Access to the abdominal aorta and its visceral trunks is possible through several approaches.
Dissections of five cadavers performed during three National Surgical Anatomy courses applied to Aorta, Hepatobiliopancreatic and Digestive Surgery. Videos and pictures were taken throughout the dissections and showed different abdominal aorta approaches.
Abdominal aorta and visceral trunks approaches: longitudinal inframesocolic access, supraceliac clamping, celiac trunk dissection, superior mesenteric artery approaches (retroperitoneal after Kocher menoeuvre, supramesocolic or inframesocolic), Cattell-Braasch manoeuvre and mattox manoeuvre: retrorenal and prerenal.
Correct knowledge of the intraabdominal anatomy is necessary to perform all the abdominal aorta surgical approaches. Cadaveric dissection could help to achieve this objective. Cardiovascular and digestive surgeons need to know the possible strategies in order to choose the one which is best suited for each patient.
Los cirujanos cardiovasculares y del aparato digestivo deberían estar al corriente de las múltiples alternativas de abordaje de la aorta abdominal y sus troncos viscerales.
Artículo narrativo, ilustrado y dinámico de las diferentes maniobras quirúrgicas descritas con este objetivo.
Disección de 5 cadáveres realizadas durante tres cursos nacionales de Anatomía Quirúrgica aplicada a aorta integral, Cirugía hepatobiliopancreática y Cirugía abdominal digestiva.
Maniobras quirúrgicas descritas: abordaje aórtico inframesocólico longitudinal, abordaje aórtico supracelíaco, abordaje del tronco celíaco, tres tipos de abordaje de la arteria mesentérica superior: retroperitoneal tras maniobra de Kocher, supramesocólico e inframesocólico, maniobra de Cattell-Braasch y dos tipos de maniobra de Mattox: retrorrenal y prerrenal.
El conocimiento profundo de la anatomía intraabdominal es fundamental para la actuación quirúrgica sobre la aorta abdominal y el entrenamiento en cadáver a partir de la anatomía quirúrgica vascular y del tubo digestivo podría ayudar a desarrollar las habilidades quirúrgicas de los cirujanos en formación.
Abdominal aorta approaches can be intimidating if the surgeon is not familiarized with the different techniques described for its approach and control. Cardiovascular and digestive system surgeons should be aware of the multiple approach options.1,2
