The presence of liver metastases in sarcomatous tumors is associated with poor prognosis. However, in selected patients, surgical resection has been suggested as a tool to improve survival rates. The aim of our study is to describe postoperative and oncological outcomes after liver resection.
MethodsA retrospective unicentric study was conducted including patients diagnosed with hepatic metastases from soft tissue sarcoma who underwent hepatic resection between 2003−2019. The inclusion criteria were the presence of resectable disease, including synchronic and metachronic lesions. The presence of extra-hepatic controlled disease was not considered unresectable.
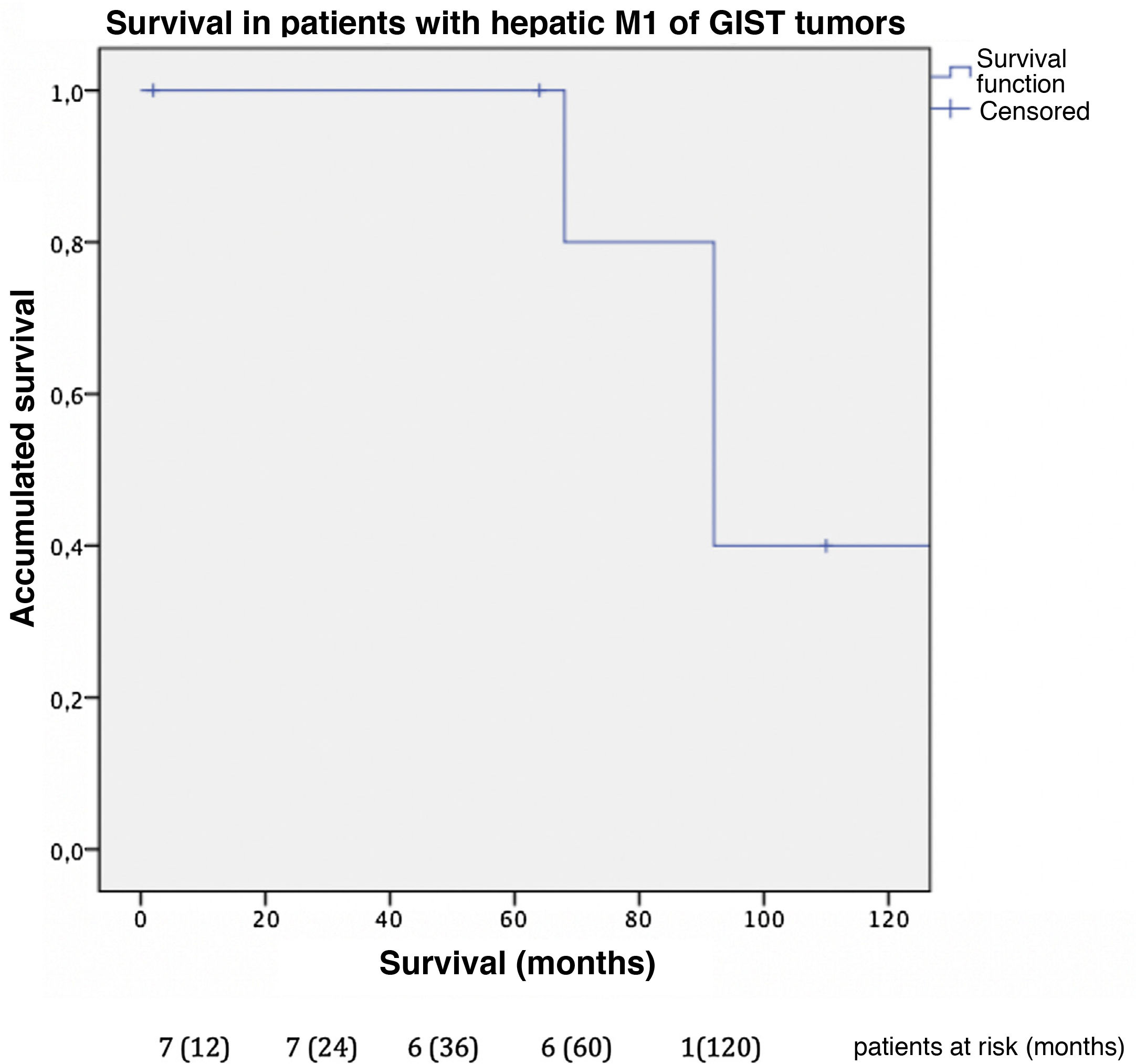
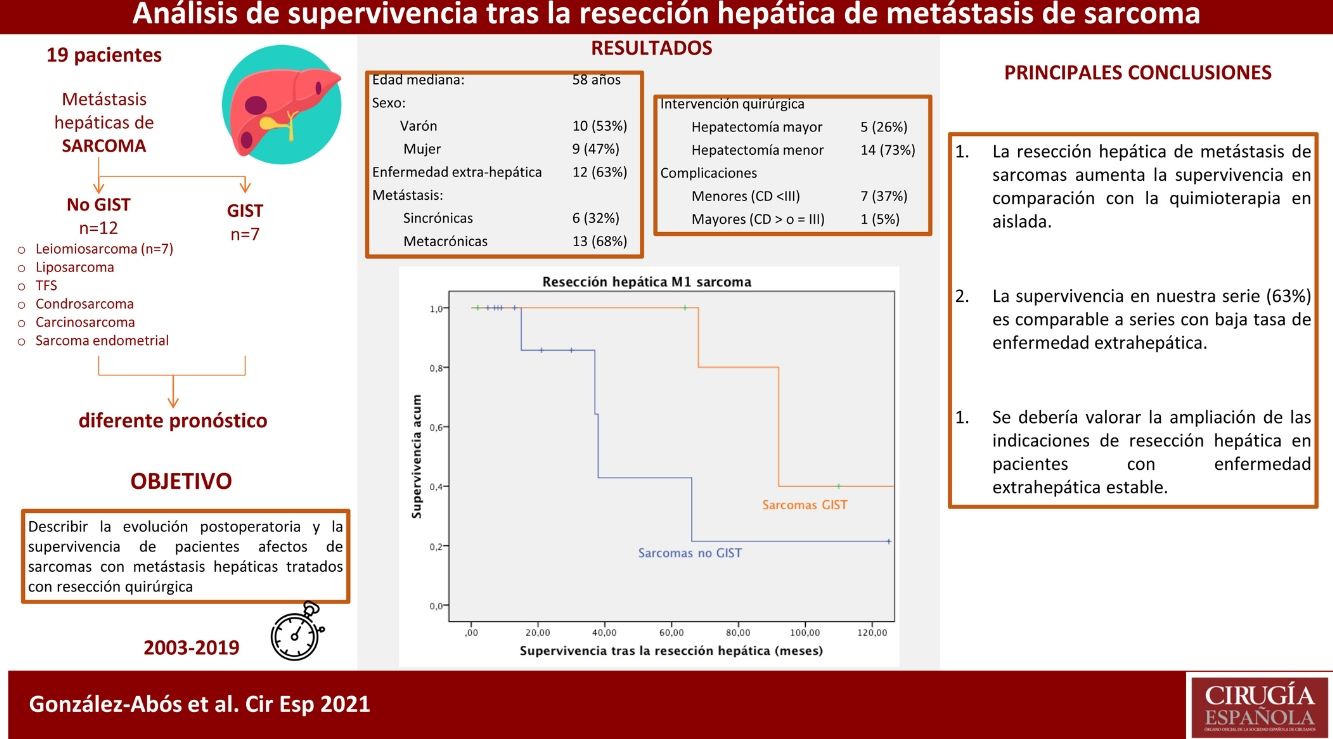
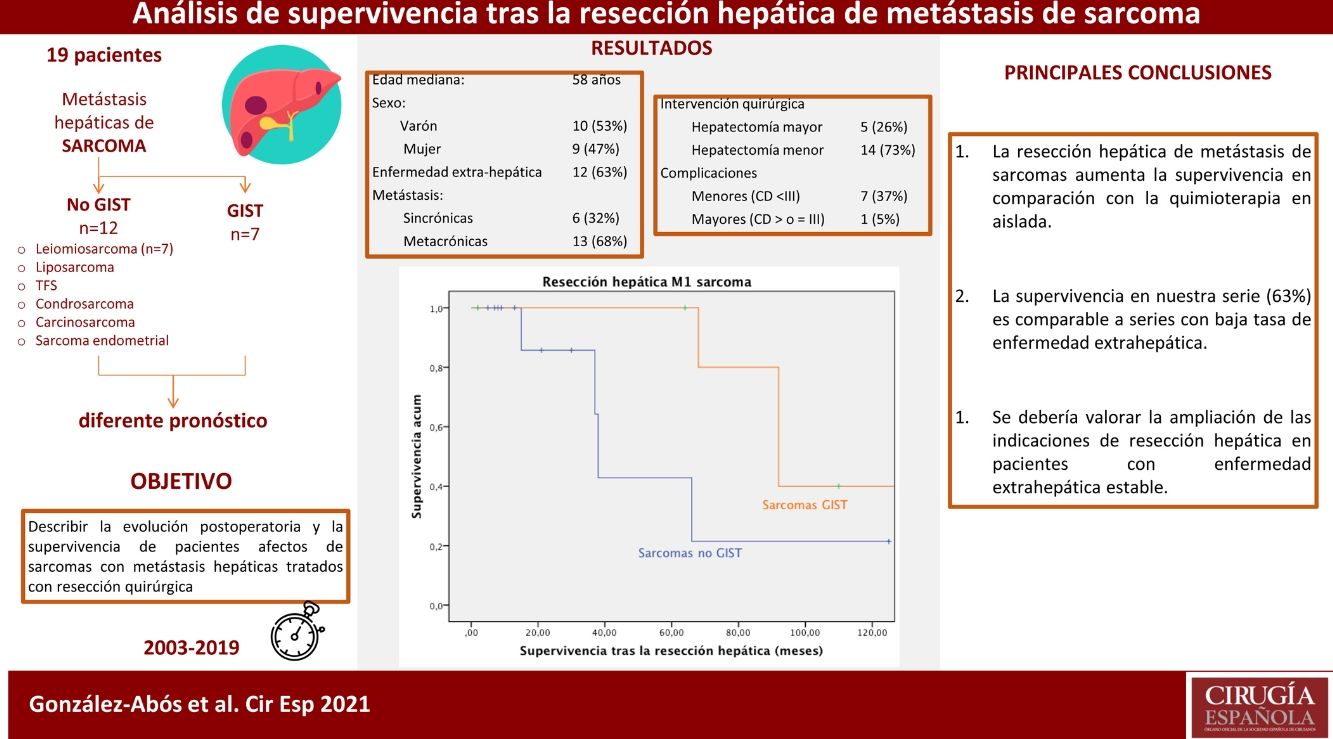
ResultsNineteen patients underwent liver resection for liver metastasis of 7 different sarcomatous subtypes. Median age was 58-years. Liver metastases were diagnosed a median 25 months after primary tumor diagnosis. Six patients (32%) suffered of synchronic metastases and 12 (63%) were affected of extrahepatic disease. Major hepatectomy was done in 5 (26 %) patients, 8 (42%) minor complications were described. Median follow-up was 33 months. Survival analysis was performed independently for, GIST tumors and non-GIST sarcomas. One, three and five-year survival rate was 100%, 85.7% and 42.9% in non-GIST sarcomas, while Five and ten-year survival rate was 100% and 40% in GIST, respectively.
ConclusionSurgical approach of liver metastases of sarcomatous tumors seems to be useful in order to improve survival in selected patients, while been associated to low complications rate. In our cohort, extrahepatic disease rate is high in comparison with series published before, nevertheless survival is comparable. These results support performing surgical resection in selected patients with stable extrahepatic disease.
La presencia de metástasis hepáticas en pacientes con sarcomas se asocia a peor pronóstico, aunque en casos seleccionados la resección de dichas metástasis se ha propuesto para aumentar la supervivencia. El objetivo de este estudio es describir la evolución postoperatoria y los resultados oncológicos tras la resección hepática.
MétodosSe presenta un estudio retrospectivo unicéntrico, se incluyen pacientes diagnosticados de metástasis hepáticas de sarcoma intervenidos quirúrgicamente entre el 2003−2019. Los criterios de inclusión fueron la presencia de enfermedad resecable, la presencia de enfermedad extra-hepática controlada no se consideró criterio de irresecabilidad.
