The Spanish Rectal Cancer Project of the Spanish Association of Surgeons was established in 2006. The main objective of this observational study was to assess the results obtained by the hospitals trained in the period 2006–2011, in order to evaluate whether this initiative has allowed achievement of the observed quality standards in the Norwegian Rectal Cancer Project.
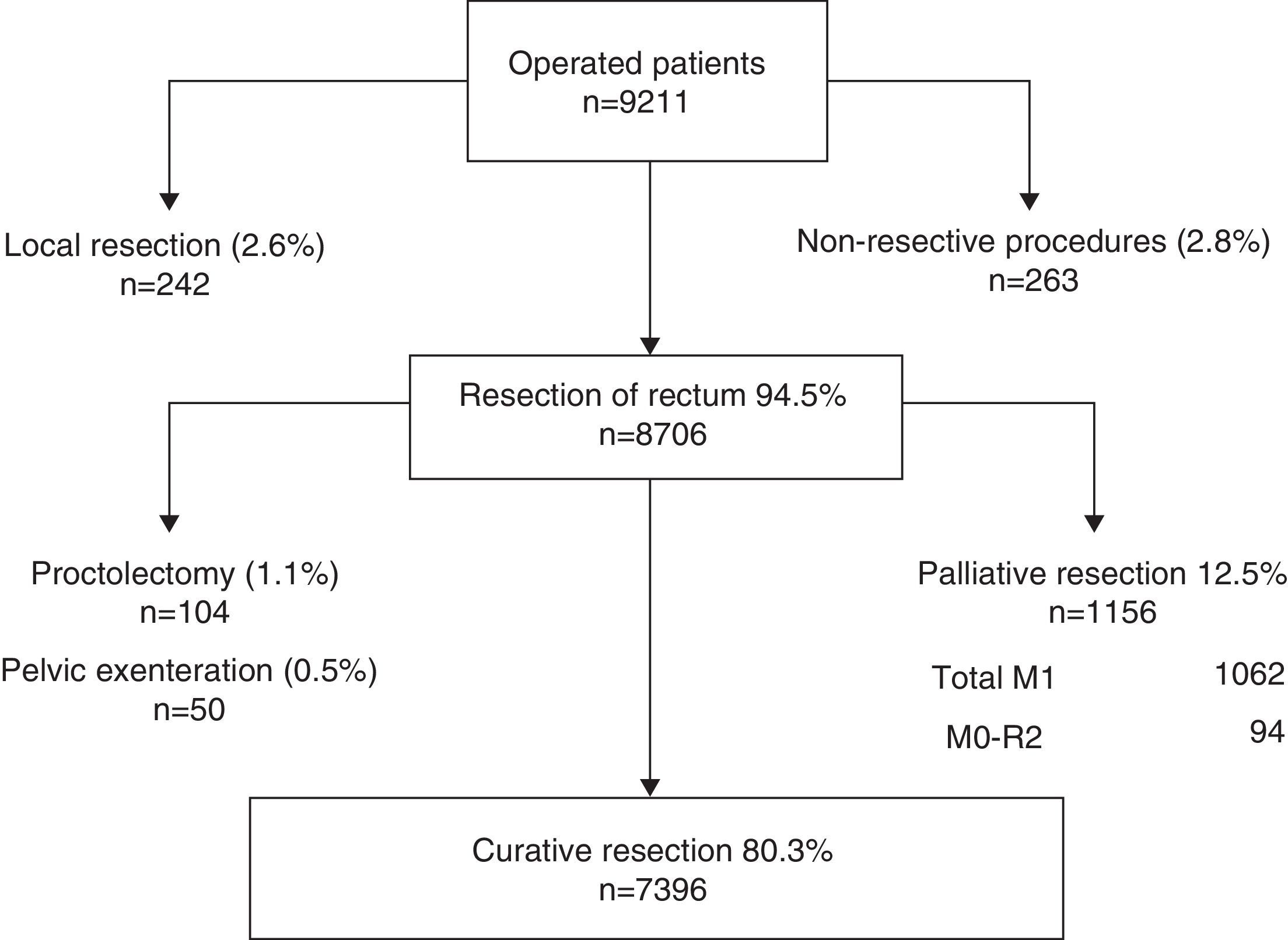
MethodsBetween March 2006 and June 2012 a cohort of 10,006 patients treated during 2006–2011 in 79 hospitals were included in the project registry. In 8.706 (94.5%) patients a rectal resection was performed. In 1.156 patients diagnosed with metastases or an R2-resection, the resection was considered palliative. The number of rectal resections with curative intent analysed was 7.396 (80.3%). The outcome measures of the programme effectiveness were local recurrence, metastases and survival.
ResultsAfter a median follow-up period of 19.0 months (interquartile range [8.00–33.0]), local recurrence rate was 7.7 (9.1–6.2); metastases, 23.4 (25.6–21.1), and mortality 25.9 (28.1–23.7).
ConclusionThis study shows that the oncological results achieved by the Spanish hospitals participating in the Rectal Cancer Project of the Spanish Association of Surgeons are similar to those observed in the Norwegian Colon and Rectal Cancer Project.
El Proyecto del Cáncer de Recto de la Asociación Española de Cirujanos se inició en el año 2006. El objetivo principal de este estudio observacional ha sido evaluar los resultados conseguidos por los hospitales formados en el periodo 2006-2011 para valorar si esta iniciativa ha permitido conseguir los estándares de calidad observados en el Norwegian Rectal Cancer Project.
MétodosEntre marzo de 2006 y junio de 2012 se ha incluido en el registro una cohorte de 10.006 pacientes tratados por 79 hospitales incluidos en el proyecto entre 2006 y 2011. En 8.706 (94,5%) se practicó una resección del recto. La resección se consideró paliativa en 1.156 pacientes, con metástasis en el diagnóstico o porque la resección fue R2. El número de resecciones de recto con intención curativa analizado fue 7.396 (80,3%). Las medidas de resultado fueron: las tasas de recidiva local, metástasis y supervivencia.
