MRI image with pancreatic graft n the right iliac fossa and renal graft in the left iliac fossa.
The current regimen of prophylaxis for bacterial infection includes ceftazidime and teicoplanin, as well as micafungin for fungal infection, valganciclovir for CMV (donor+/recipient−) and trimethoprim-sulfamethoxazole for Pneumocystis carinii. Prophylaxis for graft thrombosis has changed over the years, initially using dextran or iv heparin, and currently low-molecular-weight heparin (enoxaparin, 4000IU/day) 24h after transplantation and acetylsalicylic acid (100mg/day) after the 5th–7th days. Post-transplant measures involved maintaining normal hemodynamics, with periodic determinations of glycemia, creatinine, ions and complete blood count, performing an echo Doppler on the second day to confirm the vascularization of the grafts. In case of doubts of vascular permeability, CT angiography was performed. The patients were treated in the postoperative period with parenteral nutrition until the resumption of oral intake. Glycemia >150mg/dL was treated with insulin.
Immunosuppression consisted of quadruple therapy: induction with antibodies (1.5mg/kg/day thymoglobulin for 7 days, or basiliximab 20mg on the day of transplantation and 20 on the 4th day), tacrolimus (dose to maintain levels between 10 and 15ng/mL during the first 6 months and then between 5 and 10ng/mL), azathioprine for 3 months, which was later replaced by mycophenolate mofetil (MMF) and corticosteroids (125mg, day 1; 100mg, day 2; 20mg, day 3; and progressive reduction to 5mg/day one year later). Acute rejection was suspected due to clinical symptoms, elevated creatinine, decreased amylase activity in urine (duodenal-bladder bypass), and was confirmed by renal biopsy. Acute rejection was treated with corticosteroid bolus 250–500mg and antibodies (thymoglobulin or basiliximab).
Dysfunction or delay in graft function was considered when the patient transiently required insulin or treatment with oral antidiabetic drugs to maintain normal blood glucose levels. The diagnosis of graft pancreatitis was based on clinical criteria (fever, abdominal pain, rebound, ileus), hyperamylasemia, peritoneal drainage >250mL/day with amylase >1000IU/L, duodenitis due to cystoscopy in bladder bypass, pancreatic edema, inflammation and peripancreatic fluid on CT images or confirmation of these findings by laparotomy.21
Graft loss was defined as the permanent need for insulin with doses similar to those prior to transplantation or when the patient died from any cause.
Statistical AnalysisTo describe the qualitative variables, absolute and relative frequencies were used as percentages. The quantitative variables with normal distribution were expressed as mean and standard deviation, whereas when the distribution was not normal, the median was used with 25th and 75th percentiles. The normality of the quantitative variables was studied with the Kolmogorov–Smirnov test. Patient and graft survivals were calculated using the Kaplan–Meier actuarial method.
In addition, a multivariate Cox regression model was constructed to jointly analyze patient and graft survival, evaluating each of the variables. Significant or clinically relevant variables in the univariate analysis were analyzed using the Cox regression model. The final model was described with the hazard ratio, providing its 95% confidence interval and P value. A P <.05 was considered statistically significant. The data of this study have been collected and processed for analysis using the SPSS 15.0 statistical program.
