In last 20 years, lymph node staging procedures in breast cancer have been modified. The objective of this study is to describe the evolution of these procedures at our hospital.
MethodsA prospective observational study that included women with breast cancer who were treated surgically between 2001 and 2017. Four groups were identified according to the therapeutic regimen and 3 study periods defined by the lymph node dissection.
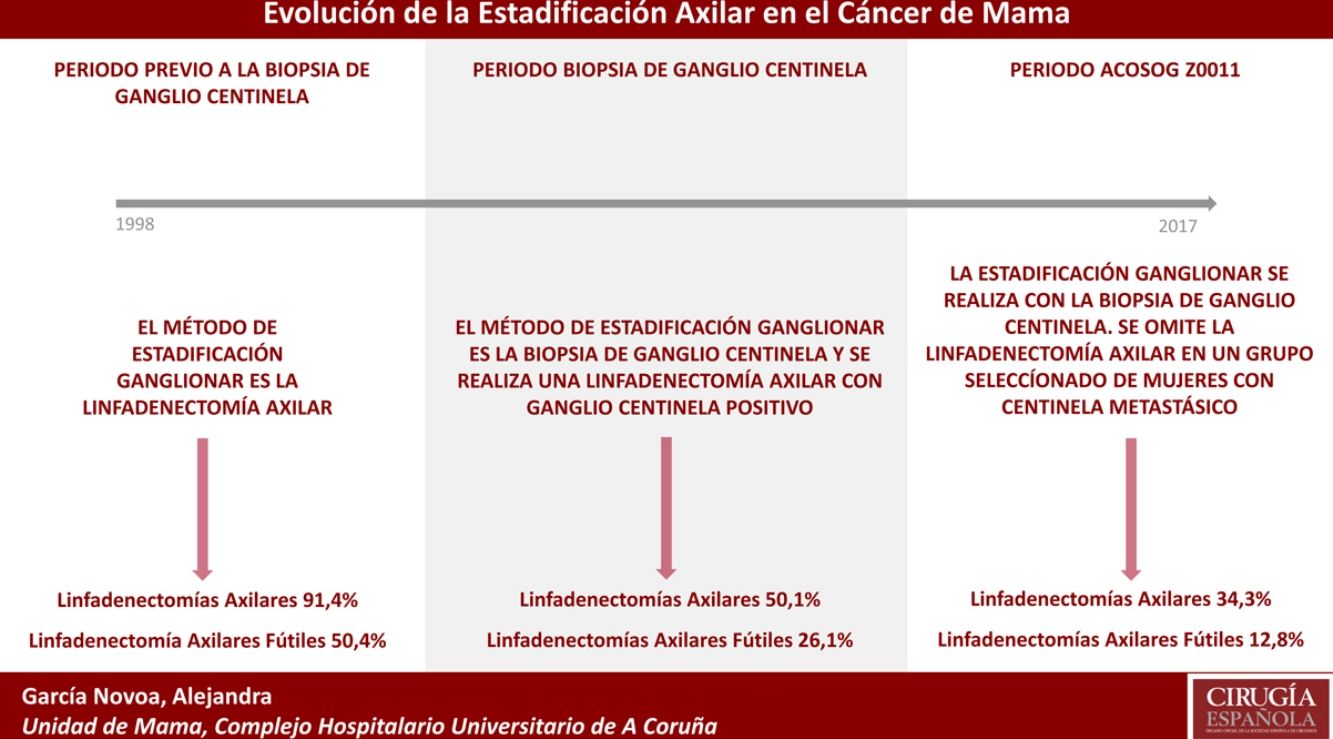
Results1319 patients met the inclusion criteria. Primary conservative surgery was the most frequent therapy (54.13%), and 615 (46.62%) axillary lymph node dissections (ALND) were performed in the 20-year study period. The percentage of ALND decreased progressively over time, going from 91% in the first period to 34% in the last period. The futile ALND fell to 6.6% in the last year. In the primary conservative surgery, no futile ALND was performed in the last two years.
ConclusionThe introduction of sentinel lymph node biopsy and the ACOSOG Z0011 criteria have modified the indication for ALND. Thus, ALND without involvement have been reduced, thereby avoiding the associated morbidity. The study demonstrates the progressive decrease in the indication of lymphadenectomy in the different study groups, similar to reports by other authors. Several clinical trials have described that these changes have not negatively impacted survival.
En los últimos 20 años los procedimientos de estadificación ganglionar en el cáncer de mama se han modificado. El objetivo de este estudio es describir la evolución de estos procedimientos en nuestro centro.
MétodosEstudio prospectivo observacional que incluye a las mujeres con cáncer de mama intervenidas entre el 2001 y el 2017. Se identificaron 4 grupos según el esquema terapéutico utilizado y 3 periodos a estudio definidos por las indicaciones de la linfadenectomía.
ResultadosMil trescientos diecinueve pacientes cumplieron los criterios de inclusión. La cirugía conservadora primaria fue el esquema terapéutico más frecuente (54,13%) y se realizaron 615 linfadenectomías axilares (46,62%) durante los 20 años estudiados. El porcentaje de linfadenectomías axilares disminuyó progresivamente en el tiempo, pasando del 91% en el primer periodo al 34% en el último periodo. Las linfadenectomías axilares fútiles descendieron al 6,6% en el último año. En la cirugía conservadora primaria no se realizó ninguna linfadenectomía axilar fútil los 2 últimos años.
ConclusiónLa introducción de la biopsia de ganglio centinela en 2001 y de los criterios ACOSOG Z0011 han modificado la indicación de la linfadenectomía axilar. Así, se han disminuido las linfadenectomías axilares sin afectación, evitando la morbilidad que asocia este procedimiento, especialmente linfedema. El estudio refleja el descenso progresivo de la indicación de la linfadenectomía en los diferentes grupos a estudio, similar a lo expuesto por otros autores. En diversos ensayos clínicos se ha descrito que estos cambios no han impactado negativamente en supervivencia.
Breast cancer is the most frequent malignant tumor in women. Its incidence seems to be increasing in developed countries, where the risk of developing breast cancer during a woman's lifetime is 12% (one in 8 women).1,2 It is the first cause of cancer death in women worldwide; however, early diagnosis and advances in treatment have reduced mortality from this cause.3
In recent decades, the treatment of breast cancer has evolved and requires a multidisciplinary perspective that provides adequate systemic control of the disease, a conservative surgical approach and a greater concern for the satisfaction and quality of life of these women. In this context, staging procedures and axillary treatment have also been modified, and the systematic indication of axillary lymph node dissection (ALND) has been replaced by an easily reproducible procedure with lower morbidity: sentinel lymph node biopsy (SLNB). Several clinical trials have analyzed the feasibility of this technique for lymph node staging and reported a sensitivity greater than 88%, a specificity of 100% and a false negative (FN) rate of less than 10%.4–8
Recently, the published results from the ACOSOG Z0011 clinical trial9,10 have demonstrated similar overall and disease-free survival in a select group of patients with metastatic involvement of the sentinel lymph node (SLN) without ALND. These findings have been corroborated by other studies11–13 and have modified routine clinical practice by omitting lymph node dissection in a large number of patients with metastatic involvement in the axilla.