ResultadosDiecinueve pacientes se sometieron a resección hepática de 7 tipos distintos de estirpes sarcomatosas. La mediana edad fue de 58 años. Las metástasis se diagnosticaron 25 meses de media tras el primario, 6 (32%) presentaron lesiones sincrónicas y 12 (63%) estaban afectos de enfermedad extra-hepática. Se realizó hepatectomía mayor en 5 (26%) pacientes; se describieron 8 (42%) complicaciones menores. La mediana de seguimiento fue de 33 meses. El análisis de supervivencia se realizó estratificando en dos grupos, la supervivencia fue del 100%, 85,7% y del 42,9% al año, a los 3 años y a los 5 años, en los no GIST y del 100% y del 40% a los 5 y 10 años en los GIST.
ConclusiónEl abordaje quirúrgico de las metástasis hepáticas de sarcoma parece aumentar la supervivencia en pacientes seleccionados asociando pocas complicaciones. En nuestra serie, la tasa de enfermedad extrahepática es elevada en comparación con series previas, no obstante la supervivencia es equiparable. Dichos resultados apoyan la resección hepática en pacientes con enfermedad extrahepática estable.
Sarcomas are malignant tumors originating in the mesenchymal tissue, with a histological variability that includes more than 80 different pathological subtypes1. They affect 1% of patients diagnosed with solid neoplasms and represent some 2% of mortality associated with neoplasms1. The prognosis of sarcomas is variable depending on clinical factors, histological type and grade, and especially the presence of metastases. The appearance of liver metastases is an unfavorable prognostic factor2; its low incidence and clinical heterogeneity make it difficult to determine the optimal treatment or to identify prognostic factors that would estimate survival. From a clinical-pathological standpoint, 2 entities can be differentiated, with different evolutions and treatments: gastrointestinal stromal tumors (GIST), and sarcomas themselves, with a wide spectrum of subtypes and locations.
Most non-GIST sarcomas originate in the extremities (60%), followed by the retroperitoneum (15%–20%). About half are leiomyosarcomas and liposarcomas. Up to 16% of patients with retroperitoneal sarcoma and 62% of patients with visceral sarcomas develop liver metastases.
In general, advanced GIST are tumors with little sensitivity to conventional chemotherapy but usually respond to tyrosine-kinase inhibitors (TKI), providing a very significant improvement in survival. In metastatic non-GIST sarcomas, treatment with chemotherapy (based on anthracyclines) is standard, and overall survival is around 18 months3.
Surgery for metastatic disease is an option in selected cases, with proven benefits in the resection of lung metastases2. In recent years, surgical resection has been proposed for sarcoma metastases of the liver4; however, unlike hepatic lesions of colorectal origin, the role of surgery is not yet well defined5–7.
The main objective of this study is to describe the postoperative progress and survival of patients with sarcomas and liver metastases treated with surgical resection.
MethodsWe conducted a single-center retrospective study analyzing patients diagnosed from 2003 to 2019 with sarcomas and liver metastases who underwent surgical resection with curative intent performed by the Sarcoma Unit (CSUR) at our hospital, a national tertiary referral center and university hospital. The study included patients with synchronous and metachronous metastases, with resectable liver disease. Synchronous metastases were defined as those diagnosed at the time of diagnosis of the primary tumor.
The indication for surgical resection was decided by a multidisciplinary committee in accordance with the hospital protocol and based on patient characteristics, radiological findings, and response to previous treatment. In patients with controlled extrahepatic disease, the presence of extrahepatic disease was not a criterion for unresectability. Controlled disease was defined as that which remained stable after completing neoadjuvant therapy or presented a partial or complete response in the follow-up imaging test. Cases were evaluated using the RECIST criteria. Patients with disease progression after chemotherapy were excluded from surgical treatment.
Preoperative targeted treatment with TKI was administered to patients with GIST tumors. Patients with non-GIST sarcomas received preoperative chemotherapy depending on the histologic type and disease extension.
The radiological response to preoperative chemotherapy treatment was evaluated according to RECIST criteria8,9, which establishes 3 groups; a first group consisting of patients with partial response to treatment, showing a reduction in radiological disease equal to or greater than 30%; a second group classified as stable disease, including patients without partial response or progression; and a third group consisting of patients with radiological progression of more than 20%, despite treatment. The patients included in our study were part of the first and second groups.
The surgical technique was determined by the multidisciplinary committee based on each individual patient. Anatomical resection >3 segments was considered major resection10. Radicality was defined according to the pathological standards of the International Union Against Cancer: R0 (complete microscopic resection); R1 (residual microscopic disease); or R2 (residual macroscopic disease)9,11.
The surgical specimens were sent to the Pathology Department, cut into 0.5 cm-thick slices and fixed in formalin. Representative samples of tumor tissue, non-tumor tissue, and surgical margins were embedded in paraffin for microscopic analysis. A positive resection margin was defined as the presence of tumor cells <1 mm from the transection line. The number of tumors and their sizes were confirmed by macroscopic analysis. Vascular invasion, degree of tumor differentiation, status of the resection margins, and the presence of additional nodules were analyzed under the microscope using hematoxylin-eosin stain. The AJCC Cancer Staging system was used for tumor staging9,11.
Complications were defined according to the Clavien-Dindo (CD) classification, grouping the CDI-CDII types as minor complications and the CDIII-CDV types as major complications12. The complications of each patient were recorded; when there was more than one, the severest was used for the CD classification. Postoperative mortality was defined as death in the first 90 days after surgery.
A uniform follow-up protocol was carried out after surgery, which included an abdominal CT scan every 3 months for the first 2 years, and every 6 months after the third year.
Progression-free time was defined as the time between the first resection of liver lesions and the first radiological image showing either progression of persistent disease or local/distant recurrence. Persistent disease was defined as the presence of stable neoplastic disease not resected due to technical impossibility or lack of indication. Recurrence was defined as the appearance of new hepatic or distant lesions compatible with metastases of sarcomatous origin or the presence of local recurrence in imaging tests. Progression was defined as the appearance of recurrence in patients without residual disease and the appearance of new lesions or the growth of stable lesions in patients with residual disease. Radiologically compatible lesions with metastases did not require biopsy.
Overall survival was defined as the time between the first liver resection and death, regardless of the cause of death. The 2 patient cohorts of GIST and non-GIST sarcoma were analyzed independently.
Statistical analysisThe results are presented as percentage for the discrete variables and as median and range for the quantitative variables with non-normal distribution, given the small sample size.