The following is a description of the different maneuvers to access the aorta and its visceral branches from a transperitoneal approach, each accompanied by illustrative images and an educational video.3
Five cadavers were dissected: three prepared in formaldehyde, one prepared fresh and the other using the Thiel technique. The specimens were obtained in accordance with Spanish national legislation and regulations.
The dissections were performed during three training courses in Applied Surgical Anatomy:
- -
Applied Surgical Anatomy: The integral aorta. Applied Surgical Anatomy Unit of the Department of Human Anatomy and Embryology of the University of Valencia (Director: IMG)
- -
Hepatobiliopancreatic Applied Surgical Anatomy. Applied Surgical Anatomy Unit of the Department of Human Anatomy and Embryology of the University of Valencia (Director: LSO)
- -
Digestive Abdominal Surgical Anatomy in Cadavers. Miguel Hernández de Elche University (Director: AA)
Surgical maneuvers were performed by three cardiovascular surgeons, two general and digestive system surgeons specialized in hepatobiliary-pancreatic surgery and liver transplantation, and two general and digestive system surgeons specialized in colorectal surgery.
During the description of each of the approaches, the technical characteristics, practical aspects, indications, advantages and disadvantages are explained and discussed in order to reduce the possibility of intraoperative and postoperative complications.
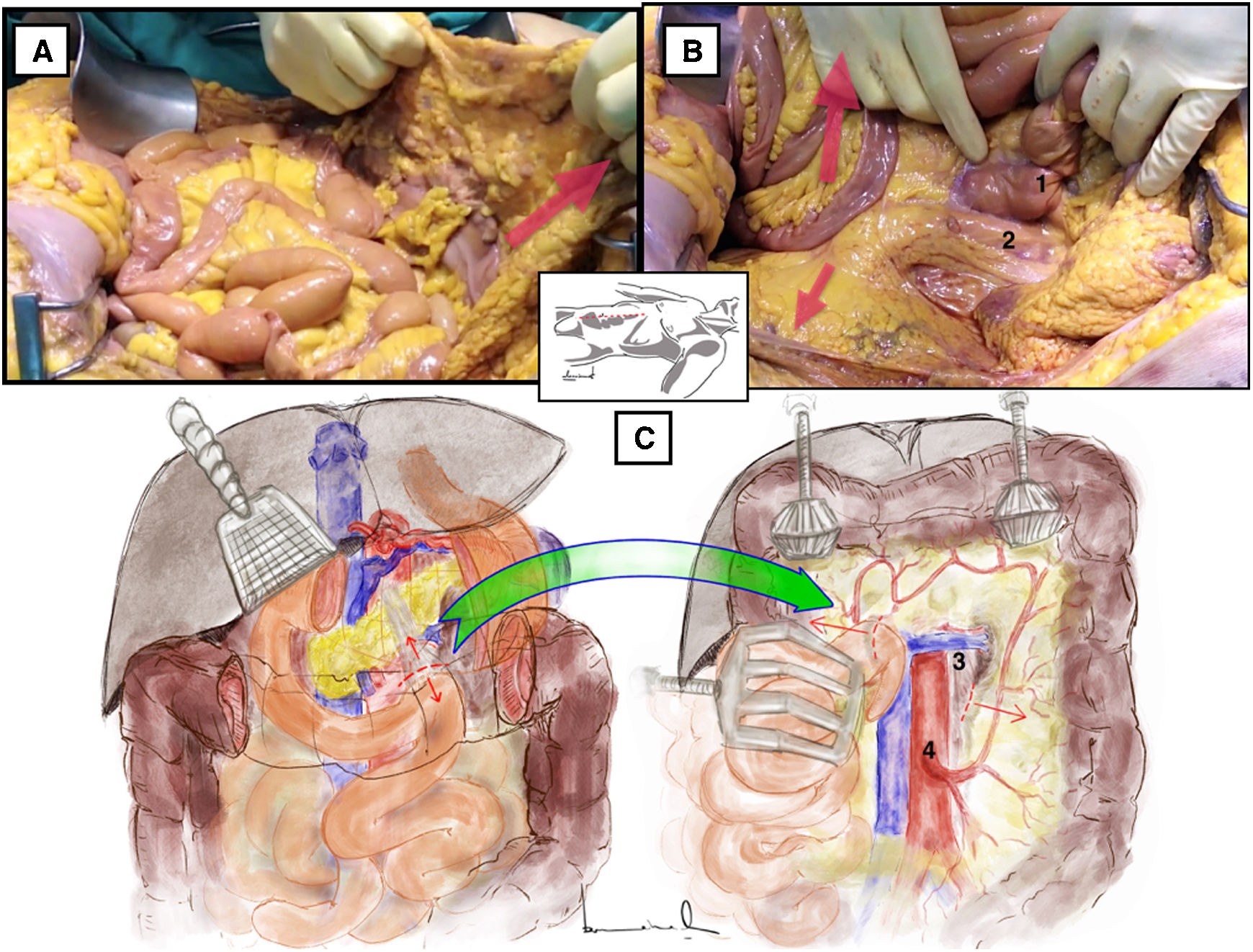
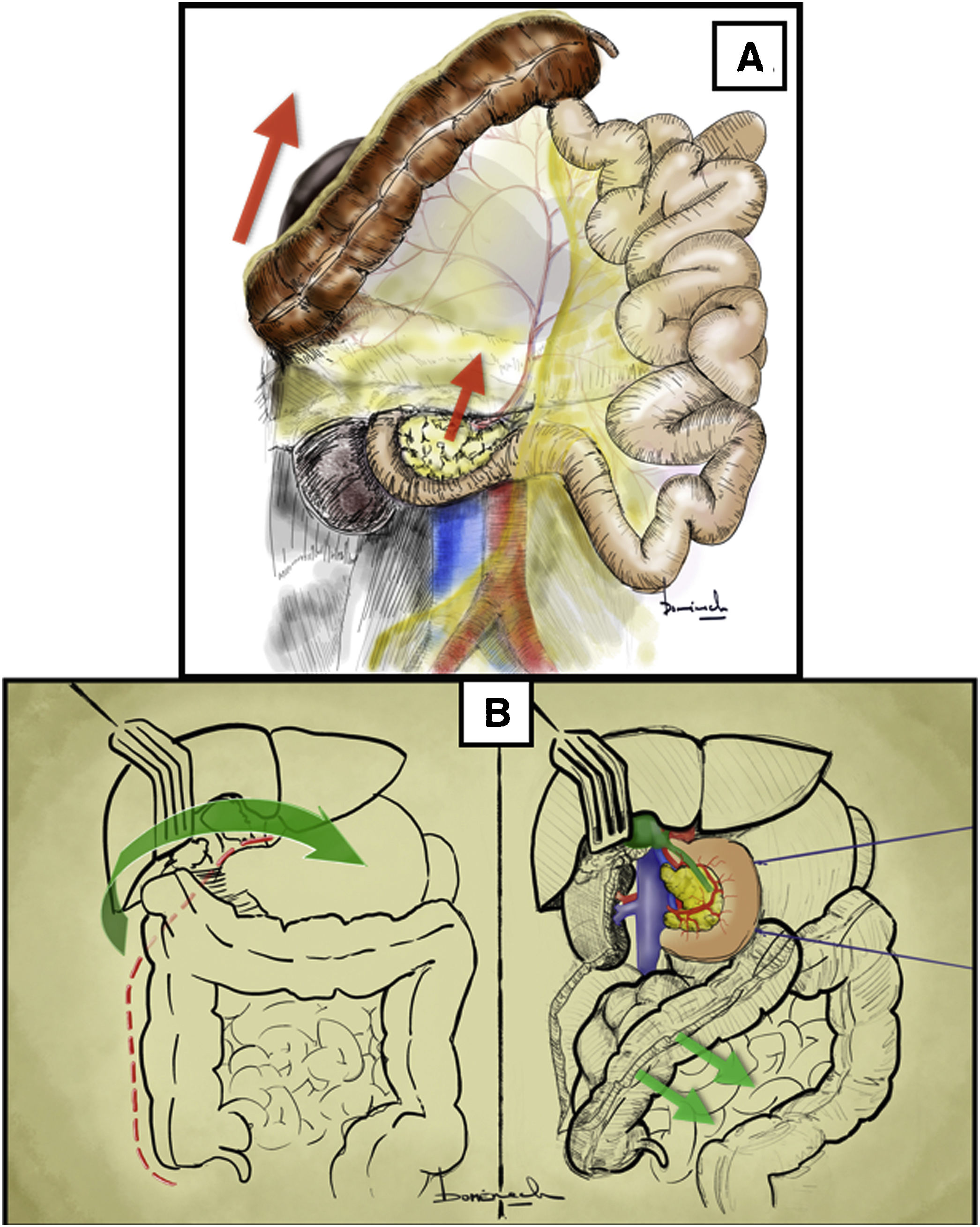
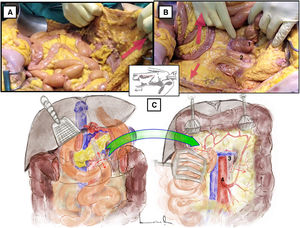
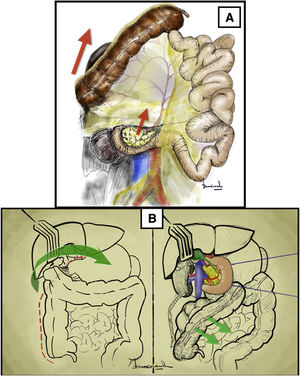
Longitudinal inframesocolic aortic approach3–5 (Appendix A [material available in the electronic version], Video 1)Surgical techniqueAfter transperitoneal midline laparotomy, the transverse colon and greater omentum are retracted towards the ribcage (Fig. 1A) and the intestinal bundle is retracted laterally to the right, allowing for identification of the root of the mesentery and the ligament of Treitz. Subsequently, retraction of the sigmoid colon towards the left pelvis clearly exposes the retroperitoneum covering the aorta (Fig. 1B).
