Gastric outlet obstruction is a complication of advanced tumors. It causes upper gastrointestinal obstruction, with progressive malnutrition and reduced survival. Currently, gastrojejunostomy or stent placement (SP) are feasible alternatives for the treatment of malignant gastric outlet obstruction. The aim of this study is to compare the efficacy and survival of both techniques.
MethodsSingle-center observational and prospective study of 58 patients with gastric outlet obstruction who underwent surgical treatment with stomach-partitioning gastrojejunostomy (SPGJ) or SP with self-expanding intraluminal prostheses between 2007 and 2018.
ResultsThirty patients underwent SPGJ and 28 SP. The mean age of the first group was significantly lower (69 vs. 78 years, respectively; P=.001). There were no statistically significant differences in terms of sex, perioperative risk or tumor etiology. Postoperative complications were non-significantly higher in the SPGJ group (P=.156). SP was associated with a shorter hospital stay (P=.02) and faster oral intake (P<.0001). However, SP had significantly higher rates of persistent and recurrent obstruction (P=.048 and 0.01, respectively), poorer energy targets (P=.009) and shorter survival (9.61 vs. 4.47 months; P=.008).
ConclusionsSPGJ presents greater luminal permeability, better oral intake and greater survival than SP. SP is preferable for non-surgical patients with a limited short-term prognosis.
El síndrome de obstrucción antroduodenal es una complicación presente en neoplasias avanzadas. Se caracteriza por clínica de obstrucción gastrointestinal alta, con desnutrición progresiva, y se asocia con una disminución de la supervivencia. La derivación mediante gastroyeyunostomía y el tratamiento endoscópico (TE) son las alternativas para el tratamiento del síndrome de obstrucción antroduodenal. El objetivo de este estudio es comparar la eficacia y la supervivencia de ambas.
MétodosEstudio monocentro, observacional y prospectivo de 58 pacientes con síndrome de obstrucción antroduodenal que recibieron tratamiento quirúrgico mediante gastroyeyunostomía con separación gástrica parcial (GYSGP) o TE con prótesis enterales autoexpandibles entre los años 2007-2018.
ResultadosA 30 pacientes se les realizó GYSGP y a 28 pacientes TE. La edad media de los pacientes con GYSGP fue significativamente menor (69 vs 78 años, p=0,001). No hubo diferencias en cuanto al sexo, el riesgo anestésico-quirúrgico ni la etiología de la neoplasia. Las complicaciones posprocedimiento fueron superiores, aunque no significativas, en el grupo de GYSGP (p=0,156). El TE se asoció con una menor estancia hospitalaria (p=0,02) y una mayor precocidad de la tolerancia oral (p<0,0001). Sin embargo, los pacientes presentaron tasas más altas de obstrucción persistente y recurrente (p=0,048 y 0,01, respectivamente), unos peores objetivos energéticos (p=0,009) y una supervivencia menor (9,61 vs 4,47 meses, p=0,008).
ConclusionesLa GYSGP obtiene una mayor permeabilidad luminal, una mejor tolerancia a la vía oral y una mayor supervivencia. El TE estaría recomendado para pacientes no subsidiarios de la cirugía con un pronóstico limitado a corto plazo.
Gastric outlet obstruction (GOO) is a complication of advanced-stage malignant tumors that cause stenosis of the antrum and duodenum to varying degrees of extension.1–3 While invasive pancreatic cancers are responsible for 15%–20% of cases, other entities include gastric, duodenal, or biliary tract neoplasms, hepatocellular carcinoma, lymphomas or metastases.3,4
Typical symptoms include nausea, vomiting and oral intolerance, leading to dehydration, malnutrition, deterioration of the patient's general condition and quality of life. In addition, it delays the administration of chemotherapy treatments. For these reasons, GOO reduces patient survival compared to the natural history of the disease.2,4
Accurate and effective treatment of GOO is necessary to restore oral intake, improve quality of life and continue with systemic treatments.2–5
There are 2 pillars in the treatment of GOO: surgery and endoscopic treatment (ET). Gastrojejunostomy (GJ) or stomach-partitioning gastrojejunostomy (SPGJ) are standard treatments. Surgery presents good functional results and symptomatic relief rates higher than 70%; however, the associated morbidity (13%–55%) and mortality (2%–36%) rates are also high.2,4,5 The SPGJ procedure published by Kaminishi et al. shows lower rates of delayed gastric emptying (DGE) and shorter hospital stays.6,7
The first publication of ET was in 1992.5 Its main advantages are: less invasive treatment, faster symptomatic relief and shorter hospital stay. However, it also presents higher rates of long-term luminal obstruction, requiring endoscopic re-interventions. Other complications of endoscopic treatment are stent migration, hemorrhage and perforation.1–4
Numerous publications4–8 have compared GJ and ET for the palliation of GOO. Despite the greater long-term efficacy of surgery, there is controversy when determining the exact indications for both procedures.