ResultadosCon una mediana de seguimiento de 19,0 (rango intercuartílico [8,00-33,0]) meses, la tasa de recidiva local fue 7,7 (9,1-6,2), la de metástasis en el seguimiento de 23,4 (25,6-21,1) y la de mortalidad 25,9 (28,1-23,7).
ConclusiónEste estudio ha permitido conocer que los resultados oncológicos de los hospitales españoles que participan en el Proyecto del Cáncer de Recto de la Asociación Española de Cirujanos son similares a los observados en el Norwegian Colon and Rectal Cancer Project.
Rectal cancer treatment results in Spain are unknown.
In 2002, the Spanish Association of Surgeons (AEC, for its initials in Spanish) conducted a voluntary survey in 43 hospitals from all regions on the results of colorectal cancer treatment,1 which revealed that mesorectal excision was not used routinely. Moreover, pathological variables, such as perforation rate and circumferential resection margin invasion–currently considered essential to assess the outcome of surgery –were not collected in this survey. Oncological outcome variables, such as local recurrence (LR), metastasis, and survival rates, were also not collected.
To determine, and possibly to improve, the treatment results of this disease in Spain, in 2006, the AEC introduced an audited teaching programme2 inspired by the Norwegian Colon and Rectal Cancer Project,3 with which Norway had achieved, for the whole population, LR and survival rates similar to the rates published by centres of excellence worldwide.4
We considered the results achieved in Norway as the benchmark to evaluate our country's project results, given the lack of previous data in Spain.
The aim of this study was to evaluate the results achieved by multidisciplinary teams (MDTs) created in the hospitals from 2006 to 2011 to assess whether this audited educational initiative allowed the achievement of quality standards observed in the Norwegian Rectal Cancer Project.
MethodsCoursesSince 2006, the AEC has sponsored annual courses to train MDTs. The rationale for this project has been described previously.5 The following issues were discussed in the courses: the foundation of mesorectal excision surgery; specimen handling; how to prepare the report according to the method of Quirke,6 emphasising the importance of studying the circumferential resection margin; and the assessment of mesorectal quality and the standardisation of magnetic resonance imaging (MRI) according to the method of Brown.7 All of these courses were based on live demonstrations.
RegistryA centralised registry was established with a specific database. Patients were entered into the database into 4 categories: (1) non-operated, (2) non-resective operations: exploratory laparotomy or laparoscopy, only stoma and bypasses, (3) local resection, and (4) rectal resection: anterior resection, abdominoperineal resection, Hartmann operation, proctocolectomy, and pelvic exenteration.
Each hospital appointed a surgeon responsible for collecting and sending data to the registry. The following variables were included in the database: patient characteristics, type of surgery, postoperative complications, and neoadjuvant and adjuvant therapy; follow-up data included LR, metastasis, and mortality rates.
Tumour stage was determined by the TNM classification (American Joint Committee on Cancer [AJCC] stages i–iv; 5th edition).8
The project was approved by the Ethics Committees of the centres included in the study.
Definitions and StandardsRectal tumours (CIE 20) were defined as those arising in the last 15cm of the large intestine measured from the anal verge by rigid proctoscopy or MRI.
Palliative resections were defined as those that were conducted on patients with metastases diagnosed in the preoperative study or during the operation, associated or not with the presence of microscopic residual tumour in the pelvis (R2).
All other resections were considered curative resections, regardless of whether the specimen had microscopic invasion of the distal or circumferential margins (≤1mm) or whether the rectum or the tumour had been perforated during surgery.
LR was defined as pelvic disease recurrence, including the anastomosis and perineal wound, regardless of whether the patient had distant metastases. An isolated recurrence in the ovaries was considered as metastasis.
Follow-upFollow-up information was sent to the registry annually. Also annually, the registry sent a report to each hospital of their results compared with the average of the hospitals included in the project. The latter information was published on the AEC website.9
Statistical AnalysisContinuous variables are presented as means and standard deviations or medians with interquartile ranges (quartile 1/quartile 3). Categorical variables are presented as absolute values and percentages. Categorical variables were compared using the χ2 test of independence. For the analysis of continuous variables, Student's t-test and the Kruskal–Wallis test were used.
The results related to LR, metastasis, and survival rates were presented as the total numbers of events (considering that patients were at risk of experiencing the events listed until death, loss to follow-up by change of residence, or termination of follow-up at 5 years). The incidences of these events were estimated using the Kaplan–Meier method.
After assessing the proportionality and linearity of the hazard ratios (HRs), event-specific HR modelling was performed using the Cox proportional-hazards regression model. Potential confounders, such as age, sex, tumour stage, and neoadjuvant and adjuvant therapy, were included in the models. HR is presented with a 95% confidence interval (95% CI).
All significant variables were included in the final analysis. Confounding variables with a marginal association (P<.15) were included in the model and were only excluded if they did not significantly change the probability of the model or the estimates of the other included variables. If a variable was significant in the analysis of LR but not on survival or vice versa, it was included in both regression models.
Data were analysed using the R statistical package, version 2.11 (R Foundation for Statistical Computing, Vienna, Austria).
ResultsGeneralA partial analysis of the results of this project with a different survey methodology has been published recently.15 MDTs of 92 centres were created between 2006 and 2011. Of these MDTs, 13 abandoned the project for not complying with the commitments that required them to send data on new patients and follow-up information on operated patients. Another 4 MDTs left the project for the same reasons but reapplied for inclusion and repeated the training process once they solved their problems.
The results shown include those provided by the 79 hospitals whose MDTs were organised between 2006 and 2011, in the period between March 2006 and June 2012.
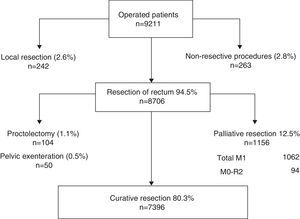
A total of 10,006 patients were enrolled. Of these, 639 did not undergo surgery and the records of 151 lacked complete information, so they were not taken into account for the analysis.