Longitudinal inframesocolic approach: (A and B) demonstration on a fresh cadaver. Red arrows indicate the direction of traction of the transverse colon, intestinal bundle, and sigmoid colon to achieve correct exposure of the infrarenal aorta. 1: duodenojejunal angle; 2: aorta. (C) The red dashed line indicates the release of the Treitz angle to achieve correct exposure. The anatomical structures exposed after opening the pre-aortic retroperitoneum are shown. 3: left renal vein; 4: inferior mesenteric artery. The color of the figure can only be seen in the electronic version.
Dissection is begun with the release of the duodenojejunal angle or angle of Treitz, which allows us to mobilize the 4th portion of the duodenum to the right and move the duodenopancreatic block cranially (Fig. 1C).
The incision over the retroperitoneum anterior to the aorta provides exposure of the anterior aortic wall. The extension of the retroperitoneal incision cranially towards the proximal neck of the aorta allows for the left renal vein (LRV) to be identified, which marks the upper limit of the aortic dissection6 (Fig. 1C).
Distal exposure of the abdominal aorta is achieved by extending the retroperitoneal incision caudally across the right anterior surface of the aorta. The inferior mesenteric artery is normally located on the left anterior surface.
Indications/advantagesThe longitudinal inframesocolic approach of the abdominal aorta is the most commonly used approach by vascular surgeons in cases of infrarenal aortic aneurysm.
Practical aspectsDepending on the distal extension of the aneurysm, the retroperitoneal incision may need to be extended below the iliac vessels.7
When performing aortic clamping, careful dissection of the aorta helps minimize inadvertent injury to the lumbar arteries exiting the posterior aorta. The identification of the renal arteries at this point guides us in the determination of the proximal clamping site.8
In terms of proximal clamping, a length of at least 1 cm of ‘healthy’ or non-aneurysmal infrarenal aorta is normally required to avoid adrenal clamping.2 Sometimes large lymphatic channels are found that cross over the neck of the aorta.3 Ligation of these lymphatic vessels or their coagulation with vascular sealants helps prevent postoperative lymphatic leakage. If it is necessary to improve exposure to perform this clamping, the inferior mesenteric vein could be ligated and divided, and the venous drainage of the left colon would depend on the pericolic marginal arch that drains into the middle colic vein.
Regarding the distal clamping of the aorta, attention must be paid to the proximity of the cava while the aorta is dissected. The cava is closer to the distal aorta and recedes slightly as we advance proximally. Circumferential dissection of the aorta is rarely necessary and risks injury to these lumbar arteries.
Circumferential dissection of the iliac arteries is not usually necessary as it exposes the underlying iliac veins to possible injury.
When the aneurysm encompasses the common iliac arteries, distal control is usually achieved beyond the iliac bifurcation. This entails the dissection of the external and internal iliac arteries, for which we will need to perform extensive decollation of the sigmoid. In these situations, the distal anastomosis is normally constructed at the iliac bifurcation.
DisadvantagesDuring this maneuver, the evisceration of the intestinal bundle can cause hemodynamic instability due to the manipulation of the superior mesenteric plexus, while also favoring intestinal venous congestion due to venous compression.
On rare occasions, the LRV may be in the retroaortic position, so correct preoperative knowledge of the patient’s anatomy with a CT-angiography is essential to avoid catastrophic venous injuries due to the proximal clamp (renal blood flow is 1200 mL/min).9 Occasionally, division of the LRV is necessary to expose the proximal infrarenal aorta. If so, it should be divided as close as possible to its junction with the vena cava to protect its venous drainage through the adrenal, gonadal and renolumbar veins.
The inferior mesenteric artery can be damaged in this approach or be chronically occluded in abdominal aortic aneurysms. In these cases, the vascularization of the left and sigmoid colon depends on the pericolic marginal vascular arcade that originates in the middle colic artery.10
The superior hypogastric plexus is located just above the aortic bifurcation, so extreme caution should be taken not to injure it in order to avoid alterations in sexual function. If exposure and clamping of the iliac vessels is necessary, it must be taken into account that the left common iliac vein crosses below the right common iliac artery.11
Supraceliac aortic approach2,12 (Appendix A, Video 2)Surgical techniquePatient in supine position with a roller located at T12 to elevate the spine. The round and falciform ligaments are divided and a subcostal retractor is placed at the costal margin.
Mobilization of the left hepatic lobe after division of the left hepatic coronary and triangular ligaments, without reaching the left suprahepatic vein.