The survival and progression-free time analysis was performed using Kaplan Meier survival curves. The analysis was divided according to the 2 subgroups, separating GIST from non-GIST sarcomatous tumors. Data analysis was performed using the Statistical Package for the Social Sciences version 21.0 for MacOS (SPSS, Inc, Chicago, IL, USA).
ResultsNineteen patients underwent surgical resection for liver metastases from sarcoma (2003–2019). Their characteristics are summarized in Table 1. Ten (53%) men and 9 (47%) women were included in the study; median age was 58 years (range: 38–80).
Patients and tumor characteristics.
| Characteristics | Value depending the cell strain (n = 19) | |
|---|---|---|
| Non-GIST sarcomas (n = 12) | GIST sarcomas (n = 7) | |
| Age, median (range), years | 60 (49−68) | 44 (40−71) |
| Male sex, n (%) | 4 (33) | 6 (86) |
| Preoperative ASA | ||
| II | 0 | 0 |
| II | 6 | 2 |
| III | 6 | 3 |
| IV | 0 | 2 |
| Number of liver lesions, n (range) | 1 (1−2) | 4 (2−8) |
| Primary tumor location | ||
| Abdominal, n (%) | 9 (75) | 7 (100) |
| Retroperitoneal | 3 (25) | 0 |
| Intraabdominal | 6 | 7 (100) |
| Small intestine | 1 | 5 |
| Colon | 1 | 2 |
| Stomach | 1 | 0 |
| Ovarian and endometrial | 3 | 0 |
| Skeletomuscular | 3 (25) | 0 |
ASA: American Society of Anesthesiologists preoperative classification; GIST: gastrointestinal stroma tumor.
A pathological review was performed, shown in Table 1. Sixteen (84%) of the primary tumors were located in the abdomen, 3 (16%) were retroperitoneal in origin, and 3 (16%) had extra-abdominal origin (Table 1).
After histopathological analysis, the patients were stratified into 2 groups. The non-GIST sarcoma group included 12 patients, and the GIST groups included 7.
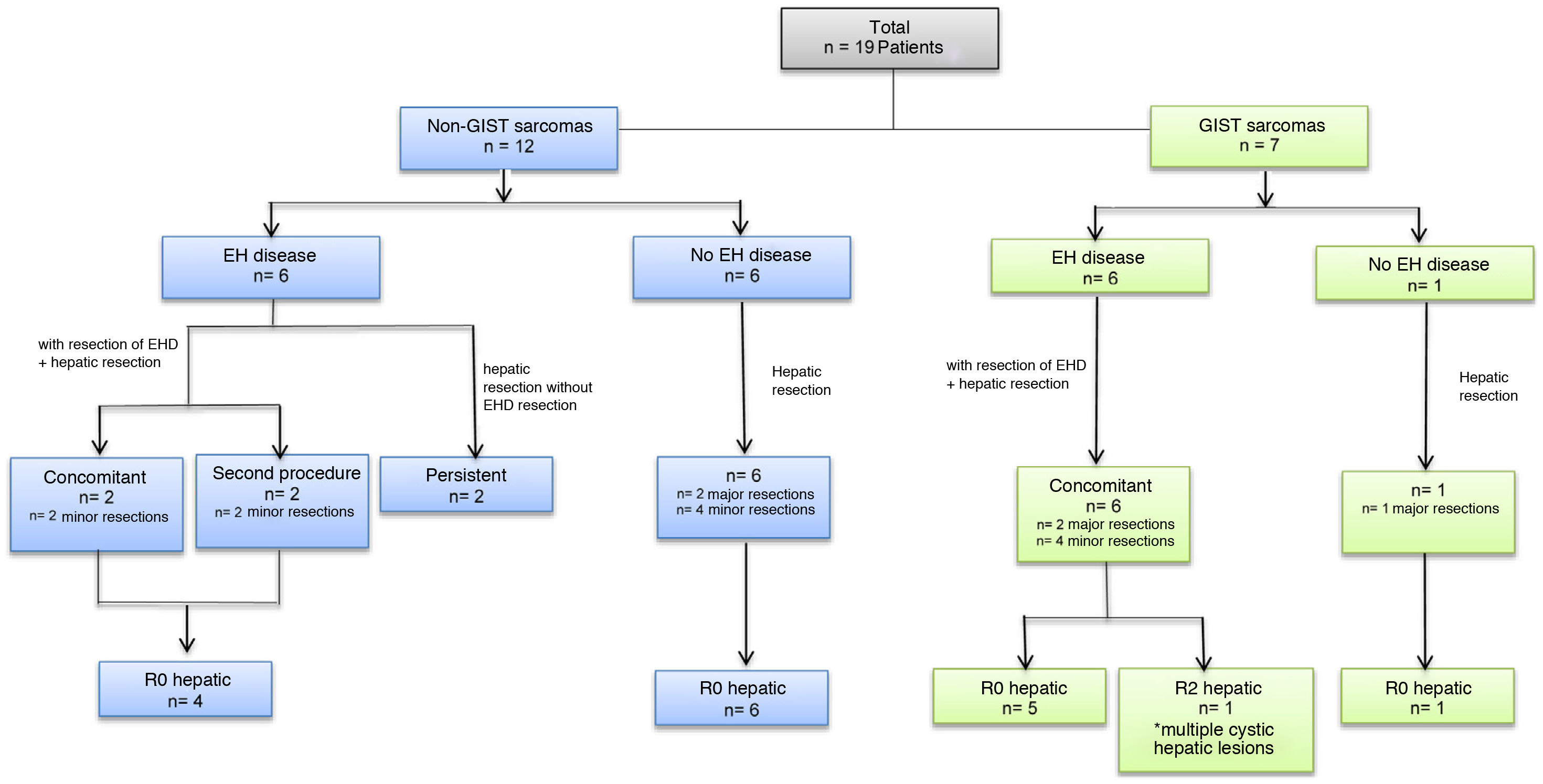
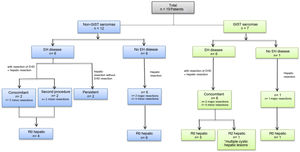
All metastatic lesions were diagnosed preoperatively by high-resolution abdominal CT and/or MRI. A PET/CT study was completed in all patients. The median time interval until diagnosis of liver metastases was 27 months (range: 12–186 months) after the diagnosis of the primary tumor in non-GIST tumors and 39 months (range: 22–58 months) in GIST tumors (Table 2). Six (32%) patients presented synchronous metastases: 4 GIST and 2 non-GIST. Twelve patients (63%) had extrahepatic disease at the time of liver disease diagnosis, 6 of which (31.5% of the total) had liver metastases from non-GIST sarcomas and 6 (31.5%) from GIST (Fig. 1, Table 2). In 10 patients (52.6%) the extrahepatic disease was resected; this was done concomitantly during the same operation in 8 patients, and in a second procedure in 2 patients (Fig. 1, Table 3). The former underwent lung resection after recovery from liver surgery, and the latter underwent salvage surgery for a single brain metastasis after adjuvant chemotherapy and radiotherapy. The 2 patients who did not undergo resection of extrahepatic disease presented millimetric lesions that were controlled with chemotherapy; one presented pulmonary disease, and the other parailiac lymph node disease (Table 3).