The aim of our study is to compare the Kaminishi SPGJ, modified by our group, with ET for the palliation of GOO.
MethodsOurs is an observational, longitudinal and prospective study using a retrospective database at the Complejo Asistencial Universitario in Salamanca from 2007 to 2018.
The sample consisted of 58 patients with GOO secondary to unresectable malignant neoplasm at the time of intervention; 28 patients underwent ET and 30 patients SPGJ.
ET was performed with uncoated enteral self-expanding metal stents (SEMS) as well as partially coated stents (Wallstent® by Boston Scientific, and Hanarostent® pylorus/duodenum Kim's flare, by M.I. Tech).
SPGJ was performed with a modified Kaminishi technique, which was previously published by our group.8 A supraumbilical laparotomy was used to access the peritoneal cavity. Gastrolysis of the greater curvature of the stomach was performed proximally and distal to the tumor. Subsequently, we performed a partial gastric division, perpendicular to the greater curvature and up to 3cm from the lesser curvature with a tri-stapler (Endo GIA™ 60mm, from Medtronic). The reconstruction of the tract was done with a side-to-side Roux-en-Y anastomosis (transmesocolic or supramesocolic position) on the posterior gastric side, 2cm from the proximal branch and parallel to the inverted V. The loop anastomosis was also created with a tri-stapler. Finally, a dual-lumen nasogastric tube was inserted with gastric suction (Compat® Stay-Put 9/18 FR, Nestlé Health Care Nutrition) for early enteral nutrition.
Data were collected for several demographic variables: age, sex, etiology of the GOO, disease stage (potentially resectable or unresectable) and estimation of the anesthetic-surgical risk using the ASA classification. Data regarding the procedure were also collected, including the surgical approach and type of stent.
The postoperative variables collected were: postoperative complications, persistence of the obstruction, recurrence, time to initiation of oral intake, type of tolerance according to a modified GOO assessment scale (0: no food, 1: semi-liquid food, 2: puréed food, 3: normal diet) and need for the placement of a new stent.
Postoperative complications were grouped using the Clavien–Dindo classification. Persistence of the obstruction was classified as a technical failure, while recurrent obstruction was classified as neoplastic progression.
Data was also collected for patient survival in both groups.
Statistical AnalysisQuantitative variables are presented as mean and standard deviation or as median and range, according to their distribution. The categorical variables are expressed as number and percentage. To verify the normality of the distribution, the Kolmogorov–Smirnov test was used. The analysis between the quantitative and categorical variables was done with the Student's t test or the Mann–Whitney U test, according to their distribution. The relationship among qualitative variables was checked by the Chi-squared test. For the survival analysis, the Mantel–Cox test was performed. Kaplan–Meier survival curves were used for the variables age, sex, type of procedure and ASA classification, using the Log-rank test for the analysis.
The variables that were clinically significant and had a P<.2 were studied using a Cox regression model. The hazard ratio and 95% confidence intervals were expressed.
The level of significance α was established at 5%, and the analysis was made with the SPSS® Statistical Package version 25 (SPSS Inc., Chicago, IL, USA).