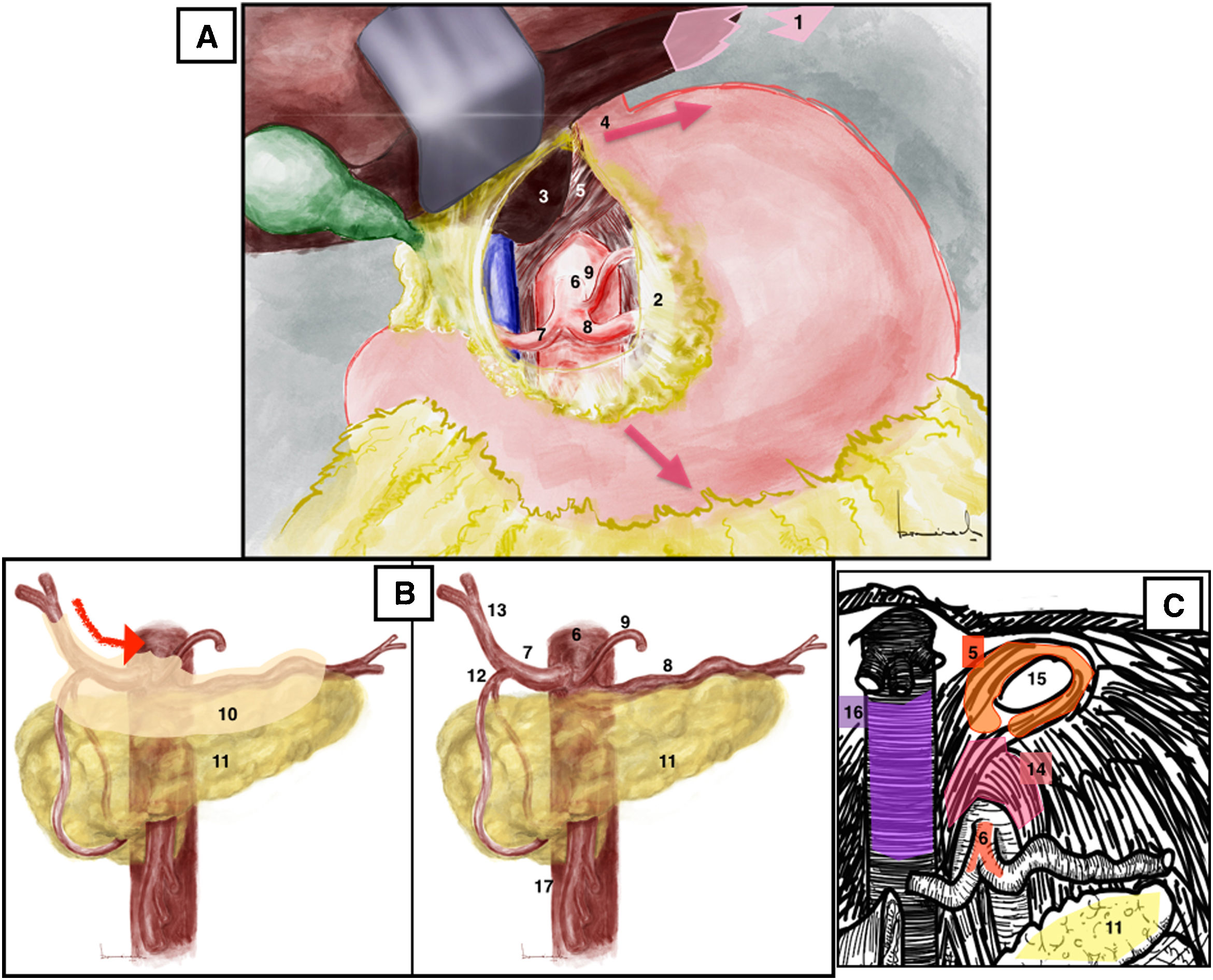
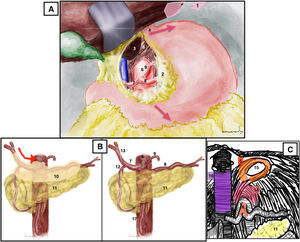
Opening of the lesser omentum to the phrenoesophageal membrane, which gives access to the caudate lobe of the liver and the celiac aortic region. The esophagus (identified by the presence of a nasogastric tube) and the two vagus nerves are retracted to the left. The aorta is exposed by dividing the right pillar of the diaphragm (Fig. 2A). The supraceliac aorta can already be clamped, if necessary. Manual palpation at the diaphragmatic hiatus allows us to identify the supraceliac aorta.
(A) Supraceliac approach. Red arrows mark the direction of esophagogastric traction. (B) Celiac trunk approach. Red arrow: direction of opening of the peritoneum that covers vascular structures. (C) Celiac trunk approach, identification of the arcuate ligament. 1: left triangular ligament of the liver; 2: lesser omentum or hepatogastric ligament; 3: caudate lobe of the liver; 4: gastroesophageal junction; 5: diaphragmatic crura; 6: celiac trunk; 7: common hepatic artery; 8: splenic artery; 9: left gastric artery; 10: peritoneum covering vascular structures; 11: pancreas; 12: gastroduodenal artery; 13: proper hepatic artery; 14: arcuate ligament; 15: esophageal hiatus; 16: vena cava; 17: superior mesenteric artery. The color of the figure can only be seen in the electronic version.
In emergency situations with active bleeding from one of the main aortic branches, in which an infrarenal aortic clamp cannot be used due to the characteristics of the patient or the intraoperative situation.
In ruptured abdominal aortic aneurysms, especially with a rupture located in the anterior wall, proximal control should be performed immediately after opening the peritoneal cavity at the supraceliac aorta.
In programmed surgery, due to the need for revascularization of visceral trunks (hepatic artery, superior mesenteric artery), renal arteries or femoral arteries.