The flowchart of the performed operations is shown in Fig. 1. Of the 9211 patients operated on, 8706 (94.5%) underwent resection of the rectum. Of these patients, resection was considered palliative in 1156 (12.5%) cases due to metastases or because it was an R2 resection. Furthermore, to compare the results of this study with the results of the Norwegian Colon and Rectal Cancer Project, proctocolectomies and pelvic exenterations were excluded from the analysis so that the number of rectal resections with curative intent analysed was 7396 (80.3%).
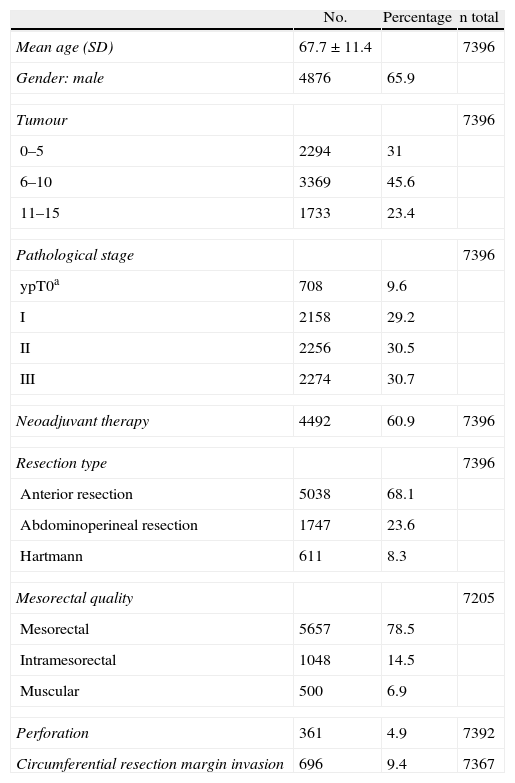
The patient and tumour characteristics, the types of surgery, and the disease data are shown in Table 1.
Characteristics of the 7396 Patients Studied.
| No. | Percentage | n total | |