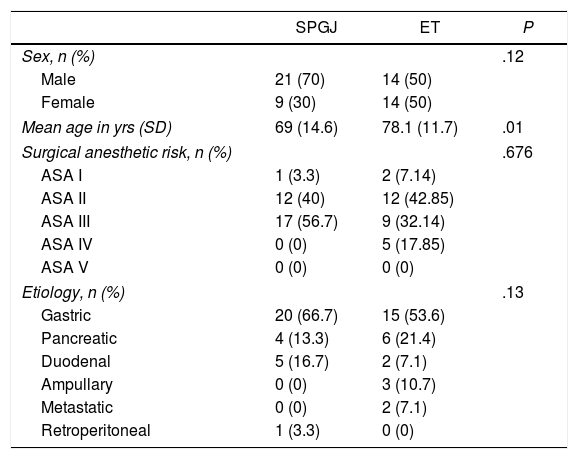
ResultsThe demographic characteristics of the 58 patients are shown in Table 1. In 30 patients, SPGJ was performed, while 28 patients underwent ET. In the SPGJ group, 70% of patients were male.
Demographic Characteristics of the Sample.
| SPGJ | ET | P | |
|---|---|---|---|
| Sex, n (%) | .12 | ||
| Male | 21 (70) | 14 (50) | |
| Female | 9 (30) | 14 (50) | |
| Mean age in yrs (SD) | 69 (14.6) | 78.1 (11.7) | .01 |
| Surgical anesthetic risk, n (%) | .676 | ||
| ASA I | 1 (3.3) | 2 (7.14) | |
| ASA II | 12 (40) | 12 (42.85) | |
| ASA III | 17 (56.7) | 9 (32.14) | |
| ASA IV | 0 (0) | 5 (17.85) | |
| ASA V | 0 (0) | 0 (0) | |
| Etiology, n (%) | .13 | ||
| Gastric | 20 (66.7) | 15 (53.6) | |
| Pancreatic | 4 (13.3) | 6 (21.4) | |
| Duodenal | 5 (16.7) | 2 (7.1) | |
| Ampullary | 0 (0) | 3 (10.7) | |
| Metastatic | 0 (0) | 2 (7.1) | |
| Retroperitoneal | 1 (3.3) | 0 (0) | |
ASA: American Society of Anesthesiologists; SD: standard deviation; SPGJ: stomach-partitioning gastrojejunostomy; ET: endoscopic treatment.
The mean age of the patients with SPGJ was 69 years (standard deviation 14.6), which was significantly lower than the age of patients with ET (78 years [standard deviation 11.7], P=.01). There were no differences between sexes (P=.12) nor in the anesthetic-surgical risk of both groups (P=.676).
All patients were diagnosed with GOO of neoplastic origin, with gastric tumors being the most frequent (53.6% in the ET group and 66.7% in the SPGJ group) (P=.13).
The patients in the surgery group underwent an open approach, and SPGJ was performed according to the described technique. In the ET group, bare-metal SEMS were placed in 82.1%, while 17.9% received coated SEMS.
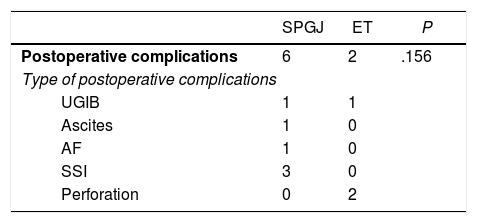
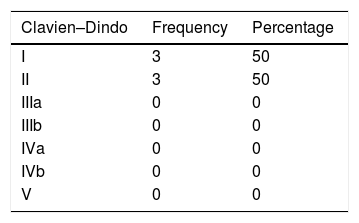
The SPGJ presented a higher rate, although not significant, of postoperative complications (P=.156) (Table 2). Postoperative complications were grouped according to the Clavien-Dindo classification (Table 3).
Postoperative Complications.
| SPGJ | ET | P | |
|---|---|---|---|
| Postoperative complications | 6 | 2 | .156 |
| Type of postoperative complications | |||
| UGIB | 1 | 1 | |
| Ascites | 1 | 0 | |
| AF | 1 | 0 | |
| SSI | 3 | 0 | |
| Perforation | 0 | 2 | |
AF: atrial fibrillation; SPGJ: stomach-partitioning gastrojejunostomy; UGIB: upper gastrointestinal bleeding; SSI: surgical site infection; ET: endoscopic treatment.
The rates of persistent obstruction were higher in the ET group (14.3% vs. 0%, respectively, P=.048) compared to the SPGJ group. The rates of recurrent obstruction were also higher (8.7% and 6.7%, respectively, P=.1); 7.1% required the placement of a new SEMS.