Practical aspectsIn obese patients with a narrow chest, the laparotomy could be extended to a partial median sternotomy.
For correct exposure, the right pleural cul-de-sac is moved cranially to provide access to the supraceliac aorta. If accidental opening of the pleura occurs, a pleural drain should be placed.
In supraceliac aortic clamping, it is useful to tighten the clamp against the spine due to the high pressures that the aorta reaches and thus prevent the aorta from “expelling” the clamp.
Slow release of the aortic clamp at the supraceliac level helps prevent sudden drops in blood pressure.
DisadvantagesWe may find an aberrant left hepatic artery originating at the left gastric artery (10%–25%), which must be respected when we divide the lesser omentum or hepatogastric ligament.
This clamping can cause severe hemodynamic changes. The slow release of the clamp helps prevent sudden drops in blood pressure.2,12
There is a risk of gastroesophageal reflux when dividing the right diaphragmatic crura in patients with previous esophageal cardia anomalies.
Celiac trunk approach2 (Appendix A, Video 3)Surgical techniqueThe approach can be begun with the identification of the common hepatic artery after palpating thrill on the upper edge of the pancreas, or it can be initiated with the identification of the proper hepatic artery in the hepatic hilum and continue towards the celiac artery until the junction with the gastroduodenal artery. At this point, the opening of the peritoneum that covers the common hepatic artery will lead us to the origin of the splenic artery and the left gastric artery (Fig. 2B). The opening of the arcuate ligament will facilitate the exposure of the origin of the celiac trunk (Fig. 2C).
Indications/advantagesThe hepatic artery is the branch of the CT most frequently used as a target for splanchnic revascularization in cases of celiac artery occlusive disease.
The splenic artery could be treated for a splenic aneurysm.
It is essential to know this maneuver for the surgical treatment of pancreatic neoplasms.
Practical aspectsThe approach of the celiac trunk can be started in two different anatomical-surgical areas. The first is by directly detecting the common hepatic artery at the upper edge of the pancreas by direct palpation, and the second is by dissecting the proper hepatic artery at the hepatic hilum. Subsequently, the opening of the peritoneum along the upper edge of the pancreas will direct us towards the origin of the celiac trunk. Due to intraoperative needs, the left gastric artery could be divided since the collateral circulation from the gastroepiploic arcade is usually sufficient.13
DisadvantagesDissection and exposure of the celiac trunk should be carried out close to the aortic wall to avoid incursions into the periaortic nerve tissue that makes up the celiac plexus.
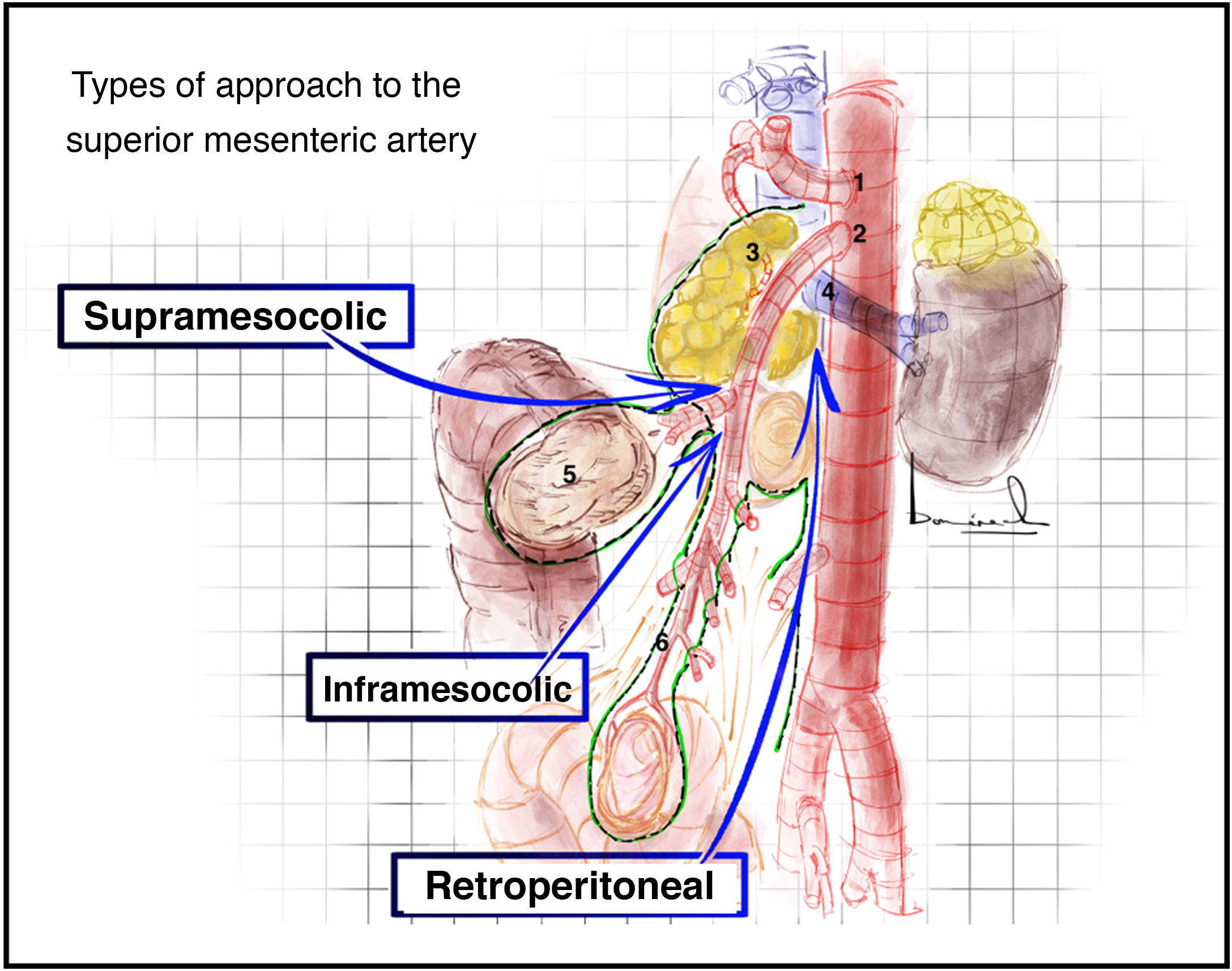
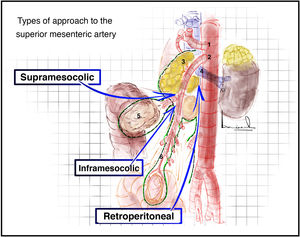
Superior mesenteric artery approach2,14 (Appendix A, Video 4)There are three main superior mesenteric artery (SMA) approaches: retroperitoneal, supramesocolic, and inframesocolic (Fig. 3).