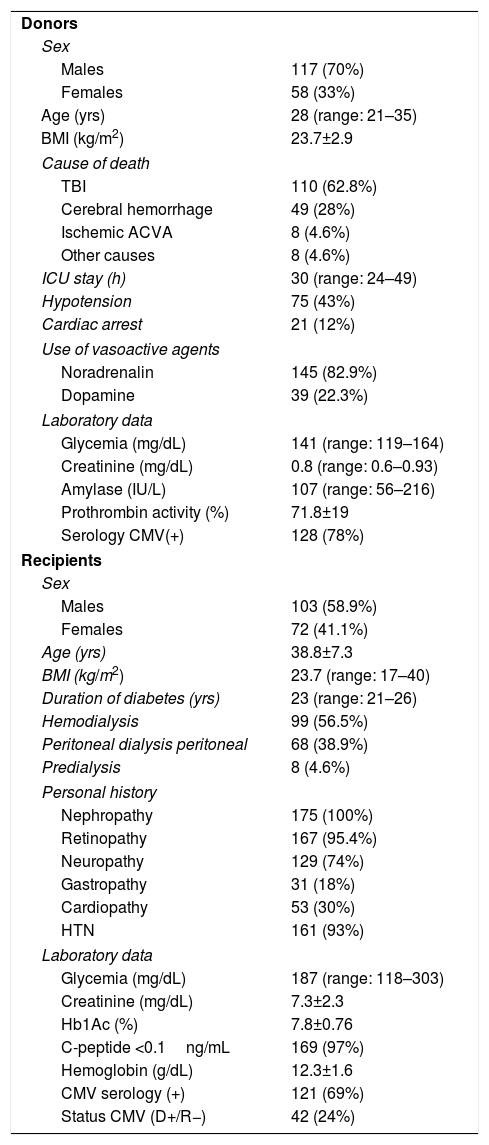
ResultsDonor and Recipient CharacteristicsThe majority of the donors in the series were men (70%), with a median age of 28 years. The main cause of death was head trauma, 12% presented cardiac arrest, and the median ICU stay was 30h. As for the recipients, 58.9% were male and the mean age of the series was 38.8±7.7. The median duration of diabetes was 23 years, with 99 (56.5%) patients on hemodialysis, 68 on peritoneal dialysis (38.9%) and 8 on predialysis (4.6%). The remaining variables are shown in Table 1.
Donor and Recipient Characteristics.
| Donors | |
| Sex | |
| Males | 117 (70%) |
| Females | 58 (33%) |
| Age (yrs) | 28 (range: 21–35) |
| BMI (kg/m2) | 23.7±2.9 |
| Cause of death | |
| TBI | 110 (62.8%) |
| Cerebral hemorrhage | 49 (28%) |
| Ischemic ACVA | 8 (4.6%) |
| Other causes | 8 (4.6%) |
| ICU stay (h) | 30 (range: 24–49) |
| Hypotension | 75 (43%) |
| Cardiac arrest | 21 (12%) |
| Use of vasoactive agents | |
| Noradrenalin | 145 (82.9%) |
| Dopamine | 39 (22.3%) |
| Laboratory data | |
| Glycemia (mg/dL) | 141 (range: 119–164) |
| Creatinine (mg/dL) | 0.8 (range: 0.6–0.93) |
| Amylase (IU/L) | 107 (range: 56–216) |
| Prothrombin activity (%) | 71.8±19 |
| Serology CMV(+) | 128 (78%) |
| Recipients | |
| Sex | |
| Males | 103 (58.9%) |
| Females | 72 (41.1%) |
| Age (yrs) | 38.8±7.3 |
| BMI (kg/m2) | 23.7 (range: 17–40) |
| Duration of diabetes (yrs) | 23 (range: 21–26) |
| Hemodialysis | 99 (56.5%) |
| Peritoneal dialysis peritoneal | 68 (38.9%) |
| Predialysis | 8 (4.6%) |
| Personal history | |
| Nephropathy | 175 (100%) |
| Retinopathy | 167 (95.4%) |
| Neuropathy | 129 (74%) |
| Gastropathy | 31 (18%) |