The aim of this study is to describe the evolution of staging procedures and axillary treatment in the last 20 years at our hospital and the main factors that have led to these changes.
MethodsA prospective, observational study of patients with invasive breast cancer treated surgically between January 1, 1998 and December 31, 2017. Patients excluded from the study were: men, patients diagnosed with carcinoma in situ, recurrences in the same breast or with distant metastasis at diagnosis, as well as patients without axillary staging or with an unknown radiotherapy or chemotherapy regimen.
Statistical AnalysisThe evolution of surgical procedures for lymph node staging was analyzed, determining the annual percentage of ALND and futile ALND. The term ‘futile ALND’ was used for ALND in which no metastatic involvement of axillary fat was found. IBM SPSS Statistics 22.0 and Microsoft Excel version 16.0 programs were used for data collection and statistical analysis.
Four study groups were established according to the treatment regimen used: (1) primary breast-conserving surgery; (2) primary mastectomy; (3) breast-conserving surgery after neoadjuvant surgery; and (4) mastectomy after neoadjuvant treatment. Three study periods were established according to the lymph node staging method used. The ‘pre-SLNB period’, comprised from the beginning of the study until the introduction of SLNB. In primary surgery, SLNB was used starting mid-2001, and then in the primary systemic treatment groups in 2005. The ‘SLNB period’ extends from the introduction of SLNB up to the application of the ACOSOG Z0011 criteria at our hospital. As of 2012, in patients with primary systemic treatment, SLNB was performed once chemotherapy was completed. The last period (‘post-Z0011’) began with the introduction of Z0011 criteria in February 2010. The IBCSG 23-01 and modified ACOSOG Z0011 criteria (patients with breast-conserving surgery after neoadjuvant therapy) were introduced in March 2012 and April 2013, respectively.
Surgical method. The decision of the type of breast surgery was agreed upon in the multidisciplinary committee of the unit, following the criteria for oncological resection valid in each period. For the identification of the SLN, technetium 99 and/or patent blue dye were used as tracers. The SLN was defined as the node with an isotopic count 10 times higher than the baseline and/or colored blue or with a bluish lymphatic duct.
Pathological study. The ALND study was performed with inclusion in paraffin and subsequent hematoxylin–eosin staining. A single cut was made to each node, studying both sides. The SLN study was initially performed by freeze-cutting and, since January 2011, with the One-Step Nucleic Acid Amplification (OSNA) method, except for patients with CK19 negative tumors and women treated with neoadjuvant therapy.
Adjuvant therapy. The adjuvant treatment plan was in accordance with the unit protocol for the management of breast cancer patients, based on international clinical guidelines14,15 and the immunohistochemical findings in the tumor tissue. Irradiation of the lymph node chains was carried out in patients with extracapsular lymph node involvement and with more than 3 affected axillary lymph nodes. Patients with involvement of 1–3 lymph nodes were assessed individually by the radiation committee.
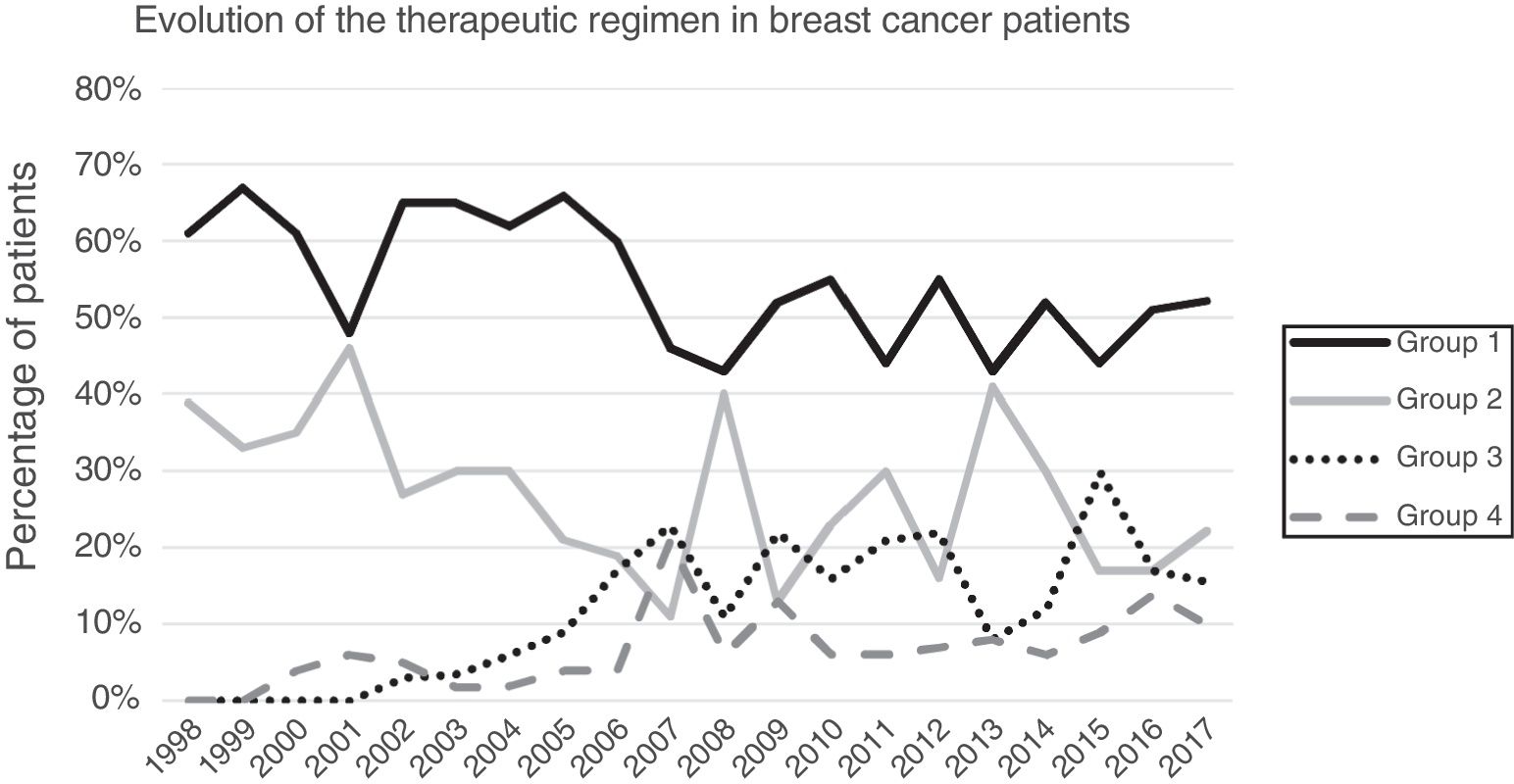
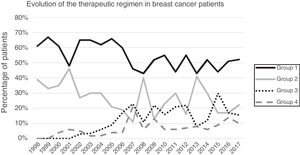
ResultsA total of 1649 patients were operated on during the study period, 1319 of which met the inclusion criteria while 313 were excluded (169 benign pathologies, 100 carcinomas in situ, 27 distant metastases at the time of diagnosis, 25 phyllodes/sarcomas and 9 men). Primary breast-conserving surgery was the most frequent therapeutic scheme (54.13%), followed by primary mastectomy (25.62%). Surgery after primary systemic treatment increased progressively over time, representing 26% of the interventions in 2017 (Fig. 1).