| Mean age (SD) | 67.7±11.4 | 7396 | |
| Gender: male | 4876 | 65.9 | |
| Tumour | 7396 | ||
| 0–5 | 2294 | 31 | |
| 6–10 | 3369 | 45.6 | |
| 11–15 | 1733 | 23.4 | |
| Pathological stage | 7396 | ||
| ypT0a | 708 | 9.6 | |
| I | 2158 | 29.2 | |
| II | 2256 | 30.5 | |
| III | 2274 | 30.7 | |
| Neoadjuvant therapy | 4492 | 60.9 | 7396 |
| Resection type | 7396 | ||
| Anterior resection | 5038 | 68.1 | |
| Abdominoperineal resection | 1747 | 23.6 | |
| Hartmann | 611 | 8.3 | |
| Mesorectal quality | 7205 | ||
| Mesorectal | 5657 | 78.5 | |
| Intramesorectal | 1048 | 14.5 | |
| Muscular | 500 | 6.9 | |
| Perforation | 361 | 4.9 | 7392 |
| Circumferential resection margin invasion | 696 | 9.4 | 7367 |
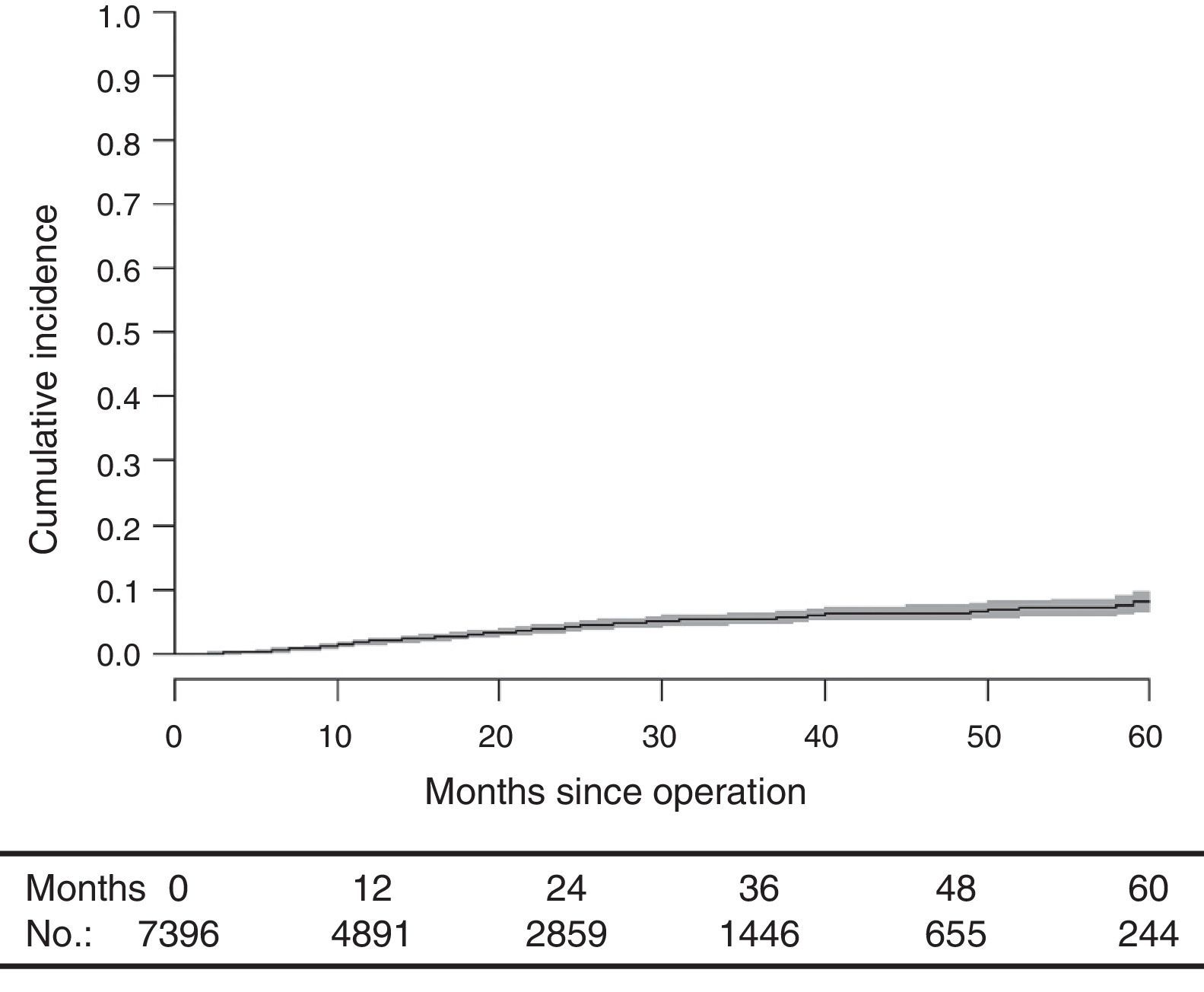