| Cardiopathy | 53 (30%) |
| HTN | 161 (93%) |
| Laboratory data | |
| Glycemia (mg/dL) | 187 (range: 118–303) |
| Creatinine (mg/dL) | 7.3±2.3 |
| Hb1Ac (%) | 7.8±0.76 |
| C-peptide <0.1ng/mL | 169 (97%) |
| Hemoglobin (g/dL) | 12.3±1.6 |
| CMV serology (+) | 121 (69%) |
| Status CMV (D+/R−) | 42 (24%) |
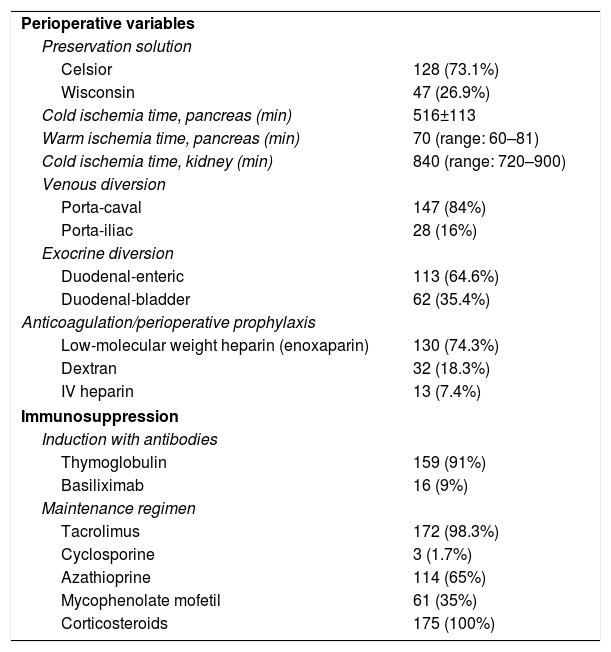
The majority of the grafts were perfused and preserved with Celsior's solution (73.1%); median cold ischemia time was 840 and median warm ischemia time was 70min. The use of portocaval shunt (84%) predominated over portal-iliac (16%), while duodenal-enteric exocrine diversion (64.6%) prevailed over duodenal-bladder (35.4%). As a prevention of graft thrombosis, we used low molecular weight heparin in most cases (74.3%). With regard to immunosuppression, most patients were induced with thymoglobulin (91%), and baseline immunosuppression therapy included tacrolimus (98.3%) and azathioprine (65%) during the first 3–4 months post-transplantation. Afterwards, azathioprine was replaced by MMF. Steroid therapy was administered to all patients (Table 2).
Perioperative Variables and Immunosuppression.
| Perioperative variables | |
| Preservation solution | |
| Celsior | 128 (73.1%) |
| Wisconsin | 47 (26.9%) |
| Cold ischemia time, pancreas (min) | 516±113 |
| Warm ischemia time, pancreas (min) | 70 (range: 60–81) |
| Cold ischemia time, kidney (min) | 840 (range: 720–900) |
| Venous diversion | |
| Porta-caval | 147 (84%) |
| Porta-iliac | 28 (16%) |
| Exocrine diversion | |
| Duodenal-enteric | 113 (64.6%) |
| Duodenal-bladder | 62 (35.4%) |
| Anticoagulation/perioperative prophylaxis | |
| Low-molecular weight heparin (enoxaparin) | 130 (74.3%) |