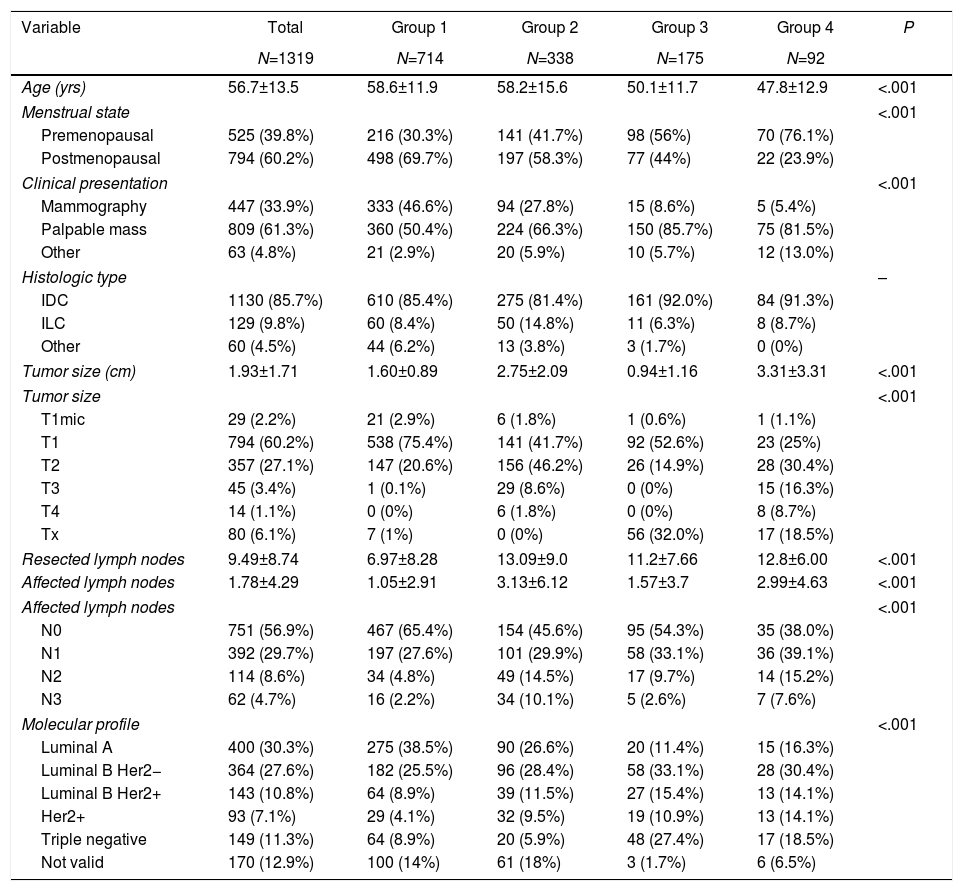
Clinicopathological characteristics. Table 1 shows the clinical and pathological characteristics of the patients. Mean patient age was 56.7 years, and the women with neoadjuvant chemotherapy were significantly younger. The most common form of presentation in all groups was a palpable mass, representing more than 80% in the groups with neoadjuvant chemotherapy. No differences were observed in the histological type according to the groups. In group 1, the most frequent molecular subtype was luminal A (38.5%), unlike the other groups with a predominance of luminal subtype B (Her2 negative). In the breast-conserving surgery groups, smaller tumor sizes were observed (P<.001), while the groups with neoadjuvant chemotherapy had a higher percentage of lymph node involvement (P<.001).
Clinical and Anatomical Pathology Characteristics.
| Variable | Total | Group 1 | Group 2 | Group 3 | Group 4 | P |
|---|---|---|---|---|---|---|
| N=1319 | N=714 | N=338 | N=175 | N=92 | ||
| Age (yrs) | 56.7±13.5 | 58.6±11.9 | 58.2±15.6 | 50.1±11.7 | 47.8±12.9 | <.001 |
| Menstrual state | <.001 | |||||