Plane posterior to the duodenum and head of the pancreas (Fig. 4A) after a Cattell Braasch or Kocher maneuver (which will be explained later).
(A) Retroperitoneal approach after duodenal-pancreatic traction towards the patient’s left shoulder. (B) Identification of the gastrocolic trunk of Henle by means of a supramesocolic approach of the superior mesenteric artery. The red arrow indicates the direction of dissection to approach the superior mesenteric artery. (C) Simulation of the inframesocolic approach in formaldehyde-treated cadaver, superior traction of the transverse colon and inferior traction of the first jejunal loop. 1: superior mesenteric artery; 2: superior mesenteric vein; 3: left renal vein; 4: aorta; 5: vena cava; 6: right gastroepiploic vein; 7: anterosuperior pancreaticoduodenal vein; 8: right superior colic vein; 9: middle colic vein; 10: jejunal veins; 11: transverse colon; 12: mesocolon; 13: mesentery; 14: first jejunal loop. The color of the figure can only be seen in the electronic version.
Between the 2nd portion of the duodenum, head of the pancreas and the transverse mesocolon (Fig. 4B). In this approach, the more lateral situation of the superior mesenteric vein means that we must access and divide the gastrocolic trunk of Henle to identify the superior mesenteric vein and thus be able to mobilize it and reach the SMA that is medial to it.
Indications/advantagesSMA access for embolectomies in the presence of acute occlusive mesenteric ischemia.
Identification and assessment of possible infiltration of the SMA and superior mesenteric vein in pancreatic neoplasms.
Possible maneuver to use if it is decided to perform a right hemicolectomy with D3 lymphadenectomy for right colon cancer.15
Practical aspectsDuring the supramesocolic approach maneuver, the vascular variability of the gastrocolic trunk of Henle can make access to the superior mesenteric vein difficult. Identification of the superior right colic vein, which drains from the right colic flexure to the gastrocolic trunk of Henle, facilitates this maneuver (Fig. 4B). It is present in 95% of patients and is the first vein that we will encounter if we perform this approach laterally.16
DisadvantagesIt is important to respect the middle colic vein to avoid venous necrosis of the transverse colon. Nevertheless, if it were divided, the venous return through the marginal arteries towards the ileocolic vein and left colic vein is usually sufficient.
Inframesocolic approachSurgical techniqueBetween the transverse mesocolon and the mesentery of the first jejunal loops (Fig. 4C). Superior traction of the transverse mesocolon and inferior traction of the mesentery should be performed. The visceral peritoneum of the mesentery is opened, and the superior mesenteric vessels are identified.
Indications/advantagesThis maneuver provides quick and direct access to the SMA and offers the possibility to perform antegrade and retrograde embolectomy.
Practical aspectsTraction of the transverse mesocolon and the mesentery must be done correctly.
DisadvantagesWe must ensure that we open the visceral peritoneum of the mesentery and not the transverse colon, as we might mistakenly dissect and divide the middle colic vessels.
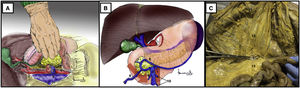
Cattell-Braasch maneuver17,18 (Appendix A, Video 5)Surgical techniqueOpening of the right parietocolic ligament from the terminal ileum ascending to the right colic flexure (both included). The ascending colon is released from the retroperitoneum through Toldt’s fascia (Fig. 5A).
Cattell-Braasch maneuver. (A) Release of the colon and duodenum through the embryological plane of Toldt’s coalescence fascia. The red arrows indicate the direction of the traction of the right colon, duodenum and pancreas. (B) Release of the hepatic flexure first, followed by Kocher’s maneuver. The green arrows indicate the direction of traction of the hepatic flexure of the colon. The color of the figure can only be seen in the electronic version.
This is followed by retroduodenopancreatic release using the Kocher maneuver.
Kocher maneuver: the assistant should medialize the second part of the duodenum with triangular forceps or manual grip to exert traction/contratraction with respect to the retroperitoneum, always in the direction of the patient’s left shoulder. The surgeon should combine blunt and scissor dissection of the adhesions of the duodenum and head of the pancreas to the retroperitoneum.
With these two movements, the duodenum and head of pancreas, intestinal bundle, cecum, ascending colon and hepatic flexure are medialized. In this maneuver, the right kidney is kept in situ.
The hilum of the liver represents the upper limit of the maneuver. The inferior mesenteric vein represents the medial limit.
The Kocher maneuver can also be performed directly with the release of the hepatic flexure and subsequent duodenal-pancreatic medialization (Fig. 5B).