| Dextran | 32 (18.3%) |
| IV heparin | 13 (7.4%) |
| Immunosuppression | |
| Induction with antibodies | |
| Thymoglobulin | 159 (91%) |
| Basiliximab | 16 (9%) |
| Maintenance regimen | |
| Tacrolimus | 172 (98.3%) |
| Cyclosporine | 3 (1.7%) |
| Azathioprine | 114 (65%) |
| Mycophenolate mofetil | 61 (35%) |
| Corticosteroids | 175 (100%) |
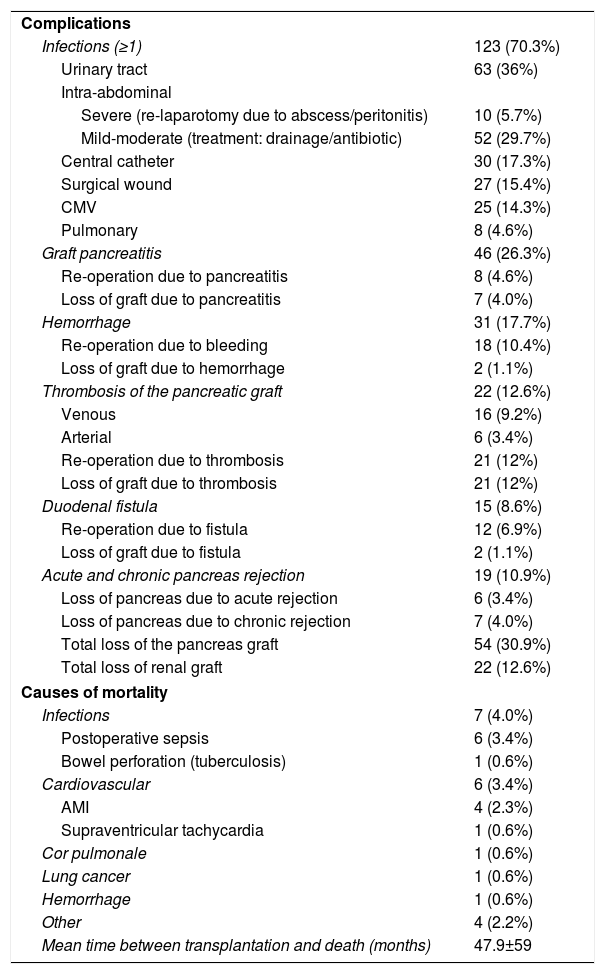
Infections were the most frequent complications after transplantation: at least one episode in 123 patients (70.3%), mainly intra-abdominal and urinary tract infections (mainly in duodenum-bladder bypasses). These were followed in frequency by graft pancreatitis (26.3%), intra-abdominal or graft hemorrhage (17.7%), overall graft thrombosis (12.6%) and duodenal fistula (8.6%).
Thrombosis of the graft was venous in 16 (9.2%) cases and arterial in 6 (3.4%); 21 patients were re-operated because of this complication, and all received another transplant because the grafts were not viable, with the exception of one graft with venous thrombosis that was diagnosed and treated intraoperatively when the laparotomy was to be closed. Acute rejection occurred in 19 (10.9%) patients, 7 (4%) of which progressed to chronic rejection. Thus, a total of 13 (7.4%) grafts were lost due to rejection (6 acute and 7 chronic). Including rejection, the overall loss of pancreas grafts was 30.9%, while the kidney graft loss was 12.6%. After a mean follow-up period of 82.7 months, 19 (10.9%) patients in the series died, the most frequent causes being infectious (7 patients; 4%) and cardiovascular (6 patients; 3.4%). The mean time between transplantation and death was 47.9±59 months (Table 3).