| Premenopausal | 525 (39.8%) | 216 (30.3%) | 141 (41.7%) | 98 (56%) | 70 (76.1%) | |
| Postmenopausal | 794 (60.2%) | 498 (69.7%) | 197 (58.3%) | 77 (44%) | 22 (23.9%) | |
| Clinical presentation | <.001 | |||||
| Mammography | 447 (33.9%) | 333 (46.6%) | 94 (27.8%) | 15 (8.6%) | 5 (5.4%) | |
| Palpable mass | 809 (61.3%) | 360 (50.4%) | 224 (66.3%) | 150 (85.7%) | 75 (81.5%) | |
| Other | 63 (4.8%) | 21 (2.9%) | 20 (5.9%) | 10 (5.7%) | 12 (13.0%) | |
| Histologic type | – | |||||
| IDC | 1130 (85.7%) | 610 (85.4%) | 275 (81.4%) | 161 (92.0%) | 84 (91.3%) | |
| ILC | 129 (9.8%) | 60 (8.4%) | 50 (14.8%) | 11 (6.3%) | 8 (8.7%) | |
| Other | 60 (4.5%) | 44 (6.2%) | 13 (3.8%) | 3 (1.7%) | 0 (0%) | |
| Tumor size (cm) | 1.93±1.71 | 1.60±0.89 | 2.75±2.09 | 0.94±1.16 | 3.31±3.31 | <.001 |
| Tumor size | <.001 | |||||
| T1mic | 29 (2.2%) | 21 (2.9%) | 6 (1.8%) | 1 (0.6%) | 1 (1.1%) | |
| T1 | 794 (60.2%) | 538 (75.4%) | 141 (41.7%) | 92 (52.6%) | 23 (25%) | |
| T2 | 357 (27.1%) | 147 (20.6%) | 156 (46.2%) | 26 (14.9%) | 28 (30.4%) | |
| T3 | 45 (3.4%) | 1 (0.1%) | 29 (8.6%) | 0 (0%) | 15 (16.3%) | |
| T4 | 14 (1.1%) | 0 (0%) | 6 (1.8%) | 0 (0%) | 8 (8.7%) | |