Perioperative characteristics.
| Characteristics | Value depending on cell strain (n = 19) | |
|---|---|---|
| Non-GIST sarcomas (n = 12) | GIST sarcomas (n = 7) | |
| Hepatic metastases, n (%) | ||
| Synchronous metastases | 2 (20) | 4 (57) |
| Metachronous metastases | 10 (80) | 3 (43) |
| Median time until appearance of first hepatic lesiona(range), months | 27 (5−27) | 27 (25−39) |
| Extrahepatic disease at the time of diagnosis of the HM, n (%) | 6 (50) | 6 (86) |
| Neoadjuvant treatment, n (%) | 4 (33) | 6 (86) |
| Hepatic resection, n (%) | ||
| Major hepatectomy | 2 (17) | 3 (43) |
| Minor hepatectomy | 10 (83) | 4 (57) |
| Liver resection margins, n (%) | ||
| R0 | 12 (100) | 6 (86) |
| R1 | 0 | 0 |
| R2 | 0 | 1 (14) |
| Complications, n (%) | 4 (33) | 4 (57) |
| Clavien-Dindo I | 1 | 0 |
| Clavien-Dindo II | 2 | 4 |
| Clavien-Dindo III | 1 | 0 |
| Clavien-Dindo IV-V | 0 | 0 |
| Complications, n (%) | 4 (33) | 4 (57) |
| Adynamic ileus | 2 | 3 |
| Wound infections (infected seroma) | 1 | 1 |
| Infected bilomab | 1 | 0 |
GIST: gastrointestinal stromal tumor; HM: hepatic metastases.
Correlation between metastasis and extrahepatic disease.
| Characteristics | Value depending on cell strain (n = 19) | |
|---|---|---|
| non-GIST sarcomas (n = 12) | Sarcomas GIST (n = 7) | |
| Extrahepatic disease at the time of diagnosis of HM, n (%) | 6 (50) | 6 (86) |
| EHD constituted by the primary tumor (synchronous) | 2 (17) | 4 (57) |
| EHD constituted by distant dissemination | 4 (33) | 2 (43) |
| Treatment of extrahepatic disease | ||
| Resection EHD concomitant to HM, n (%) | 2 (17) | 6 (100) |
| Endometrium and ovaries | 1 | 0 |
| Colon, psoas, tail of the pancreas, spleen and kidneys | 1 | 0 |
| Small intestine | 0 | 3 |
| Colon | 0 | 1 |
| Lymphadenopathic and peritoneal disease | 0 | 2 |
| Resection of EHD in second procedure, n (%) | 2 (17) | 0 |
| Single brain metastasis | 1 | 0 |
| Lung metastasis | 1 | 0 |
| EHD not resected (persistent EHD), n (%) | 2 (17) | 0 |
| Multiple millimetric lung lesions | 1 | 0 |
| Parailiac lymphadenopathies | 1 | 0 |
EHD: extrahepatic disease; GIST: gastrointestinal stroma tumor; HM: hepatic metastases.
Six (32%) patients presented bilobar liver disease, and the presentation was synchronous in half. In the cases of single-lobe involvement, the distribution was similar between the lesions that affected the right and left lobes: 8 (40%) and 5 (26%), respectively. The median number of liver nodules present at diagnosis was 1.5 nodules (range 1–10 nodules), with an average diameter of the largest nodule of 43 mm (range 6−100 mm) (Table 1).
Neoadjuvant therapy was administered in 10 (53%) patients, consisting of treatment with chemotherapy or targeted therapy (TKI). Six were GIST tumors, and 4 were non-GIST sarcomas. Response to neoadjuvant treatment was analyzed according to RECIST 1.1 criteria. Six (60%) presented stable disease, and 4 (40%) partial radiological response. Out of the 9 patients who did not receive neoadjuvant therapy, 8 presented sarcomas for which the multidisciplinary committee decided not to administer chemotherapy (due to comorbidity or low chemosensitivity), and the ninth was a patient with a GIST tumor for whom we do not have preoperative data regarding neoadjuvant therapy (Table 2).
After surgery, all GIST tumors received adjuvant treatment with TKI, and 6 of the non-GIST tumors received adjuvant treatment with multiple lines of chemotherapy and monoclonal antibodies based on their chemosensitivity.
Major hepatectomy was performed in 5 patients (26%), one of whom had previously undergone right portal vein embolization. Fourteen (74%) patients underwent minor resections. The laparoscopic approach was used in 5 patients (26%), and one required conversion to open surgery. Liver resection was performed using an ultrasonic dissector (Sonoca®) and a bipolar sealer (Aquamantys®). Simultaneous resection of extrahepatic disease was performed in 8 patients (42.1%) (Table 3 and Fig. 1). In 18 (95%) patients, negative resection margins (R0) were obtained in the liver resection; the last patient was an R2 liver resection in a patient with a GIST tumor with lymphadenopathy, peritoneal and cystic liver involvement, the latter of which diagnosed intraoperatively. Given that this patient had correct response to neoadjuvant therapy with TKI, we decided to perform cytoreductive surgery intraoperatively, partially resecting the liver disease.
Regarding the 8 patients in whom the extrahepatic disease was resected in the same operation, the margins were negative (R0) in 7 patients, with positive margins in an eighth patient.
Median hospital stay was 11 days, and postoperative mortality was 0%. Seven (36.9%) patients presented a minor complication in the postoperative period, and one patient presented a major complication (iiia; described in Table 2). All the complications were surgical, such as wound infection or adynamic ileus; there were no medical complications (Table 2).
In all patients, the median follow-up was 33 months (range: 2–222 months) after liver resection. Median progression-free time was 16 months, and 6 patients (31%) had a second liver recurrence after resection of metastatic lesions.