The ET group showed a shorter time interval until reintroduction of oral intake (most initiated tolerance the afternoon after the procedure, compared to a mean of 4.4 days in the SPGJ group, P<.0001).
Despite the earlier initiation of oral intake, only 15% of the patients with ET could be fed a normal diet, in contrast to the patients with SPGJ, 60% of whom reached the requirements (P=.009).
The median hospital stay was shorter in the ET group (4 days) compared to the SPGJ group (10 days) (P=.02).
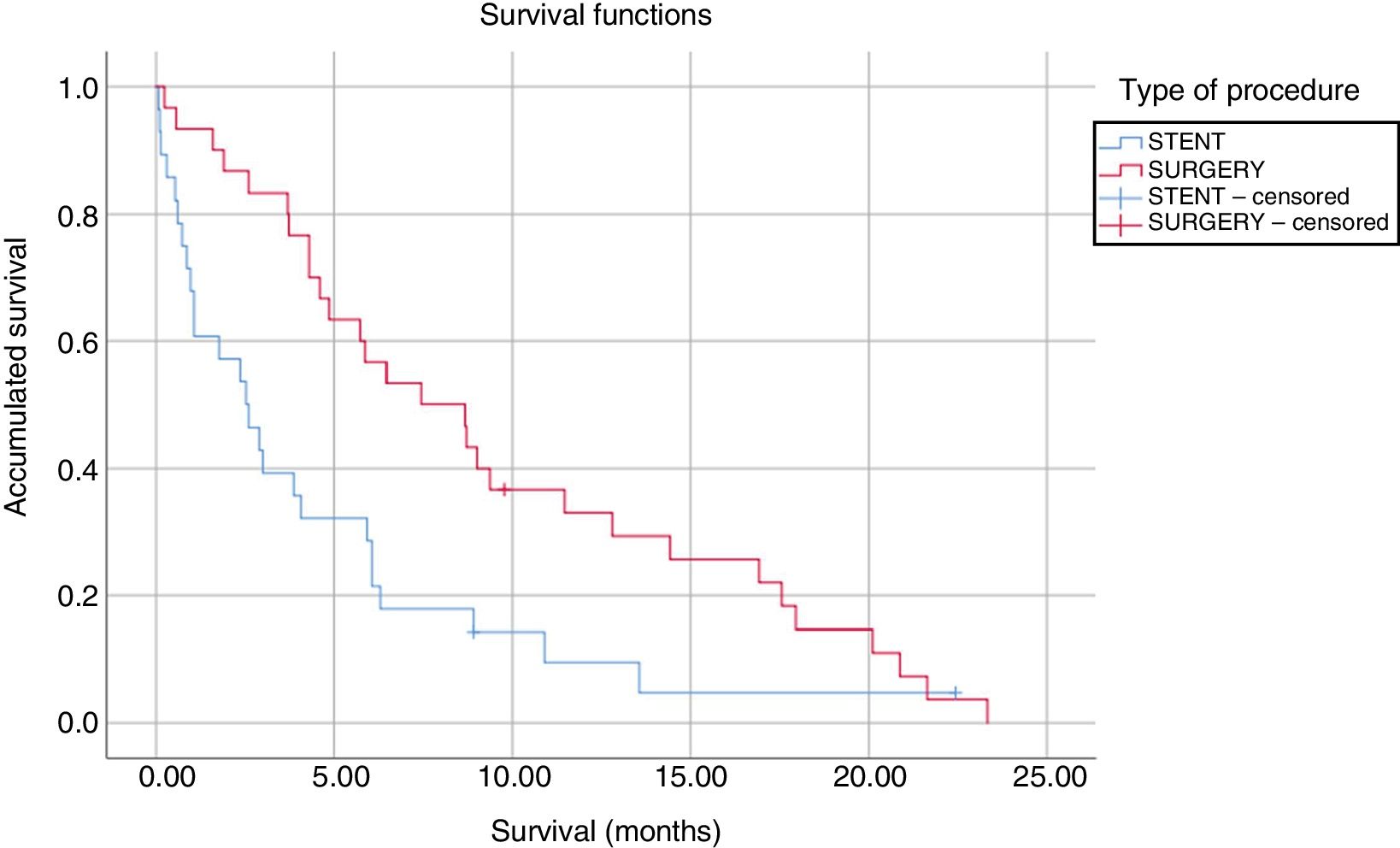
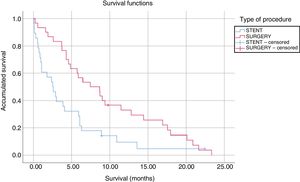
The median survival of patients undergoing SPGJ was significantly higher than that of patients treated with stents (9.61 vs. 4.47 months, P=.008) (Fig. 1). In the SPGJ group, there was one death due to sepsis secondary to liver abscesses, and the remainder died due to cancer progression. From the ET group, one patient died after infection with the influenza virus, another due to traumatic brain injury and, finally, another patient due to hemorrhagic shock. The rest died due to tumor progression. The multivariate analysis only found a significant association between the type of procedure performed (SPGJ or ET) and survival (hazard ratio 0.48, 95% confidence interval 0.27–0.83).