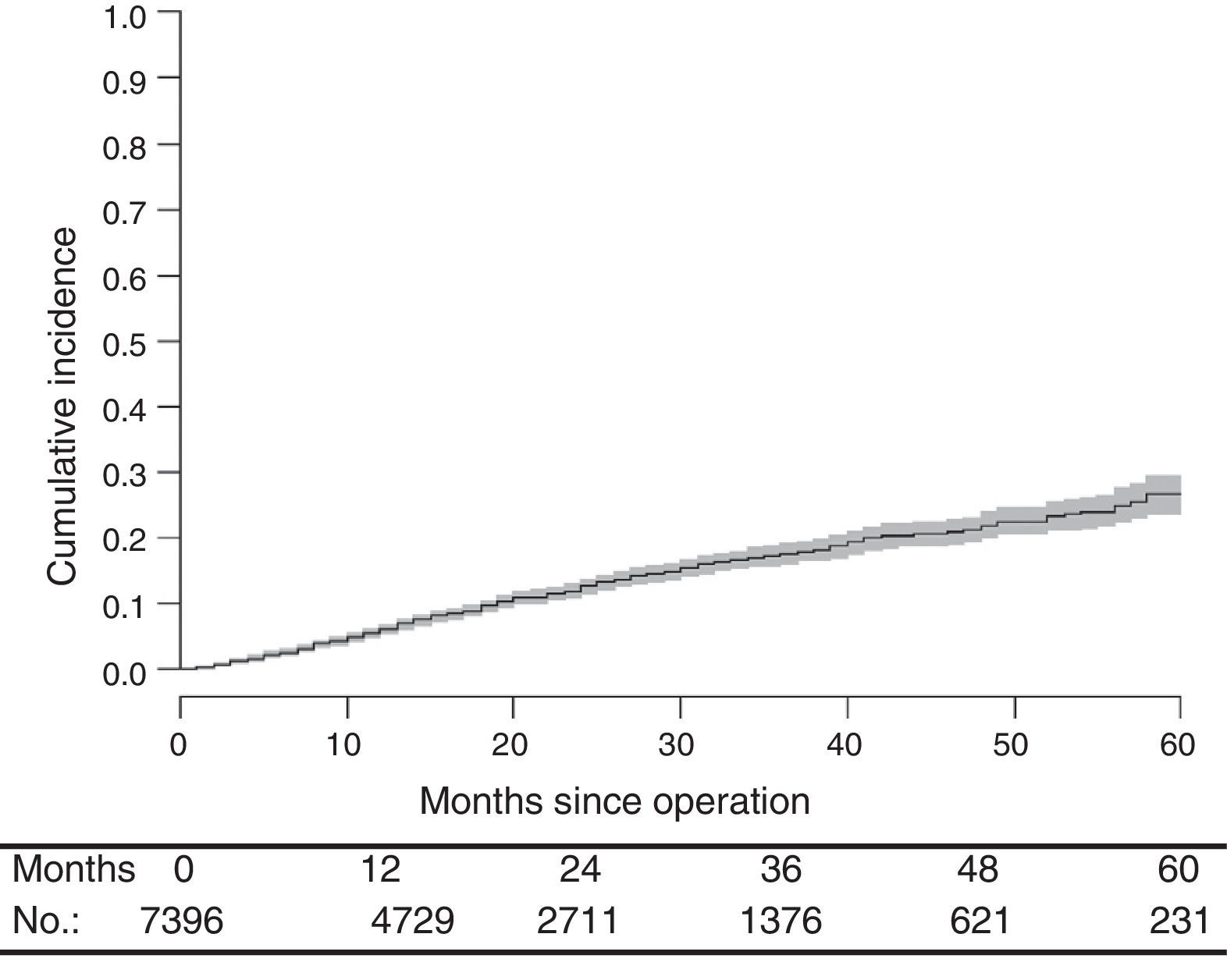
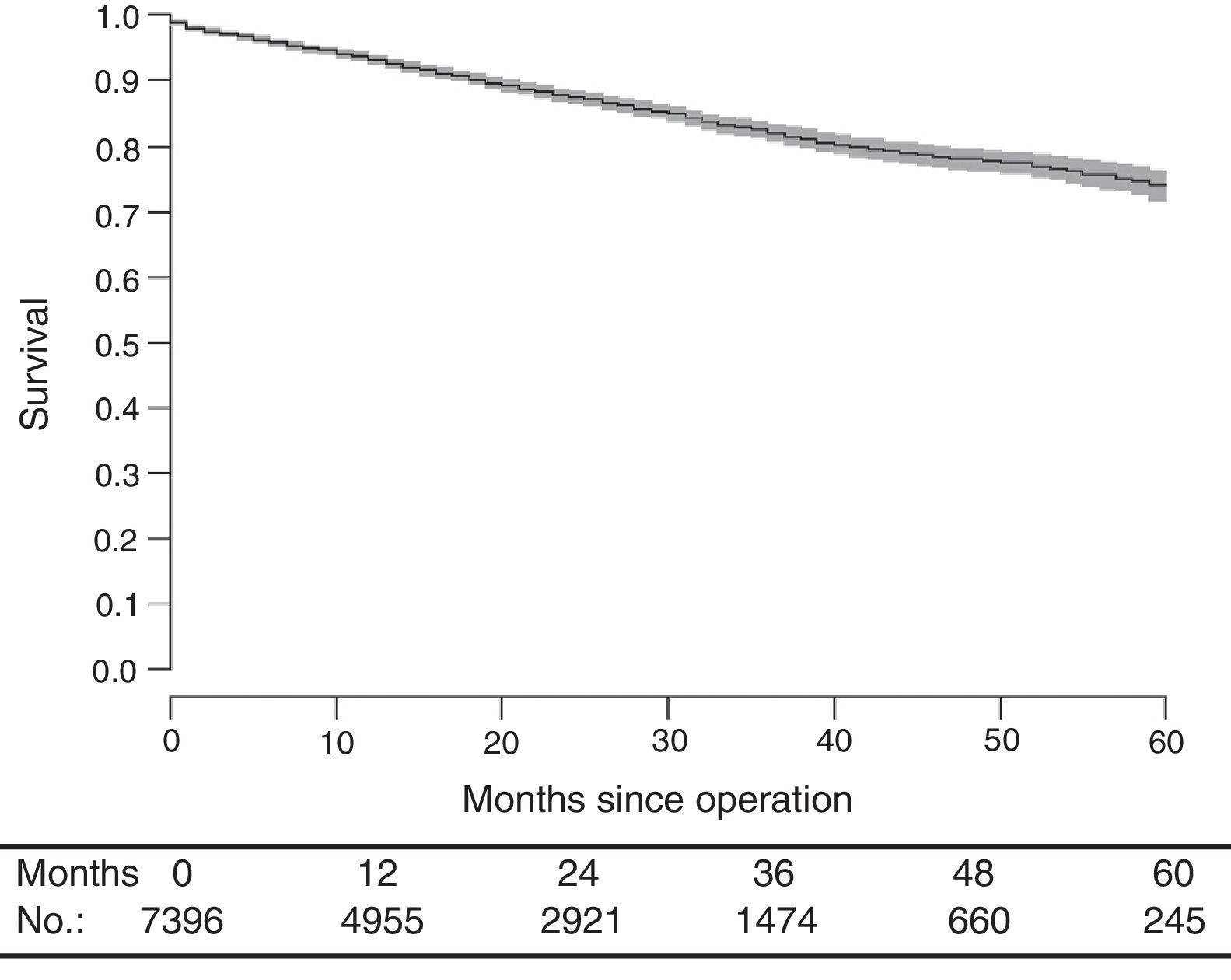
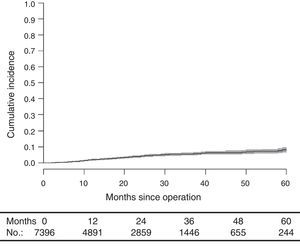
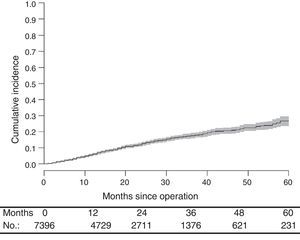
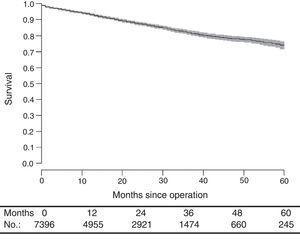
With a median follow-up of 19.0 (interquartile range 19.0 [8.00–33.0] months, the LR rate was 7.7 [9.1–6.2] (Fig. 2), the metastasis rate during follow-up was 23.4 [25.6–21.1] (Fig. 3), and the mortality rate was 25.9 [28.1–23.7] (Fig. 4).
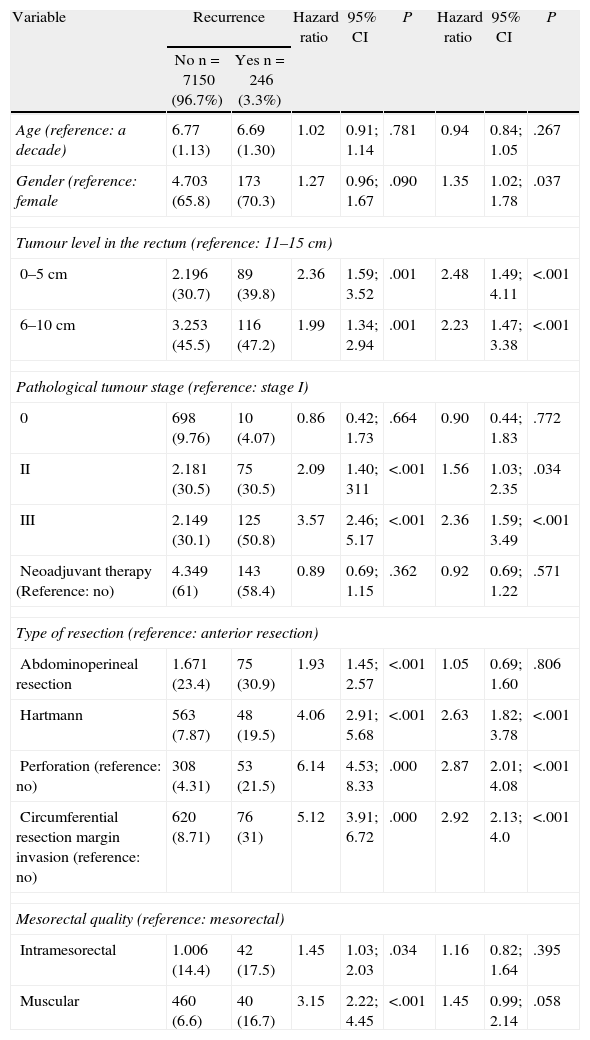
The factors that influenced the development of LR are shown in Table 2. Male gender, distal tumour location in the rectum, more advanced cancer stages, tumour or rectum perforation during surgery, circumferential resection margin invasion, and Hartmann’ procedure increased the odds of LR.