Overall survival in our cohort was 68 months from the first liver resection.
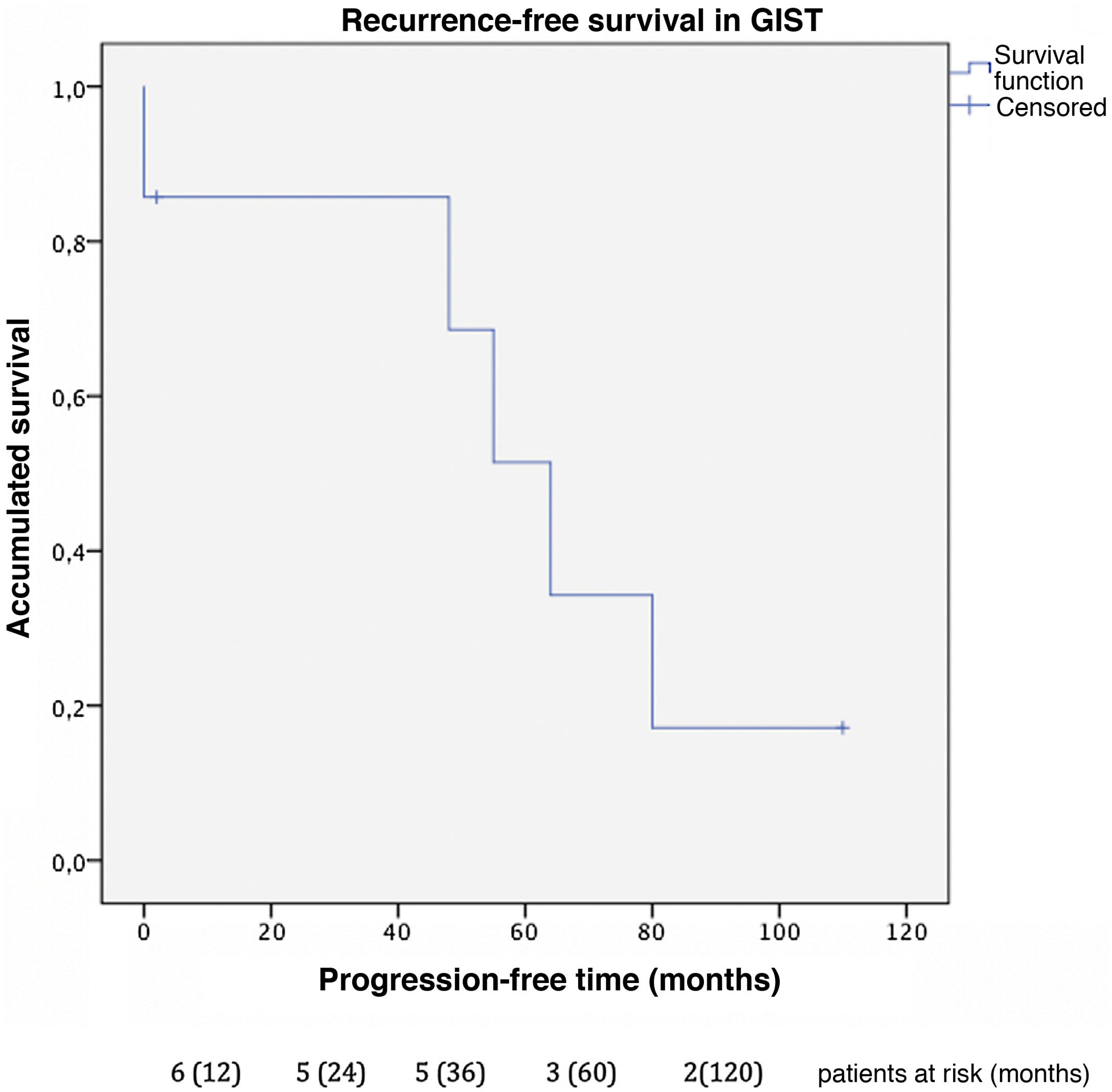
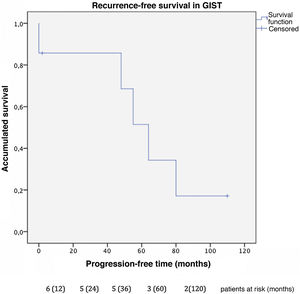
Progression-free time was 32 months in GIST (Fig. 2), with a one-year and 3-year disease-free survival of 85% and a 5-year rate of 51%. In this group, 2 patients presented new liver recurrences during follow-up and underwent surgical resection again, one of whom remained disease-free at the time of data collection.
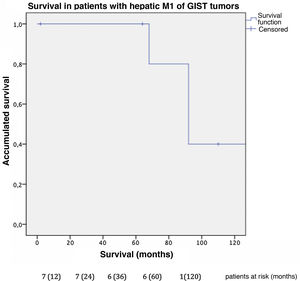
In this cohort, the overall one-, 3- and 5-year survival rate was 100% (Fig. 3); the 10-year rate was 40%. The median follow-up was 92 months, and median survival was 140 months.