| Tx | 80 (6.1%) | 7 (1%) | 0 (0%) | 56 (32.0%) | 17 (18.5%) | |
| Resected lymph nodes | 9.49±8.74 | 6.97±8.28 | 13.09±9.0 | 11.2±7.66 | 12.8±6.00 | <.001 |
| Affected lymph nodes | 1.78±4.29 | 1.05±2.91 | 3.13±6.12 | 1.57±3.7 | 2.99±4.63 | <.001 |
| Affected lymph nodes | <.001 | |||||
| N0 | 751 (56.9%) | 467 (65.4%) | 154 (45.6%) | 95 (54.3%) | 35 (38.0%) | |
| N1 | 392 (29.7%) | 197 (27.6%) | 101 (29.9%) | 58 (33.1%) | 36 (39.1%) | |
| N2 | 114 (8.6%) | 34 (4.8%) | 49 (14.5%) | 17 (9.7%) | 14 (15.2%) | |
| N3 | 62 (4.7%) | 16 (2.2%) | 34 (10.1%) | 5 (2.6%) | 7 (7.6%) | |
| Molecular profile | <.001 | |||||
| Luminal A | 400 (30.3%) | 275 (38.5%) | 90 (26.6%) | 20 (11.4%) | 15 (16.3%) | |
| Luminal B Her2− | 364 (27.6%) | 182 (25.5%) | 96 (28.4%) | 58 (33.1%) | 28 (30.4%) | |
| Luminal B Her2+ | 143 (10.8%) | 64 (8.9%) | 39 (11.5%) | 27 (15.4%) | 13 (14.1%) | |
| Her2+ | 93 (7.1%) | 29 (4.1%) | 32 (9.5%) | 19 (10.9%) | 13 (14.1%) | |
| Triple negative | 149 (11.3%) | 64 (8.9%) | 20 (5.9%) | 48 (27.4%) | 17 (18.5%) | |
| Not valid | 170 (12.9%) | 100 (14%) | 61 (18%) | 3 (1.7%) | 6 (6.5%) | |
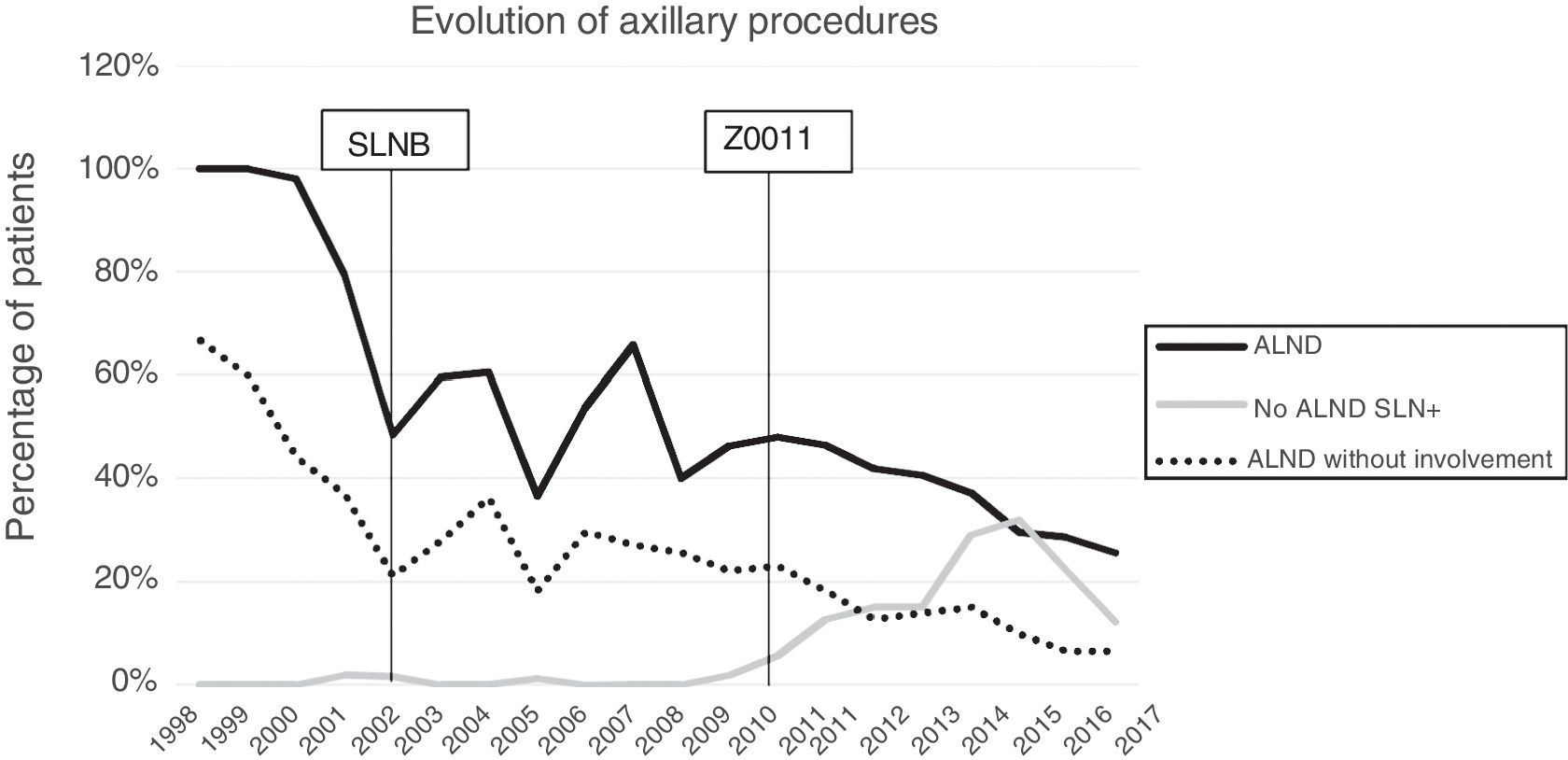
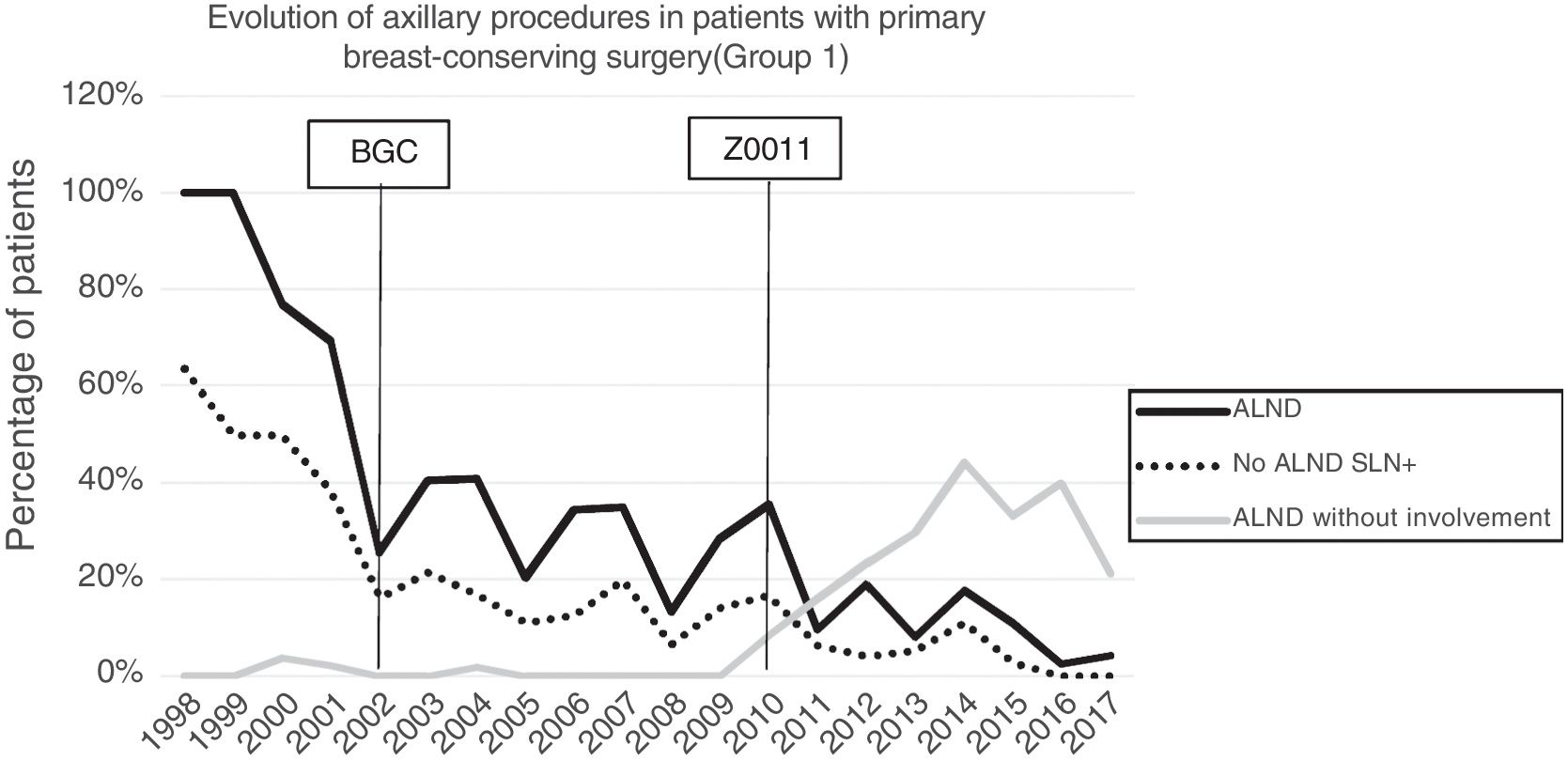