DiscussionTraditionally, the technique for the treatment of GOO was gastric bypass with GJ. However, this surgery presents DGE rates between 10 and 50%9,10 (in a study at our hospital, the rate was 33%).8 In 1925, GJ was published with antral exclusion for the palliation of GOO.11 The high rate of anastomotic ulcers, together with the need for decompression of the gastric antrum, motivated the abandonment of the technique described by Devine.6,8,11
In the 90s, SPGJ was introduced by the Kaminishi group.7 Its advantages are: limited contact of the tumor with food, improved DGE rates, reduced blind loop effect and the possibility of endoscopic access of the tumor or bile ducts.6,8
The modification proposed by our group tries to diminish the blind loop effect in the efferent loop of the GJ and favor gastric emptying.
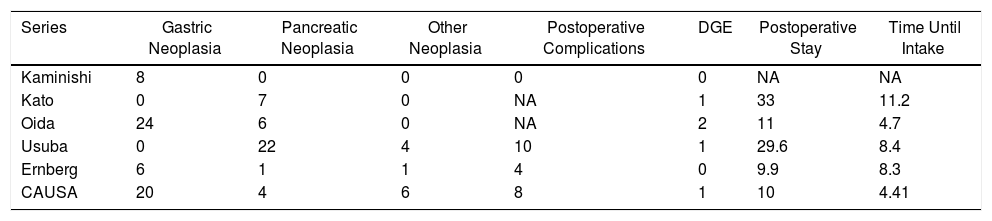
There are currently very few studies comparing SPGJ versus GJ. Most are retrospective and provide heterogeneous conclusions. In 2016, Kumagai et al.6 published a systematic review that compared both techniques, reviewing 7 retrospective studies with 207 patients diagnosed with neoplastic GOO. SPGJ presented significantly lower DGE rates and postoperative hospital stay compared to GJ. In our series, patients receiving SPGJ had a postoperative stay (10 days), DGE rate (one patient) and time to onset of oral tolerance (4.4 days) similar to those of other publications (Table 4).
Results of the Main Series About Gastrojejunostomy With Partial Gastric Separation Compared to Our Series.
| Series | Gastric Neoplasia | Pancreatic Neoplasia | Other Neoplasia | Postoperative Complications | DGE | Postoperative Stay | Time Until Intake |
|---|---|---|---|---|---|---|---|
| Kaminishi | 8 | 0 | 0 | 0 | 0 | NA | NA |
| Kato | 0 | 7 | 0 | NA | 1 | 33 | 11.2 |
| Oida | 24 | 6 | 0 | NA | 2 | 11 | 4.7 |
| Usuba | 0 | 22 | 4 | 10 | 1 | 29.6 | 8.4 |
| Ernberg | 6 | 1 | 1 | 4 | 0 | 9.9 | 8.3 |
| CAUSA | 20 | 4 | 6 | 8 | 1 | 10 | 4.41 |
CAUSA: Complejo Asistencial Universitario de Salamanca; NA: not available; DGE: delayed gastric emptying.