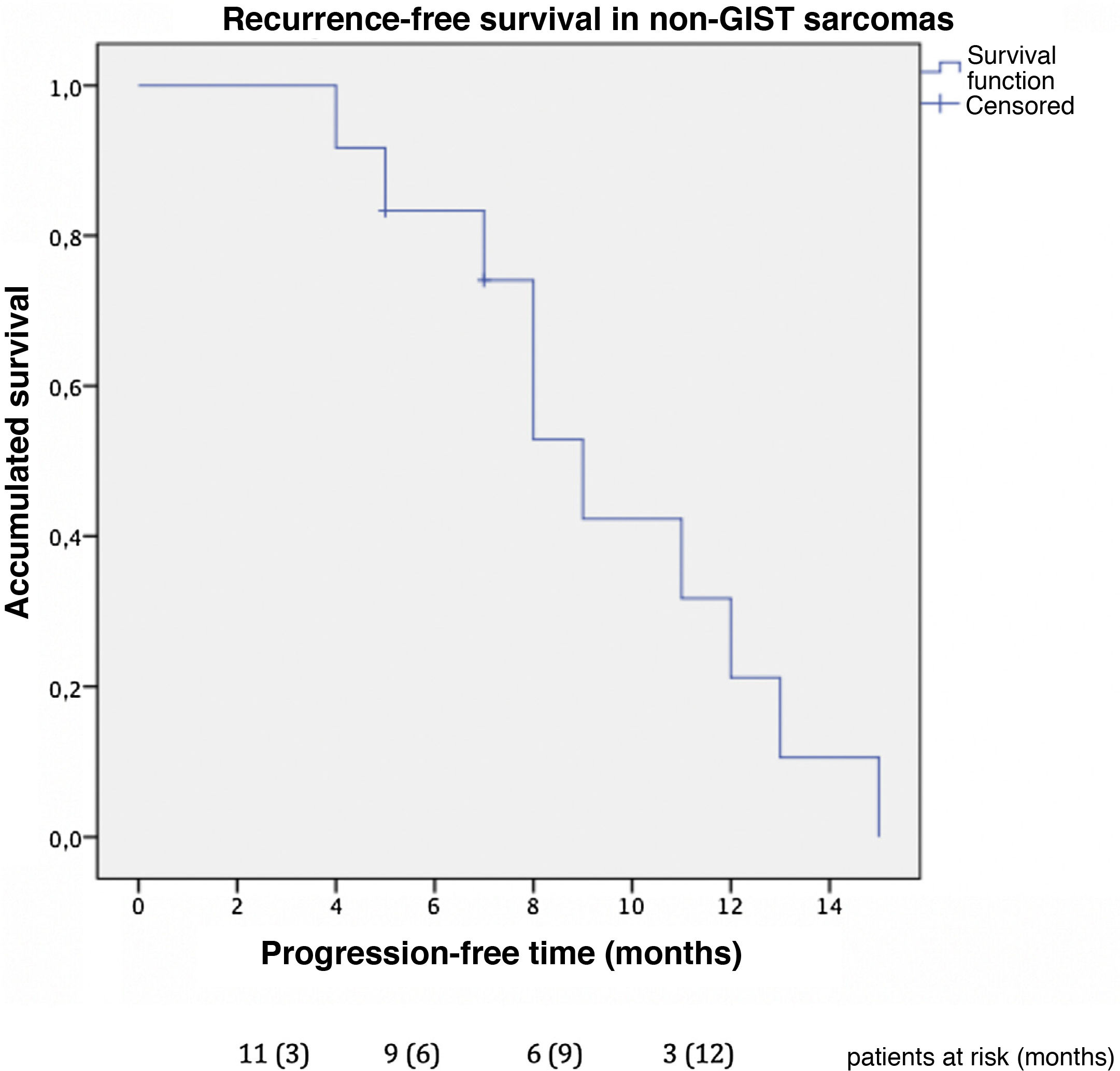
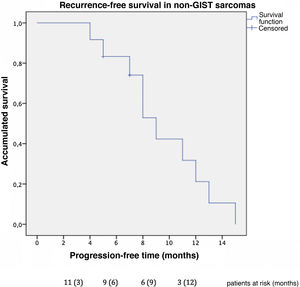
Progression-free time was 9 months in non-GIST sarcomas (Fig. 4), and the one-year progression-free survival was 31.7%. Excluding the 2 patients diagnosed with non-GIST sarcoma and persistent disease, disease-free survival reached 12 months in the subgroup of non-GIST patients. Four patients presented new liver recurrence, one of whom underwent new liver resections on 2 occasions.
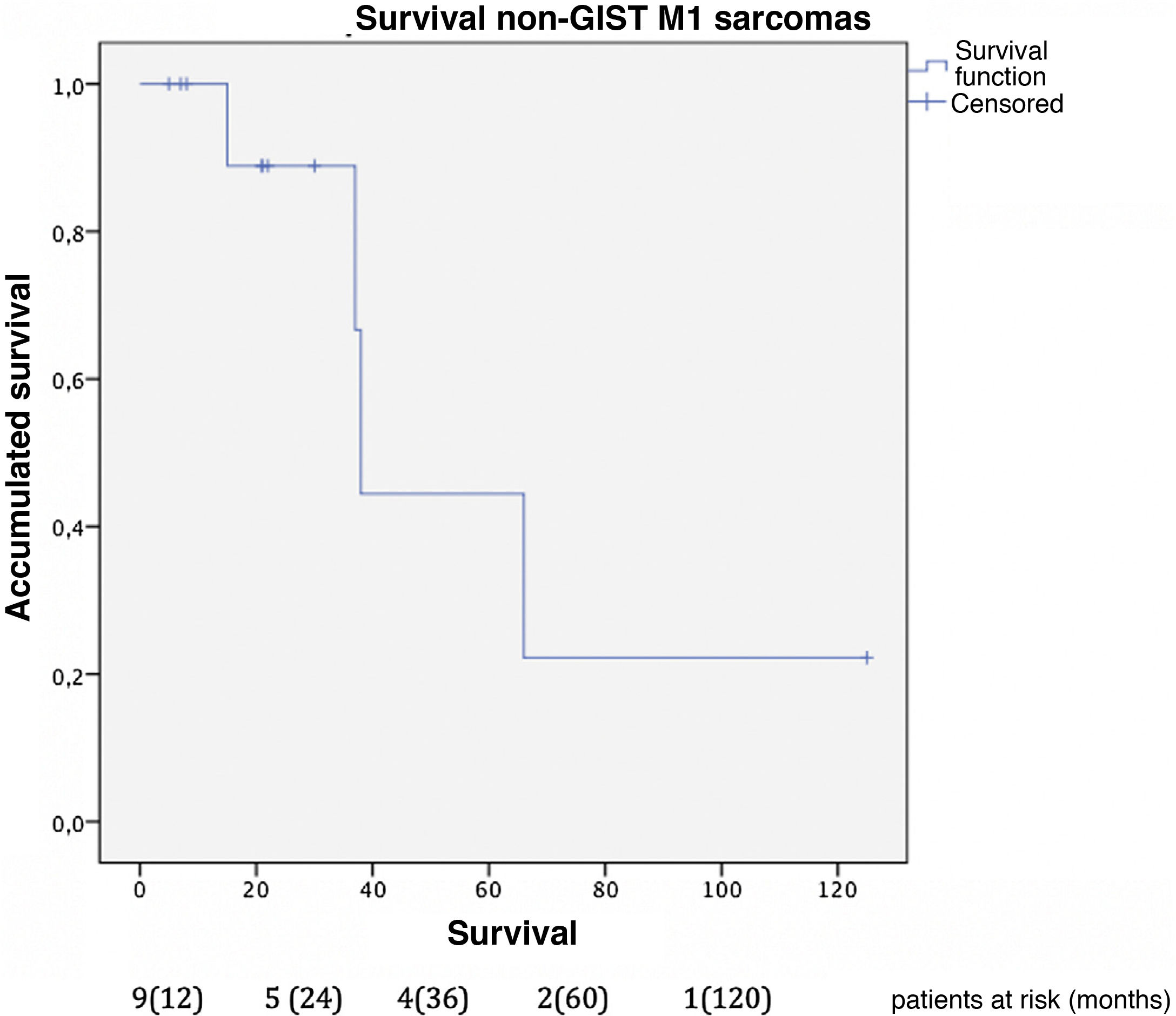
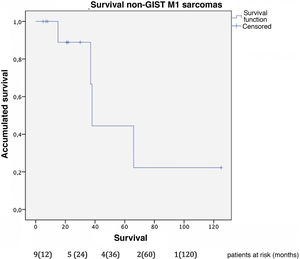
The median survival of this group was 81 months from the diagnosis of the primary disease. Overall one-, 3- and 5-year survival rates after liver resection (Fig. 5) were 100%, 85.7% and 42.9%, respectively. Median follow-up was 22 months, and median survival was 38-months after liver resection.