Risk Factors for Local Recurrence.
| Variable | Recurrence | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| No n=7150 (96.7%) | Yes n=246 (3.3%) | |||||||
| Age (reference: a decade) | 6.77 (1.13) | 6.69 (1.30) | 1.02 | 0.91; 1.14 | .781 | 0.94 | 0.84; 1.05 | .267 |
| Gender (reference: female | 4.703 (65.8) | 173 (70.3) | 1.27 | 0.96; 1.67 | .090 | 1.35 | 1.02; 1.78 | .037 |
| Tumour level in the rectum (reference: 11–15cm) | ||||||||
| 0–5cm | 2.196 (30.7) | 89 (39.8) | 2.36 | 1.59; 3.52 | .001 | 2.48 | 1.49; 4.11 | <.001 |
| 6–10cm | 3.253 (45.5) | 116 (47.2) | 1.99 | 1.34; 2.94 | .001 | 2.23 | 1.47; 3.38 | <.001 |
| Pathological tumour stage (reference: stage I) | ||||||||
| 0 | 698 (9.76) | 10 (4.07) | 0.86 | 0.42; 1.73 | .664 | 0.90 | 0.44; 1.83 | .772 |
| II | 2.181 (30.5) | 75 (30.5) | 2.09 | 1.40; 311 | <.001 | 1.56 | 1.03; 2.35 | .034 |
| III | 2.149 (30.1) | 125 (50.8) | 3.57 | 2.46; 5.17 | <.001 | 2.36 | 1.59; 3.49 | <.001 |
| Neoadjuvant therapy (Reference: no) | 4.349 (61) | 143 (58.4) | 0.89 | 0.69; 1.15 | .362 | 0.92 | 0.69; 1.22 | .571 |
| Type of resection (reference: anterior resection) | ||||||||
| Abdominoperineal resection | 1.671 (23.4) | 75 (30.9) | 1.93 | 1.45; 2.57 | <.001 | 1.05 | 0.69; 1.60 | .806 |
| Hartmann | 563 (7.87) | 48 (19.5) | 4.06 | 2.91; 5.68 | <.001 | 2.63 | 1.82; 3.78 | <.001 |
| Perforation (reference: no) | 308 (4.31) | 53 (21.5) | 6.14 | 4.53; 8.33 | .000 | 2.87 | 2.01; 4.08 | <.001 |
| Circumferential resection margin invasion (reference: no) | 620 (8.71) | 76 (31) | 5.12 | 3.91; 6.72 | .000 | 2.92 | 2.13; 4.0 | <.001 |
| Mesorectal quality (reference: mesorectal) | ||||||||
| Intramesorectal | 1.006 (14.4) | 42 (17.5) | 1.45 | 1.03; 2.03 | .034 | 1.16 | 0.82; 1.64 | .395 |
| Muscular | 460 (6.6) | 40 (16.7) | 3.15 | 2.22; 4.45 | <.001 | 1.45 | 0.99; 2.14 | .058 |
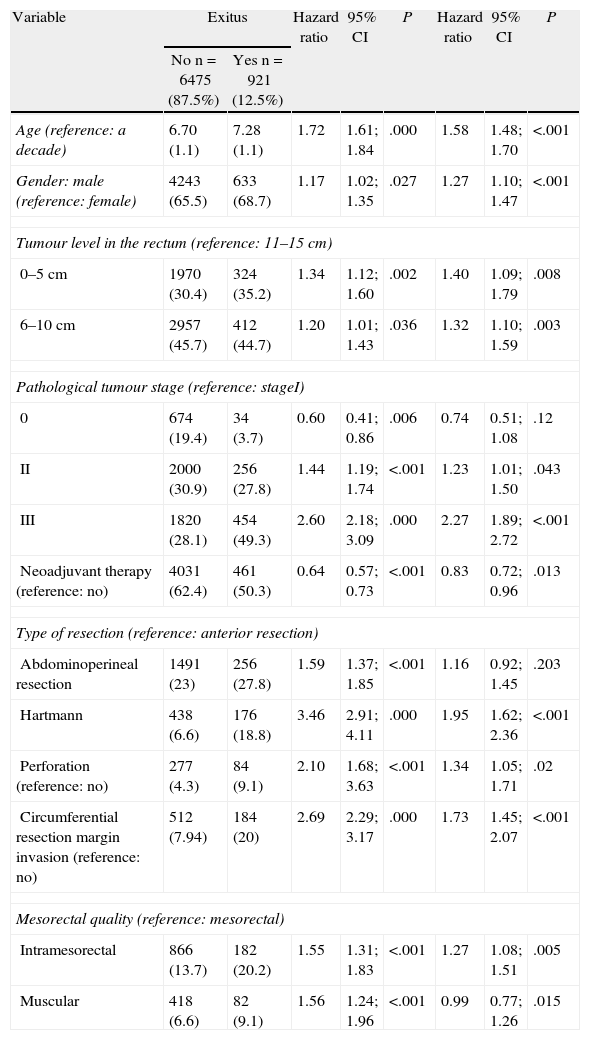
Factors influencing mortality are shown in Table 3. In addition to all of the factors mentioned for LR, the age and quality of the mesorectum also negatively influenced survival.