Complications and Post-Transplantation Mortality.
| Complications | |
| Infections (≥1) | 123 (70.3%) |
| Urinary tract | 63 (36%) |
| Intra-abdominal | |
| Severe (re-laparotomy due to abscess/peritonitis) | 10 (5.7%) |
| Mild-moderate (treatment: drainage/antibiotic) | 52 (29.7%) |
| Central catheter | 30 (17.3%) |
| Surgical wound | 27 (15.4%) |
| CMV | 25 (14.3%) |
| Pulmonary | 8 (4.6%) |
| Graft pancreatitis | 46 (26.3%) |
| Re-operation due to pancreatitis | 8 (4.6%) |
| Loss of graft due to pancreatitis | 7 (4.0%) |
| Hemorrhage | 31 (17.7%) |
| Re-operation due to bleeding | 18 (10.4%) |
| Loss of graft due to hemorrhage | 2 (1.1%) |
| Thrombosis of the pancreatic graft | 22 (12.6%) |
| Venous | 16 (9.2%) |
| Arterial | 6 (3.4%) |
| Re-operation due to thrombosis | 21 (12%) |
| Loss of graft due to thrombosis | 21 (12%) |
| Duodenal fistula | 15 (8.6%) |
| Re-operation due to fistula | 12 (6.9%) |
| Loss of graft due to fistula | 2 (1.1%) |
| Acute and chronic pancreas rejection | 19 (10.9%) |
| Loss of pancreas due to acute rejection | 6 (3.4%) |
| Loss of pancreas due to chronic rejection | 7 (4.0%) |
| Total loss of the pancreas graft | 54 (30.9%) |
| Total loss of renal graft | 22 (12.6%) |
| Causes of mortality | |
| Infections | 7 (4.0%) |
| Postoperative sepsis | 6 (3.4%) |
| Bowel perforation (tuberculosis) | 1 (0.6%) |
| Cardiovascular | 6 (3.4%) |
| AMI | 4 (2.3%) |
| Supraventricular tachycardia | 1 (0.6%) |
| Cor pulmonale | 1 (0.6%) |
| Lung cancer | 1 (0.6%) |
| Hemorrhage | 1 (0.6%) |
| Other | 4 (2.2%) |
| Mean time between transplantation and death (months) | 47.9±59 |
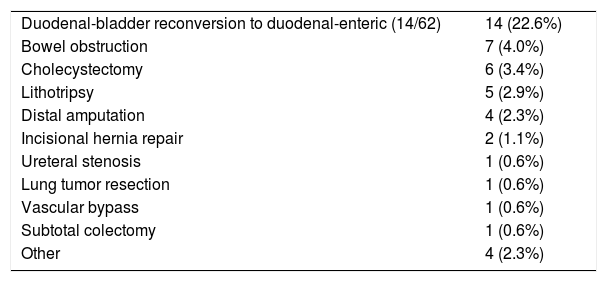