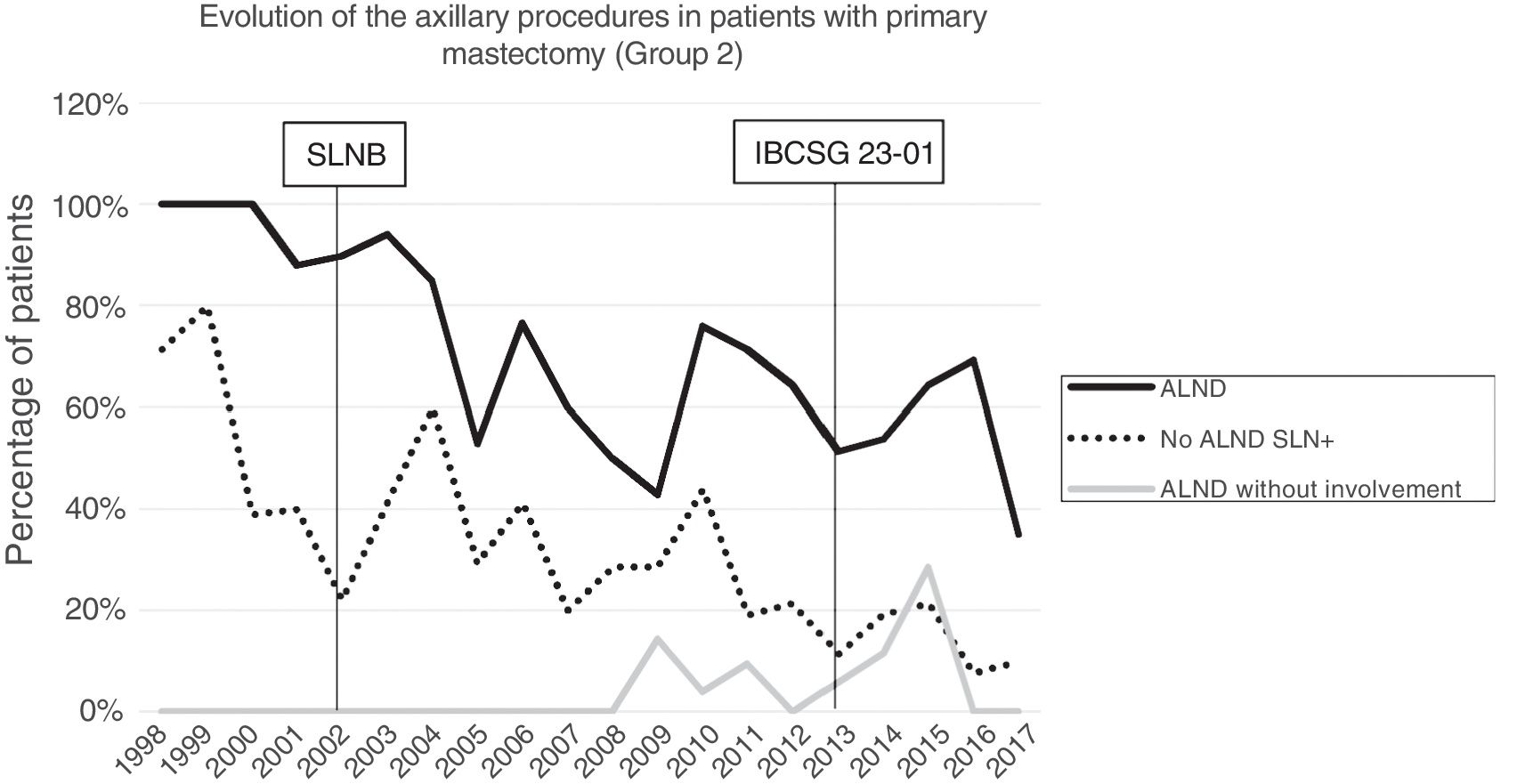
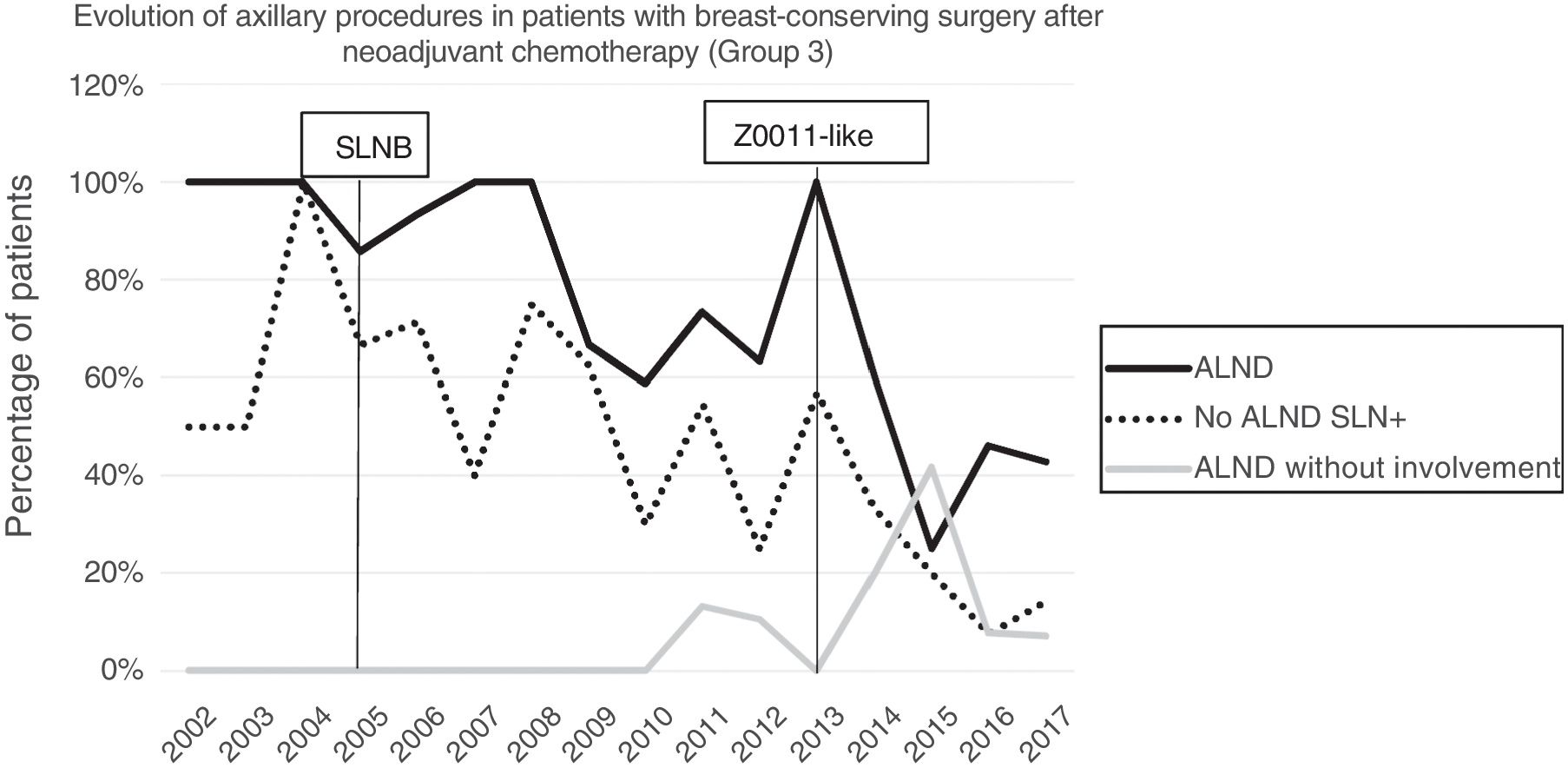
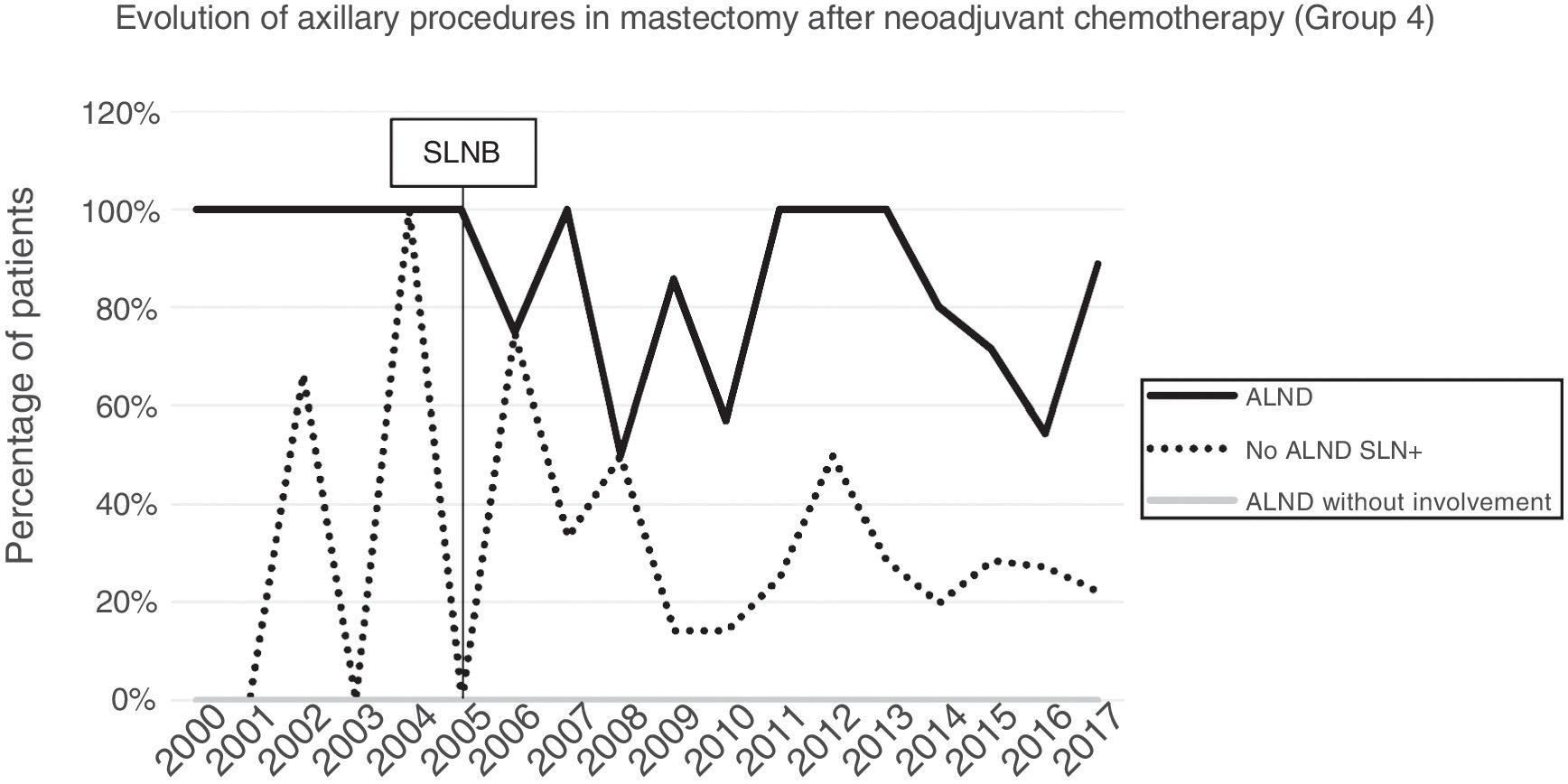
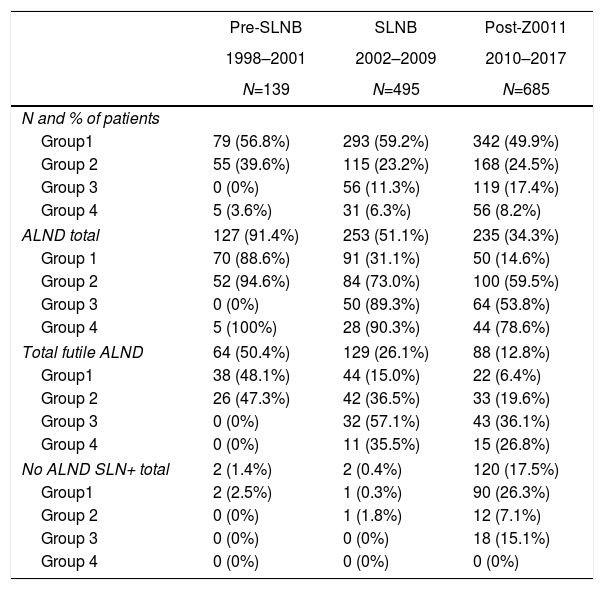
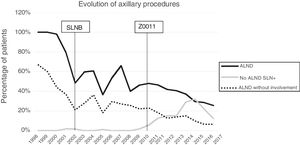
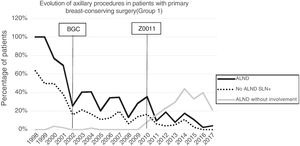
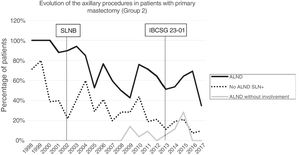
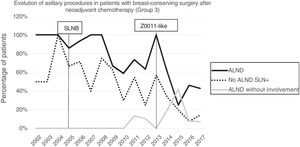
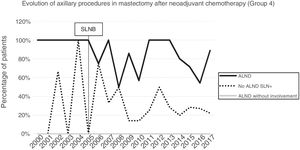
Axillary lymphadenectomy. Table 2 shows the number and percentage of ALND and futile ALND in the 3 periods. A total of 615 ALND (46.62%) were performed during the 20 years of study with differences between the groups: 29.55% in group 1; 69.8% in primary mastectomy; 65.14% in group 3 and 83.7% in mastectomy after neoadjuvant therapy The percentage of ALND decreased progressively over time, from 91% in the first period to 34% after the introduction of the ACOSOG Z0011 criteria (Fig. 2). Two factors marked the decline of ALND. First, with the introduction of the SLNB in mid-2001, ALND decreased by 50% per year. The second factor was the introduction of the Z0011 criteria, which reduced ALND to 25.6% in 2017. Group 1 showed the greatest decrease in the ALND performed (4% in 2017) (Figs. 3–6).