Risk Factors for Mortality.
| Variable | Exitus | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| No n=6475 (87.5%) | Yes n=921 (12.5%) | |||||||
| Age (reference: a decade) | 6.70 (1.1) | 7.28 (1.1) | 1.72 | 1.61; 1.84 | .000 | 1.58 | 1.48; 1.70 | <.001 |
| Gender: male (reference: female) | 4243 (65.5) | 633 (68.7) | 1.17 | 1.02; 1.35 | .027 | 1.27 | 1.10; 1.47 | <.001 |
| Tumour level in the rectum (reference: 11–15cm) | ||||||||
| 0–5cm | 1970 (30.4) | 324 (35.2) | 1.34 | 1.12; 1.60 | .002 | 1.40 | 1.09; 1.79 | .008 |
| 6–10cm | 2957 (45.7) | 412 (44.7) | 1.20 | 1.01; 1.43 | .036 | 1.32 | 1.10; 1.59 | .003 |
| Pathological tumour stage (reference: stageI) | ||||||||
| 0 | 674 (19.4) | 34 (3.7) | 0.60 | 0.41; 0.86 | .006 | 0.74 | 0.51; 1.08 | .12 |
| II | 2000 (30.9) | 256 (27.8) | 1.44 | 1.19; 1.74 | <.001 | 1.23 | 1.01; 1.50 | .043 |
| III | 1820 (28.1) | 454 (49.3) | 2.60 | 2.18; 3.09 | .000 | 2.27 | 1.89; 2.72 | <.001 |
| Neoadjuvant therapy (reference: no) | 4031 (62.4) | 461 (50.3) | 0.64 | 0.57; 0.73 | <.001 | 0.83 | 0.72; 0.96 | .013 |
| Type of resection (reference: anterior resection) | ||||||||
| Abdominoperineal resection | 1491 (23) | 256 (27.8) | 1.59 | 1.37; 1.85 | <.001 | 1.16 | 0.92; 1.45 | .203 |
| Hartmann | 438 (6.6) | 176 (18.8) | 3.46 | 2.91; 4.11 | .000 | 1.95 | 1.62; 2.36 | <.001 |
| Perforation (reference: no) | 277 (4.3) | 84 (9.1) | 2.10 | 1.68; 3.63 | <.001 | 1.34 | 1.05; 1.71 | .02 |
| Circumferential resection margin invasion (reference: no) | 512 (7.94) | 184 (20) | 2.69 | 2.29; 3.17 | .000 | 1.73 | 1.45; 2.07 | <.001 |
| Mesorectal quality (reference: mesorectal) | ||||||||
| Intramesorectal | 866 (13.7) | 182 (20.2) | 1.55 | 1.31; 1.83 | <.001 | 1.27 | 1.08; 1.51 | .005 |
| Muscular | 418 (6.6) | 82 (9.1) | 1.56 | 1.24; 1.96 | <.001 | 0.99 | 0.77; 1.26 | .015 |
Rates for operative mortality, complications, anastomotic dehiscence, and reoperations for rectal resection patients were 3, 42.1, 8.8, and 8.4%, respectively.
DiscussionThe rates of local recurrence, metastasis during follow-up, and survival observed in this study were similar to those reported in the last update of the Norwegian Colon and Rectal Cancer Project Registry: 8, 19, and 73, respectively.10,11
Despite these satisfactory results, the comparison of the results of the Spanish with the Norwegian Registry has several limitations. The Norwegian project started in 1993, and all 55 hospitals in Norway were invited to participate in it. From this initial number of hospitals, today, only 25 remain in the project. The other hospitals withdrew, voluntarily stopped operating this disease, or were forbidden to treat these patients since the year 2000, when the Norwegian health authorities funded this project.
In contrast, in the Spanish project, hospitals with less than 12 patients per year were not allowed to participate following the experiences and recommendations from the Norway12 and Sweden13 Registries because they had observed that LR rates in centres with such small case numbers doubled the rates of the centres with larger case numbers. Survival rates were also different and more statistically significant compared to centres with larger case numbers.
Moreover, our results, which were apparently similar to the results observed in Norway, can be influenced by the increased use of neoadjuvant therapies. In the Spanish registry, the rate of patients receiving this treatment is 61%, while in the Norwegian Colon and Rectal Cancer Project, this figure was 35% in the final analysis.10,11
The confounding effect that neoadjuvant therapy may have on these results can be analysed in more depth when comparing the results of a study prior to the Spanish project with results observed in another educational programme developed in Stockholm County, Sweden.14 In the latter, the LR rate at 2 years was 6% in 381 patients undergoing mesorectal excision who were treated with short-course radiotherapy. This result is comparable to the 4.7% LR rate observed in a study prior to the Spanish project on 3213 patients in which radiotherapy was used in 61% of patients,15 while the LR rate was 10% in the Norwegian project when neoadjuvant therapy was only used in 8% of patients. Therefore, the results shown may be due to either an improvement in surgery quality in our country or the more frequent use of neoadjuvant therapies.
Another limitation of the study may involve data quality. In the Norwegian registry, the pathologist mandatorily reports the presence of cancer, and both the surgeon and pathologist must report local recurrences. In addition, all patients can be followed in any hospital in the country because citizens have an ID that is used as their medical record number. In contrast, in Spain, there is no tumour registry, there is no obligation to declare recurrence, there is no sure way to follow patients as they move from hospital, and finally, each hospital registry is voluntary. To overcome these shortcomings, several initiatives have been undertaken to ensure data quality. Some important features of the project have been analysed in different studies.16–19 Finally, and perhaps considered the most significant finding, the rates of intraoperative tumour or rectal perforations, operative mortality and anastomotic dehiscence–variables that indicate the quality of the surgeon and that they are not eager to declare–were similar to the rates published in Norway in 20023 and worse than the rates observed by Wibe et al.11 in 2012: 4, 1, and 6%, respectively.