Throughout the follow-up of the series, 46 interventions were carried out: conversion of duodenal-bladder to duodenal-enteric diversion in 14 patients (22.6%) out of a total of 62 where bladder bypass was used; laparotomy due to intestinal obstruction in 7 (4%); cholecystectomy in 6 (3.5%); bladder lithotripsy in 5 (2.9%), distal amputations of lower limbs in 4 (2.3%), incisional hernia repair in 2 (1.1%) and others in 8 (4.6%) (Table 4).
Surgical Procedures During Follow-Up (n=46).
| Duodenal-bladder reconversion to duodenal-enteric (14/62) | 14 (22.6%) |
| Bowel obstruction | 7 (4.0%) |
| Cholecystectomy | 6 (3.4%) |
| Lithotripsy | 5 (2.9%) |
| Distal amputation | 4 (2.3%) |
| Incisional hernia repair | 2 (1.1%) |
| Ureteral stenosis | 1 (0.6%) |
| Lung tumor resection | 1 (0.6%) |
| Vascular bypass | 1 (0.6%) |
| Subtotal colectomy | 1 (0.6%) |
| Other | 4 (2.3%) |
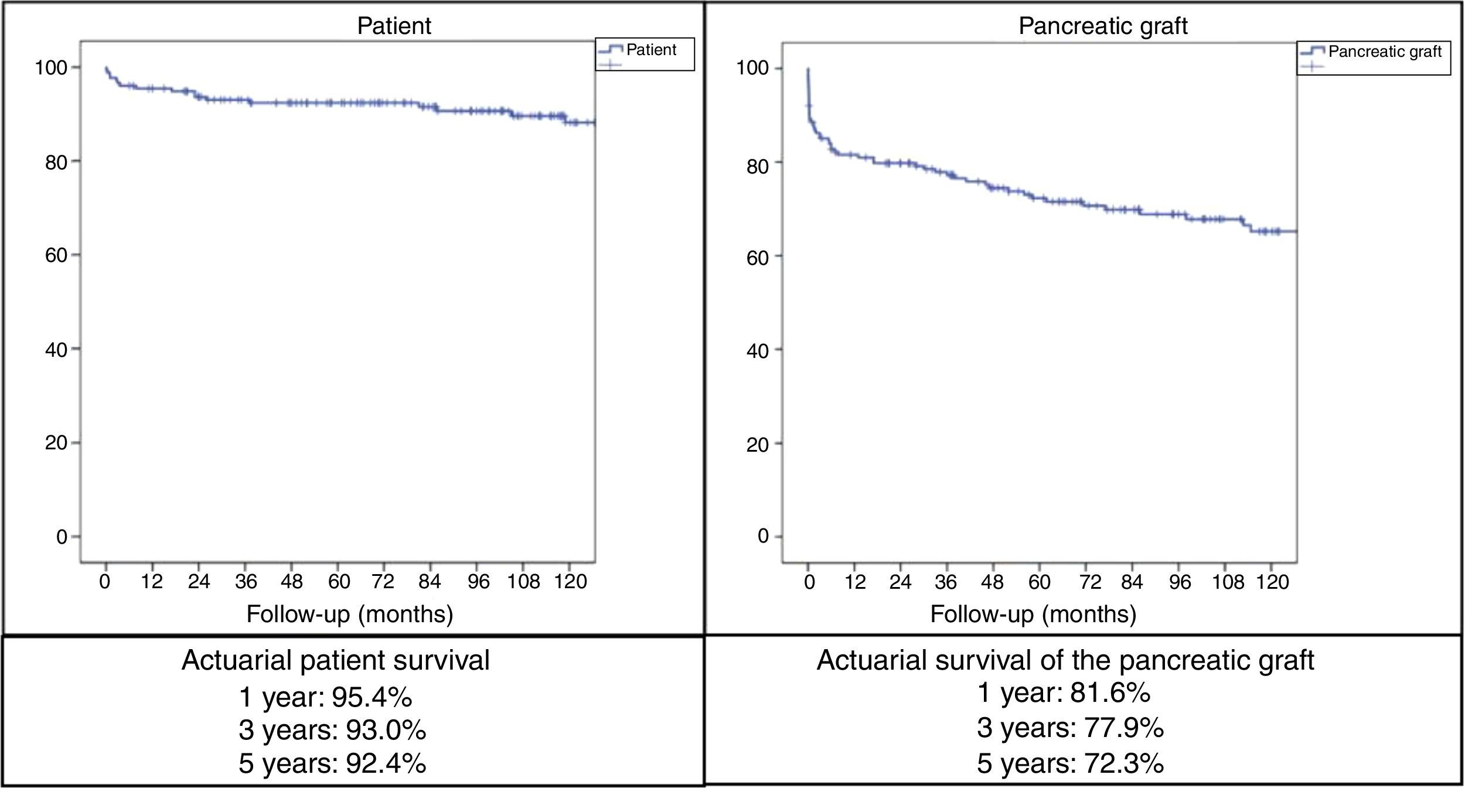
With mean follow-up of the series of 89.5 months, the actuarial 1, 3 and 5-year patient survival rates were 95.4%, 93% and 92.4%, respectively, while the actuarial 1, 3 and 5-year survival rates for the pancreatic grafts were 81.6%, 77.9% and 72.3%, respectively (Fig. 2).
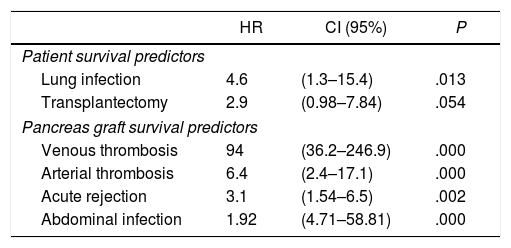
Risk Factors for Patient and Graft SurvivalThe multivariate analysis demonstrated that lung infection significantly influenced patient survival, although the need to perform transplantectomy did not reach statistical significance (P=.054). Furthermore, graft survival was significantly and adversely influenced by the presence of an arterial or venous thrombosis of the graft, acute rejection and abdominal infection (Table 5).
Cox Regression Multivariate Analysis of Patient and Pancreas Graft Survival.