ET using SEMS was first described in the 90s. Since then, its implementation has grown greatly due to the demonstration of being a safe and effective procedure12,13 that is less invasive and provides clinical success rates that are comparable to surgery.1,8
In addition, ET involves earlier onset of oral tolerance and a shorter hospital stay compared to SPGJ.1,2,5,14–16
In our series, the ET group had a shorter hospital stay compared to the patients who underwent SPGJ. However, this result could not be shown with statistical significance. The onset of oral nutrition was significantly lower in the ET group (P<.0001), similar to data from the literature. In our case, we believe that this result was influenced by the use of the nasogastric tube for early enteral nutrition.
These results agree with those of a recently published study1 that shows hospital stay rates of 9.8 days in the surgical group versus 4.7 days in the ET group. Several publications show, given the shorter hospital stay, that ET is a more cost-effective option than surgery in the early time period. However, due to the prognosis of these patients, long-term efficiency studies cannot be carried out.
Other authors have a reduction in hospital stay to become similar to post-ET stay in patients with laparoscopic SPGJ. In our study, this hypothesis could not be corroborated because all patients underwent laparotomy.
In our series, ET showed a postoperative complication rate lower than SPGJ (2 versus 6, P=.156). However, all surgical complications were in groups I and II of the Clavien–Dindo classification, these being the 2 groups with the least severity.
Jeurnink et al.2 published a higher rate of postoperative complications in the ET group compared to the surgery group (6 episodes versus 4, P=.02). However, other studies did not find significant differences.4,18
Given the similar results in terms of clinical success, resolution of symptoms and complications in the procedure, we need other criteria so that we can choose one of the 2 therapies.
To answer this question, we have compared ET and SPGJ in terms of long-term luminal permeability and survival.
The persistence and recurrence of the obstruction were higher in the ET group (14.3 vs. 0% and 8.7 vs. 6.7%, respectively). We attribute this finding to the absence of intestinal bypass, a greater probability of intra-stent tumor growth and possible stent migration. Different studies report greater intraluminal permeability in the surgery group.15
In addition, patients who underwent SPGJ had a longer survival compared to patients with ET (9.61 vs. 4.47 months, P=.008). These results are similar to reports published in the literature.1,16,17,19
The greater survival could be related to a selection bias because patients who undergo surgery generally have a better general and nutritional status. However, recent studies1 have demonstrated that the type of intervention for GOO is an independent factor in terms of survival and luminal permeability. The presence of ascites, poor nutritional status and poor general condition are factors for a poor prognosis, regardless of the type of intervention.
In our study, the ET patients were significantly older than the SPGJ recipients, a datum that could be related with a worse general state. However, multivariate analysis has shown that age did not appear as an independent predictor of survival.
Therefore, the greater survival of patients treated surgically could be related not only with their previous general condition, but also better tolerance to oral intake, less need for re-operations and the greater number of patients with chemotherapy.
Currently, surgery would be reserved for patients with acceptable surgical risk and a relatively long-term survival. ET should be performed in patients with a poorer general condition, who would benefit from a less invasive method and present a poor mid-term prognosis. Some studies established a survival cut-off point of 2 months to choose between the 2 therapies.2
To our knowledge, the present series is one of the largest published to date. In addition, there are no large studies in the literature comparing SPGJ and ET.
The main limitations of the study include the sample size, as well as the absence of certain variables of interest (nutritional and general state of the patients) that would allow us to carry out a more extensive analysis. Larger, prospective, randomized studies with clear inclusion criteria and an intention-to-treat analysis are necessary.
We therefore conclude that the SPGJ obtains better rates of luminal permeability in the long term, better tolerance to oral intake and longer patient survival compared to ET. ET would be the ideal therapeutic option for patients who are not candidates for surgical treatment with a poor short-term prognosis.
AuthorshipLuis Muñoz-Bellvís and Felipe C. Parreño-Manchado have contributed equally and should be considered senior authors.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: López-Sánchez J, Marcos Martín ÁF, Abdel-Lah Fernández O, Quiñones Sampedro JE, Álvarez Delgado A, Esteban Velasco MC, et al. Anastomosis gastroyeyunal con separación gástrica parcial frente al tratamiento endoscópico para el tratamiento del síndrome de obstrucción antroduodenal de causa maligna. Cir Esp. 2019;97:385–390.