Number and Percentage of Patients With Axillary Lymph Node Dissection per Year.
| Pre-SLNB | SLNB | Post-Z0011 | |
|---|---|---|---|
| 1998–2001 | 2002–2009 | 2010–2017 | |
| N=139 | N=495 | N=685 | |
| N and % of patients | |||
| Group1 | 79 (56.8%) | 293 (59.2%) | 342 (49.9%) |
| Group 2 | 55 (39.6%) | 115 (23.2%) | 168 (24.5%) |
| Group 3 | 0 (0%) | 56 (11.3%) | 119 (17.4%) |
| Group 4 | 5 (3.6%) | 31 (6.3%) | 56 (8.2%) |
| ALND total | 127 (91.4%) | 253 (51.1%) | 235 (34.3%) |
| Group 1 | 70 (88.6%) | 91 (31.1%) | 50 (14.6%) |
| Group 2 | 52 (94.6%) | 84 (73.0%) | 100 (59.5%) |
| Group 3 | 0 (0%) | 50 (89.3%) | 64 (53.8%) |
| Group 4 | 5 (100%) | 28 (90.3%) | 44 (78.6%) |
| Total futile ALND | 64 (50.4%) | 129 (26.1%) | 88 (12.8%) |
| Group1 | 38 (48.1%) | 44 (15.0%) | 22 (6.4%) |
| Group 2 | 26 (47.3%) | 42 (36.5%) | 33 (19.6%) |
| Group 3 | 0 (0%) | 32 (57.1%) | 43 (36.1%) |
| Group 4 | 0 (0%) | 11 (35.5%) | 15 (26.8%) |
| No ALND SLN+ total | 2 (1.4%) | 2 (0.4%) | 120 (17.5%) |
| Group1 | 2 (2.5%) | 1 (0.3%) | 90 (26.3%) |
| Group 2 | 0 (0%) | 1 (1.8%) | 12 (7.1%) |
| Group 3 | 0 (0%) | 0 (0%) | 18 (15.1%) |
| Group 4 | 0 (0%) | 0 (0%) | 0 (0%) |
SLNB: sentinel lymph node biopsy; SLN: sentinel lymph node; ALND: axillary lymph node dissection.
Futile axillary lymph node dissection. The number of futile ALND dropped from 50% in the initial period to 12.8% in the last period. In 2017, only 6.6% of futile ALND were performed. This decrease was more evident in group 1; in fact, in the last 2 years no futile ALND were performed in this group. In groups 2 and 3, there was also evidence of a decrease in the futile ALND curve, reaching 10% and 14.3%, respectively, in 2017 (Figs. 4 and 5). Group 4 presented the highest percentage of futile ALND (27.3%). In this group, ALND was not omitted with metastatic SLN. The introduction of the ACOSOG Z0011 criteria led to an increase in omitted ALND with metastatic SLN in all groups, which was especially notable in the breast-conserving surgery groups (Figs. 3 and 5).
DiscussionThe treatment of women with breast cancer has evolved over the last 2 decades, foregoing the need for radical surgery in the majority of patients. Three facts have led to this change. First, the paradigm of systemic disease proposed by Fisher,16,17 which minimizes the importance of locoregional treatment. Second, the introduction of mammography and early detection programs, which increased the number of patients with early breast disease.18,19 Finally, the description of Perou et al.20 of the different tumor subtypes, which means that specific treatments can be chosen according to the biological characteristics of the tumor.