| HR | CI (95%) | P | |
|---|---|---|---|
| Patient survival predictors | |||
| Lung infection | 4.6 | (1.3–15.4) | .013 |
| Transplantectomy | 2.9 | (0.98–7.84) | .054 |
| Pancreas graft survival predictors | |||
| Venous thrombosis | 94 | (36.2–246.9) | .000 |
| Arterial thrombosis | 6.4 | (2.4–17.1) | .000 |
| Acute rejection | 3.1 | (1.54–6.5) | .002 |
| Abdominal infection | 1.92 | (4.71–58.81) | .000 |
Ideally, pancreas donors should be between 10 and 40 years of age, have a BMI <27.5kg/m2 and brain death not caused by stroke22; our donors had a median age of 28 years. Although, due to the shortage of donors, potential donors up to 50–55 years of age are being evaluated, the use of these grafts is associated with a higher rate of surgical complications (pancreatitis and graft thrombosis) and, therefore, lower graft survival.23,24 The use of donors >45 years of age presents a 4 times greater risk for developing graft thrombosis.25 The excessive replacement of fluids due to donor hemodynamic instability is associated with graft edema, poorer microcirculation and greater graft thrombosis,23 so these donors should be ruled out for transplantation.
Donor hyperglycemia is frequent and is attributed to the release of endogenous steroids and catecholamines as well as the administration of glucose solution and corticosteroids. Therefore, in the absence of endocrine insufficiency, hyperglycemia is not an absolute contraindication for use of the graft.26 In this series, we have used a graft from a donor with a blood glucose level of 299mg/dL.
Hyperamylasemia, in the absence of pancreatic trauma, is not a contraindication for transplantation, since it is usually associated with head trauma, cerebral infarction and treatment with corticosteroids.27,28 Thus, in this experience we have used grafts of up to 807IU/L amylase with good results.
The average age of our recipients was 38.8 years, with one exceptional case of a 66-year-old with a good biological age (absence of iliac atherosclerosis), who was transplanted 10 years ago with no complications. Disease severity and comorbidity are better predictors of post-transplant complications than age per se, and similar results were obtained (patient and graft survival or complication rate) in comparative series between recipients older than or younger than 50 years of age29 and older or younger than 55.30 Obesity in pancreas transplantation is associated with greater technical difficulty, incidence of complications and recurrence of diabetes, as well as reduced patient and graft survival.31,32 The ideal situation to perform a SPKT is pre-dialysis, a fact seen in 8 (4.6%) patients in our series. Pre-transplantation peritoneal dialysis of the pancreas has been referred to as a risk factor for the development of post-transplantation intra-abdominal infections.33,34 In our experience, this incidence was also greater, although not statistically significant in the peritoneal dialysis group compared to hemodialysis, with similar patient and graft survival in both groups.35
The preservation solution we most frequently used was Celsior instead of Wisconsin, as a previous comparative study had obtained similar results with both.36 The duodenal-vesical bypass, performed at the beginning of our program, was replaced with the more physiological duodenal-enteric diversion, since the incidence of thrombosis and urinary infections with the former was significantly higher, although the patient and graft survivals were similar with both diversions.37 In recent publications, incidences of complications and graft loss have been similar with the duodenal-vesical and duodenal-enteric diversions.2,19,38
The incidence of infection in pancreas recipients can reach 80% during the first year after transplantation, with an average of two episodes of severe infection per year,39,40 and 50% of deaths are attributed to these infections.39 According to an extensive series, 70% of post-transplantation infections were intra-abdominal,41 requiring re-laparotomy in 90% of patients and graft explantation in 70%.42 Our overall incidence of infections was 70%, the most severe being abdominal and the most frequent urinary. In most cases, the presentation was after the third month post-transplantation, and most occurred in patients with duodenal-bladder diversion.43 Graft pancreatitis has been reported in 35% of cases, associated with serious complications (abscess, necrosis, fistula, collections).21 Our incidence of graft pancreatitis was 26.3%, having performed the explantation of the pancreatic graft in 7 (4%) patients due to severe pancreatitis.
Graft thrombosis presents an incidence between 8.8% and 35%. It is associated with graft loss in practically all cases, and low blood flow of the graft is the main risk factor.23,44–47 Our overall incidence of graft thrombosis was 12.6% (22 cases), more frequently venous (9.2%), resulting in the explantation of 21 grafts and preservation of only one, which was diagnosed and treated intraoperatively before closure.