When we analyzed the survival of the 6 patients with non-GIST sarcomas and extrahepatic disease, the overall one- and 3-year survival was 100%, and the 5-year rate was 50%. Specifically, the 2 patients with persistent extrahepatic disease in this group were still alive 22 months after liver resection, with 100% survival after one and 2 years. We could not determine the 3- and 5-year survival rates because these patients are still being followed up.
DiscussionWithin the limitations of a retrospective observational study, the low incidence of this type of tumor, and the small sample size, this study presents the largest published Spanish series of the surgical management of liver metastases in patients with sarcomas. It is also one of the largest single-center study cohorts.
The combined analysis of different types of sarcomas can cause bias in the results due to the difference in prognosis described between GIST sarcomas and non-GIST sarcomas, so we conducted the analysis by subgroups. Our series describes a 100% survival rate in GIST with metastatic lesions 5 years after liver resection, with a progression-free survival of 32 months. These data correlate with those obtained by the Spanish Sarcoma Research Group which, after analyzing 171 patients, shows statistically significant differences after resection of liver metastases in disseminated GIST, with an overall 5-year survival rate of 70% versus 50% in those not operated on (GEIS)4,13. It is worth remembering the improved prognosis of these tumors after treatment with TKI was introduced, as they were previously associated with low survival rates due to their low chemosensitivity. Currently, 5-year survival rates reach 100% in some series.
Regarding the patients operated on for liver metastases of non-GIST sarcomas, the overall one-year, 3-year and 5-year survival rates in our study were 100%, 85.7%, and 42.9%, respectively, after resection of liver metastases, with a median follow-up of 22 months. These results are comparable to the most representative published series. Goumard et al.14 analyzed 126 patients who underwent hepatic resection for lesions of sarcomatous origin, reporting a 5-year survival rate of 49% and a median follow-up longer than our cohort: 38 months after liver resection. Similar results have been published by Grimme et al.15, with one-year, 3-year and 5-year survival rates of 88.1%, 53.9% and 41.1%, respectively; median follow-up was 18 months. DeMatteo et al.16 reported one-year, 3-year, and 5-year survival rates of 88%, 50%, and 30%, respectively, with a median follow-up of 29 months after liver resection in 56 patients.
These studies, like our cohort, include highly selected patients in whom R0 or R1 resection of the liver disease was performed, showing no improvement in survival in R2 resections15,16; these cases were comparable to survival without liver resection. Beyond complete resection (R0 or R1), it has not been possible to determine other clear prognostic factors in this type of patients17. According to the study carried out by the European Organization for Research and Treatment of Cancer (EORTC), treatment of these patients with chemotherapy alone offers overall one-year and 2-year survival rates of 42% and 13%, respectively18. Gourmard et al.14 also describe much lower survival rates in patients who cannot undergo liver surgery, with one-year, 3-year, and 5-year survival rates of 50%, 13%, and 4%, respectively. With regards to chemotherapy as adjuvant treatment after surgery, given the great variety of histological subtypes, we have not been able to draw conclusions, and assessments would be necessary in future studies.
Regarding progression-free survival, our cohort presented earlier recurrences, with progression-free survival of 9 months in patients with persistent extrahepatic disease that was not completely resected. The subgroup of patients without residual disease after surgery presented a disease-free survival of 12 months, which is comparable to the 12 months presented by Goumard et al. and 16 months by the Grimme et al. group.
The higher proportion of synchronous lesions (up to 31%) and extrahepatic disease when the liver lesions were diagnosed (63% in our global cohort and 50% in non-GIST sarcomas, vs 20%–30% in other series) may justify earlier recurrence, without affecting overall survival. As previously stated, survival in patients with non-GIST sarcomas and extrahepatic disease is 100% after one and 3 years, and 50% after 5 years. Previous studies have established that the presence of extrahepatic disease is a poor prognostic factor, probably resulting in stricter selection of patients undergoing surgical interventions with curative intent1,18.
The results of our study reaffirm previously published data. In this series of selected patients, the resection of liver metastases in patients with stable liver disease of sarcomatous origin improves survival compared to management exclusively with chemotherapy. The low associated rates of postoperative complications are comparable to reports in the literature regarding hepatic resection of colorectal metastases19. Although it is true that the follow-up of patients with metastatic non-GIST sarcomas in our series is relatively short (median 22 months), it is comparable to the follow-up period of previous series. However, we must consider that the follow-up from the initial diagnosis of the disease is long, reaching a median of 53 months.
Unlike previous series, our cohort presented a high rate of extrahepatic disease when the liver progression was diagnosed, maintaining survival rates comparable to patients without extrahepatic disease and longer survival than patients with liver metastases who did not have surgical resection. These data could support extending the indications for liver resection in patients with stable extrahepatic disease and neoadjuvant therapy, increasing the survival of this group of selected patients, albeit with a lower progression-free survival. However, our study is limited by the low incidence of these tumors and the small sample size, reaffirming the need for multicenter studies with a larger population to confirm this hypothesis and establish homogeneous criteria for surgical resections that benefit survival.
Conflict of interestsThe authors have no conflict of interests to declare.