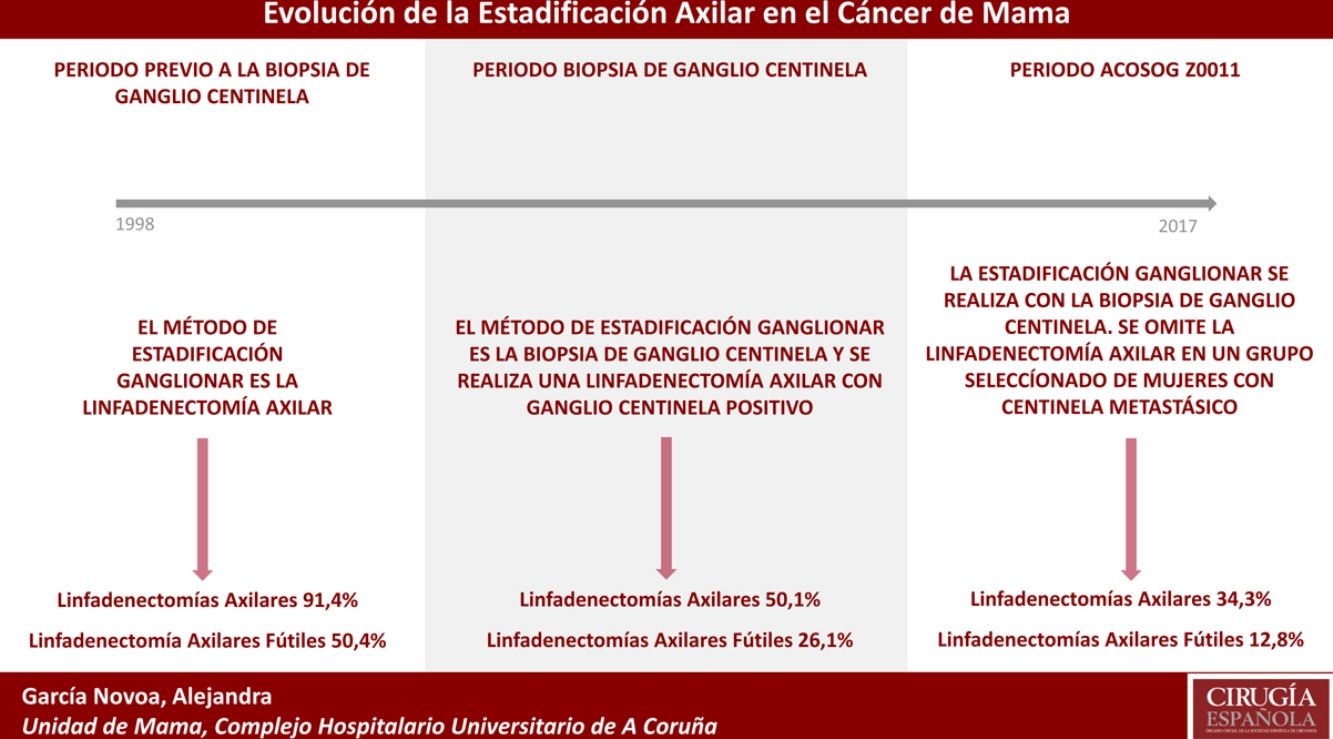
Nevertheless, lymph node involvement continues to be the most important prognostic factor in breast cancer, and lymph node staging is an essential element for planning therapeutic regimens. Initially, lymph node staging was done by clinical examination. However, physical examination is not very sensitive and is non-specific, with FN rates above 40%.21,22 Therefore, ALND became the standard axillary staging procedure, capable of providing adequate regional control of the disease. The dilemma is that ALND has a high morbidity (lymphedema and neuralgia) so it is necessary to determine which patients would benefit from this procedure in terms of survival. In this context, SLNB has emerged as an accessible technique that is easily reproducible, while offering great sensitivity and specificity, yet lower morbidity.4–8 The introduction of SLNB at our hospital has been divided into 2 periods. In the first period, corresponding to the Halsted era, ALND was performed in all women with breast cancer, obtaining more than 50% futile ALND. These data are similar to reports by other authors, who described more than 60% of ALND without metastatic involvement. Thus, Fisher et al.23 reported 62% of patients with ALND with no lymph node involvement and Louis-Sylvestre et al.24 reported 79%. Similarly, Martelli et al.25 and Rudenstam et al.26 determined lymph node metastasis in 23% and 28% of patients, respectively. These data show an overtreatment of the axilla in more than half of the patients who underwent breast cancer surgery.
The second period began in 2001 with the introduction of SLNB at our hospital, which reduced the indication of ALND to below 60% and the futile ALND rate to 21% in 2002. This phenomenon was particularly evident in patients with primary breast-conserving surgery (group 1). The progressive introduction of SLNB in the different treatment groups reduced futile ALND in all groups, but SLNB also created a problem: the possibility of FN. To reduce the FN rate, a thorough study of the SLN was done (serial sectioning, immunohistochemistry or OSNA method). However, thorough study of the SLN increased the diagnosis of small tumor foci that would have gone unnoticed in most ALND in the period prior to SLNB.27 The detection of these tumor foci even modified the TNM nomenclature, where metastatic nests smaller than 0.2mm or those with a cell count less than 250 in the molecular study are now defined as isolated tumor cells. Micrometastases are tumor deposits between 0.2mm and 2mm at their largest dimension or a count of 250–5000 cell copies in the OSNA.
Several studies28,29 have described that the incidence of residual axillary disease in patients with isolated tumor cells and micrometastases in the SLN is less than 11% and 16%, respectively; the survival of these patients is similar to those without lymph node involvement.30–33 In addition, most authors report only 1.5%–6% of patients with more than 3 metastatic nodes (pN2) if the SLN presented micrometastasis.27–29 This implies that less than 15% of patients with micrometastases will have residual disease in the axilla and that about 5% of patients will modify their lymph node stage and perhaps the indication of their adjuvant treatments (nodal irradiation).34,35 Three clinical trials9–13 have studied the repercussions of omitting ALND in patients with SLN metastasis. The first, ACOSOG Z0011,9,10 randomized women with invasive breast cancer (less than 5cm) and metastatic involvement (up to 2 SLN) to either ALND or observation after breast-conserving surgery and radiotherapy. Follow-up studies after 5 and 10 years9,10 have shown no statistically significant differences in locoregional recurrence, disease-free survival or overall survival, despite 27% residual axillary disease detected in the ALND group. Subsequently, the trials by Galimberti et al.11,12 and de Solá et al.13 have demonstrated similar results to the ACOSOG Z0011 in patients with micrometastatic involvement of the axilla. The findings of these 3 clinical trials (ACOSOG Z0011, IBCSG 23-01 and ATTRM) have modified the algorithm for axillary treatment in clinical guidelines worldwide.14,15
In our study, the application of the ACOSOG Z0011 criteria marked the beginning of the third period, during which ALND were reduced to 34% and futile ALND to less than 6.6% of patients operated on in 2017. This was particularly evident in the group with primary breast-conserving surgery, in whom no futile ALND was performed in the last 2 years. In patients with breast-conserving surgery after neoadjuvant chemotherapy, the reduction in ALND and futile ALND was delayed, due to 2 events. First, performing SLNB after chemotherapy starting in 2012, which allowed for the rescue of patients with lymph node response to systemic chemotherapy. Second, and to a lesser extent, to the omission of ALND in patients with micrometastasis of the SLN in the last 4 years. The safety of observation without ALND in this group of patients has not been assessed, and in the literature there is no evidence to support our therapeutic strategy. The results in this period are similar to reports in the literature. Caretta-Weyer et al.36 calculated a possible reduction of 38% in ALND in the population meeting Z0011 criteria. Caudle et al.37 analyzed the impact of the Z0011 criteria one year after their implementation and demonstrated a decrease in ALND with metastatic SLN from 85% to 24%. Joyce et al.38 conducted a similar study and determined a 27% reduction in ALND. Likewise, in a previous publication by our hospital,39 58% of the patients with primary breast-conserving surgery and metastatic SLN were rescued from ALND.
In conclusion, our study reflects the changes that have occurred in the last 2 decades in the staging procedures and axillary treatment of breast cancer. Evolution in breast cancer treatment has decreased the indication of ALND, thereby reducing the number of futile ALND. Women with primary breast-conserving surgery gain the greatest benefit, since futile ALND is no longer performed in this group. Presently, several clinical trials are underway40–49 that will contribute to making decisions about the indication of ALND in different scenarios. Lymph node dissection should be limited to women who will benefit from it in terms of overall survival, thus reducing the morbidity associated with this technique (lymphedema, neuralgia, paresthesia, etc.).
FundingThis study has received no specific support from public, private or non-profit organizations.
Conflict of InterestsThere are no conflicts of interests.
Please cite this article as: García-Novoa A, Acea-Nebril B, Casal-Beloy I, Bouzón-Alejandro A, Cereijo Garea C, Gómez-Dovigo A, et al. El declive de la linfadenectomía axilar en el cáncer de mama. Evolución de su indicación durante los últimos 20 años. Cir Esp. 2019;97:222–229.