Our rate of re-operations due to graft hemorrhage was 10.4%, which mainly occurred in cases with thrombotic prophylaxis using iv heparin, which has since been replaced years ago with low-molecular weight heparin. Duodenal fistulae occur in 5%–20% of duodenal-bladder diversions versus 5%–8% in duodenal-enteric diversions,48 representing 8.6% in our series, where all the fistulae were located in the lateral closure of the duodenum, not in the actual anastomosis.
When tacrolimus+MMF double therapy is used, the incidence of acute rejection is 27%,49 and pancreatic graft function is lost in 20% of patients with acute rejection in the first year.50 In our series, 19 (10.9%) patients presented acute rejection, with a total of 13 pancreatic grafts lost due to rejection (acute in 6 and chronic in 7).
Patients with SPKT have a mortality risk 2.8 times lower than those who remain on the transplant waiting list (31% mortality on the waiting list versus 12% in transplant recipients).51 When the pancreatic graft remains functional at the end of the year after transplantation, five-year patient survival is greater than 90% in all categories (SPKT, PAKT, PTA).
In the series of 1000 SPKT at the University of Wisconsin, the 5-year patient survival rate was 89% and the most frequent causes of death were cardiopulmonary (7.2%), infectious (3.4%), cerebrovascular (1.8%), renal failure (1.5%) and tumors (1.3%).52 The most important risk factor for mortality is the loss of the graft due to the resulting return to insulin treatment.53 The 5-year survival rate of our patients with SPKT was 92.4%; the most frequent causes of death were infectious and cardiovascular causes.
According to the International Pancreatic Transplant Registry (IPTR) regarding SPKT from 2008 to 2009, the 5-year kidney and pancreas graft survival rates were 81% and 73%, respectively.5 The actuarial pancreatic graft survival rates of our SPKT series after 1, 3, and 5 years were 81.6%, 77.9%, and 72.3%, respectively. The IPTR also shows that, from 2008 to 2009, SPKT had the highest 5-year graft survival rate: 73% in SPKT, 64% in PAKT and 53% in PTA. A series of factors favoring greater 5-year graft survival in SPKT was also indicated: absence of pre-transplantation dialysis, level <20% of the panel of reactive antibodies, young donors, short ischemia time and absence of acute rejection during the first year.5
During the follow-up period, as other authors have observed,54 the interventions most frequently performed in our patients (46 interventions in total) have been: reconversion of duodenal-bladder to duodenal-enteric diversion because of urological complications (infections, gallstones) or reflux pancreatitis, re-laparotomy due to intestinal obstruction, cholecystectomy, bladder lithotripsy, distal amputations, incisional hernia repair and hernia repair.
In the multivariate analysis, we observed that lung infection is a risk factor for mortality, while, as other transplant teams have pointed out, venous or arterial thrombosis,54 acute rejection54 and intra-abdominal infection54 have been confirmed as risk factors for graft loss.
In conclusion, in our 21 years of experience in simultaneous pancreatic–kidney transplants, the 5-year patient and graft morbidity and survival rates are similar to those reported in international pancreas transplant registries.
AuthorshipCarlos Jiménez-Romero: Study design; article composition; critical review and approval of the final version.
Alberto Marcacuzco Quinto: Study design and data collection.
Alejandro Manrique: Analysis and interpretation of the results; critical review and approval of the final version.
Iago Justo: Critical review and approval of the final version; study design.
Jorge Calvo: Data collection; analysis and interpretation of the results.
Félix Cambra Molero: Data collection and data analysis.
Óscar Caso: Data collection; analysis and interpretation of the results.
Álvaro García-Sesma: Data collection; analysis and interpretation of the results.
Enrique Moreno González: Study design; critical review and approval of the final version.
Conflict of InterestThe authors have to conflict of interests to declare.
Please cite this article as: Jiménez-Romero C, Marcacuzco Quinto A, Manrique Municio A, Justo Alonso I, Calvo Pulido J, Cambra Molero F, et al. Trasplante de páncreas-riñón simultáneo. Experiencia del Hospital Doce de Octubre. Cir Esp. 2018;96:25–34.