Indications/advantagesThese maneuvers expose the posterior side of the pancreas and the second and third parts of the duodenum and facilitate the retroperitoneal approach to the origin of the SMA, located just above the LRV. Excellent exposure of the right ureter and kidney with its renal vascular pedicle is also obtained. They can also expose the infrahepatic vena cava, the right gonadal vessels, and the infrarenal aorta up to their bifurcation.
Practical aspectsThe Kocher maneuver is performed by releasing the hepatic flexure of the colon and subsequent access to the retroperitoneum located behind the second part of the duodenum and pancreas. While in the Cattell-Braasch maneuver the same plane is accessed but by complete decollation of the right colon and terminal ileum, through the planes known as Toldt’s fascia (between the right colon and retroperitoneum) and Treitz fascia (between the posterior pancreatic-duodenal side and the retroperitoneum).19 Therefore, the Cattell-Braasch maneuver exposes a greater retroperitoneal surface.
DisadvantagesAs little as 2−3 cm of the SMA can be dissected by this approach.
When performing the retroperitoneal approach using the Kocher or Cattell-Braasch maneuver, traction on the pancreas should be limited to avoid tearing the junctions of the hepatic and splenic arteries, in addition to causing possible pancreatitis.
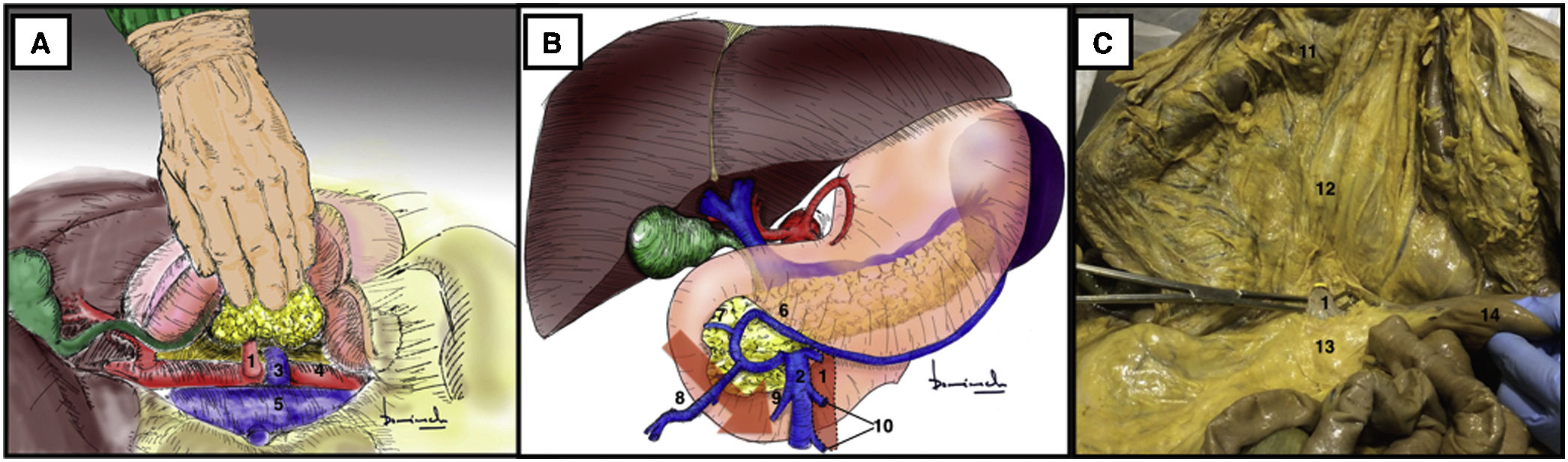
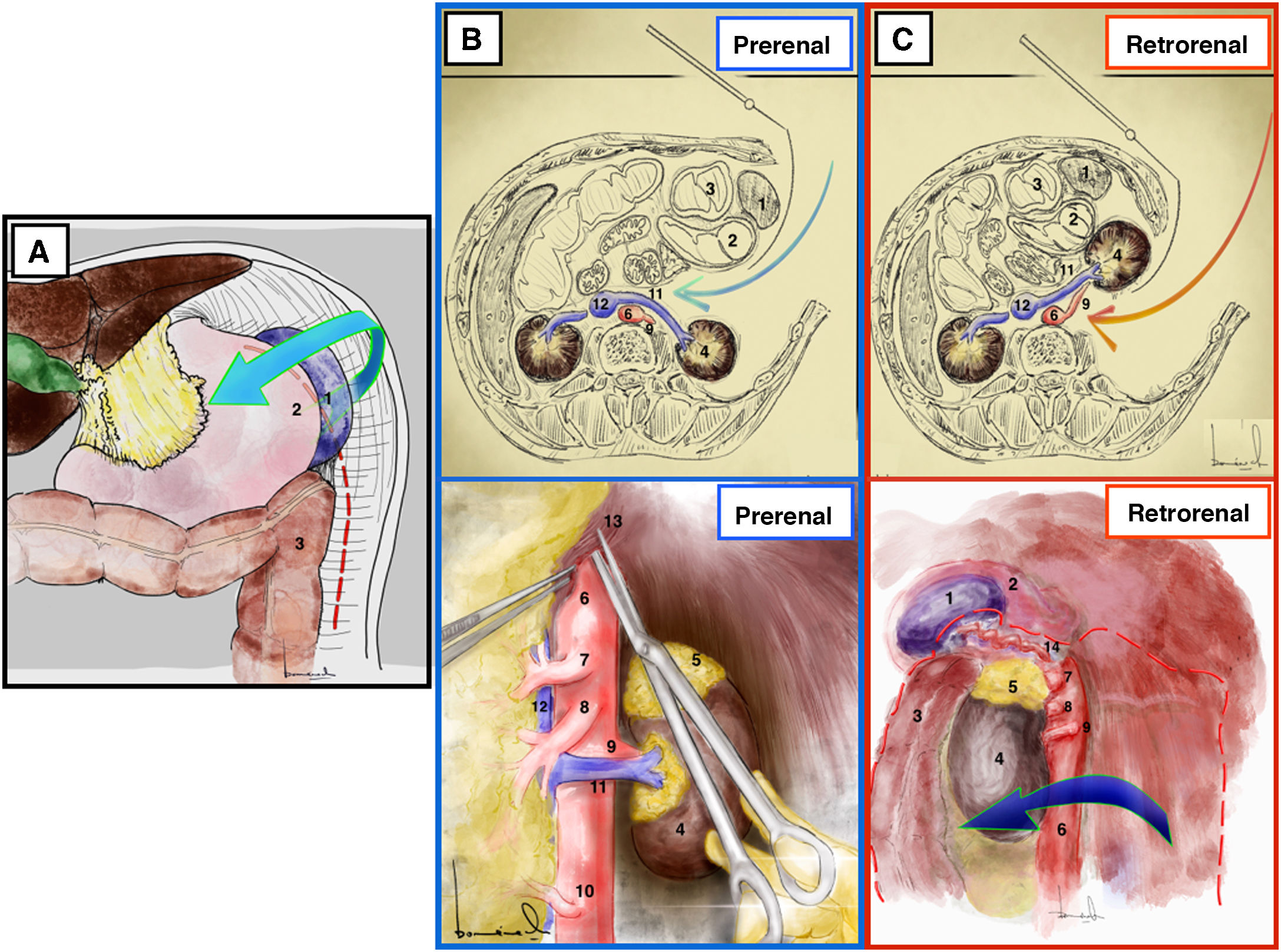
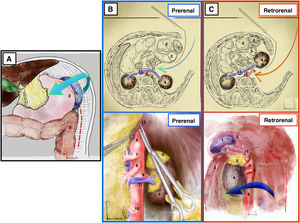
Mattox maneuver17,18 (Appendix A, Video 6)Surgical techniqueThe maneuver begins with the division of the left parietocolic ligament from the parietal sigmoid attachment to the phrenicocolic ligament, followed by en bloc mobilization of the splenic flexure of the colon together with the omental bursa and spleen after dividing its attachments to the diaphragm (Fig. 6A).
Mattox maneuver. (A) It begins with left decollation and splenic release from its adhesions to the parietal peritoneum. (B) Prerenal Mattox. (C) Retrorenal Mattox. 1: spleen; 2: stomach; 3: spleen; 4: left kidney; 5: left adrenal gland; 6: aorta; 7: celiac trunk; 8: superior mesenteric artery; 9: left renal artery; 10: inferior mesenteric artery; 11: left renal vein; 12: vena cava; 13: crura of the diaphragm; 14: splenic artery.
The release of the splenic adhesions together with the mobilization of the spleen is the most delicate step in this approach due to the risk of rupture of the splenic capsule, sometimes requiring emergency splenectomy (up to 20%).17,18
There are 2 possible approaches:
Prerenal or modified Mattox17 (Fig. 6B)Surgical techniqueThe kidney, adrenal gland, and left ureter remain at the back of the dissection. The LRV drains into the cava over the anterior side of the aorta.
Indications/advantagesIt provides exposure of a greater segment of the superior mesenteric artery, the left kidney and its entire vascular pedicle.
Practical aspectsThe left diaphragmatic pillar interferes with access to the aorta at the point where it exits the diaphragm; therefore, it can be divided or simply disconnected from the aorta after dividing the arcuate ligament.
DisadvantagesDuring the prerenal Mattox maneuver, care must be taken when separating the kidney and adrenal gland from the pancreas to avoid damaging any of these three organs. There is a greater risk of pancreatitis in the prerenal approach than in the retrorenal approach (up to 5%).17,18
Mattox or retrorenal17(Fig. 6C)Surgical techniqueThe kidney is mobilized by detaching its posterior side from the lumbar fossa and retracted forward and to the right together with the rest of the viscera. Thus, the spleen, tail of the pancreas, stomach, left colon and left kidney are medialized to the right.
Indications/advantagesThe retrorenal approach provides better access to the posterolateral side of the aorta and the left renal artery. It is used primarily to access the adrenal aorta and allows for an approach of the entire abdominal aorta.
Practical aspectsA key point during the retrorenal Mattox maneuver is the possible traction bleeding of the renolumbar veins. Therefore, if a renolumbar vein is identified, it should be ligated and divided to avoid bleeding.
DisadvantagesThe dissection is extensive, and in heparinized patients it may mean the loss of large amounts of blood intraoperatively.
Knowledge of the anatomical and surgical approaches of the aorta and its visceral branches is mandatory for both general and digestive system surgeons as well as vascular surgeons.
Through this dissection in different cadavers, the key anatomical points have been shown in order to perform these approaches correctly and thereby avoid unnecessary intraoperative morbidity in real patients. The experience contributed by different specialists has provided this document with added value.
Cadaveric training in gastrointestinal and vascular surgical anatomy could help develop the surgical skills of vascular and gastrointestinal surgeons-in-training.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Fletcher-Sanfeliu D, García-Granero A, Doménech Dolz A, Pellino G, Orbis F, Arroyo A, et al. Anatomía quirúrgica aplicada a abordajes transperitoneales de la aorta abdominal y los troncos viscerales. Artículo dinámico. Cir Esp. 2021;99:562–571.