From the results of this study, it is noteworthy that abdominoperineal resection had no influence on local recurrence or survival rates. These results, which match those of the Norwegian Colon and Rectal Cancer Project, are at odds with the current trend to consider abdominoperineal resection as a surgery that offers worse results than anterior resection.20,21 Considering that LR rates in this study were in line with studies indicating that abdominoperineal resection is a surgery that yields worse results, the only current explanation has been suggested by Wibe et al.,22 which indicates that the tumour level in the rectum and not the type of surgery is the cause of the results. However, this issue deserves a specific study.
The poor results obtained with Hartmann's procedure should also be mentioned, which, starting this year, has led to the exclusion of this surgery from the annual database analysis, as is currently the practice in Norway. As in the previous case, the causes of these results deserve further study.
The initiative conducted in Norway has promoted a change in attitude in Europe regarding the treatment of rectal cancer based on the improvement of the competence of MDTs through educational components, and an even more important factor, if possible, as is a prospective registry of the activity.23–25 Unfortunately, this cultural movement has not led to the promotion of a national registry in Spain maintained by health authorities, although this audited registration strategy has dramatically improved the results of rectal cancer treatment in countries that have implemented it.10,11,26 The observed improvements are such, that the oncological results obtained for rectal cancer have been better than the results observed in colon cancer; and as a consequence, colon surgery has also been currently divided into sectors in the countries that have implemented the initiative.
It would be difficult to argue whether the results obtained in this project are due to the educational activities or to the recording of the results and even whether the initiatives caused any improvements in each hospital. These conclusions are to be determined by the analysis of the results from the study cases.
ConclusionThis study has outlined the results of a group of Spanish hospitals that participated in the rectal cancer project of the AEC, which consisted of a teaching programme for MDTs to increase competence and a centralised results registry. This study has also revealed that local recurrence, metastasis during follow-up, and survival rates are within the quality ranges of the population registries considered as reference standards in the treatment of rectal cancer.
FundingThis project has been funded with the following research grants: FIS number 05/2276 and Department of Health, Government of Navarra58/2008.
Conflict of InterestThe authors declare no conflicts of interest.
The authors acknowledge Ana Lopez Carballedo for her invaluable work as a voluntary project secretary.
Virgen de la Arrixaca Hospital (Juan Luján Monpean); Bellvitge University Hospital (Doménico Fracalvieri, Sebastiano Biondo); Navarra Hospital Complex (Pedro Armendáriz Rubio, Mario de Miguel Velasco); Hospital Clinic of Valencia (Alejandro Espí Macías); Josep Trueta Hospital (Antonio Codina Cazador); Sagunto Hospital (María D. Ruiz Carmona); Vall d’Hebrón Hospital (Eloy Espin Basany); La Fe Hospital (Rosana Palasí Giménez); Ourense Hospital Complex (Alberto Parajo Calvo); Germans Trias i Pujol University Hospital (Ignasi Camps Ausàs, Marta Piñol Pascual); General Morales Meseguer Hospital (Enrique Pellicer Franco); Lluís Alcanyis Hospital (Vicent Viciano Pascual); Burgos Care Complex (Evelio Alonso Alonso); Hospital del Mar (Miguel Pera Román); Vigo Hospital Complex Hospital Meixoeiro (Teresa García Martínez, Enrique Casal Núñez); Salamanca Care Complex (Jacinto García García); Gregorio Marañón Hospital (Marcos Rodríguez Martín); Torrecárdenas Hospital (Ángel Reina Duarte); Valencia General Hospital (José Roig Vila); Txagorritxu Hospital (José Errasti Alustiza); Donostia Hospital (José Andrés Múgica Martirena); Reina Sofía Hospital (José Gómez Barbadillo); Carlos Haya Hospital (José Antonio Toval Mata, Manuel Ruiz López, Santiago Mera Velasco); Juan Ramón Jiménez Hospital (Ricardo Rada Morgades, Mónica Orelogio Orozco); Arnau de Vilanova Hospital of Valencia (Natalia Uribe Quintana); Jerez General Hospital (Juan de Dios Franco Osorio); Elche University General Hospital (Antonio Arroyo Sebastián), Arnau de Vilanova Hospital of Lérida (José Enrique Sierra Grañón); Santa Creu i Sant Pau Hospital (Pilar Hernández Casanovas); Santiago de Compostela University Clinical Hospital/Universidad de Santiago de Compostela (Jesús Paredes Cotoré); Jaén University Hospital (Gabriel Martínez Gallego); San Carlos Clinical Hospital (Fernando Jiménez Escobar); Cabueñes Hospital (Guillermo Carreño Villareal); Albacete General Hospital (Jesús Cifuentes Tébar), Miguel Servet Hospital (José Monzón Abad); Xeral Hospital of Lugo (Olga Maseda Díaz); Fuenlabrada University Hospital (Daniel Huerga Álvarez); University Hospital Clinic of Barcelona (Calin Pavel Mihai); Joan XXIII Hospital (Fernando Gris Yrayzoz); Virgen de las Nieves Hospital (Inmaculada Segura Jiménez, Pablo Palma Carazo); Nuestra Señora de la Candelaria Hospital (José Gregorio Díaz Mejías); Badajoz Hospital Complex (José Luis Jiménez Redondo); San Cecilio University Clinical Hospital (Francisco Pérez Benítez); Galdakao Hospital (Vicente Portugal Porras); Requena Hospital (Juan C. Bernal Sprekelsen); Alicante General Hospital (Félix Lluis Casajuana); Virgen Macarena Hospital (Luis Capitán Morales); Xeral-Cíes Hospital of Vigo (Nieves Cáceres Alvarado); Infanta Sofía Hospital (Ramón Cantero Cid, Javier Martínez Alegría); Povisa Hospital (Alfredo Estévez Diz); Virgen del Rocío Hospital (María Victoria Maestre, José Manuel Díaz Pavón); San Juan de Dios Hospital of the Aljarafe (Mónica Reig Pérez, Antonio Amaya Cortijo); Nuestra Señora de Sonsoles Hospital (José Antonio Carmona Sáez); Getafe Hospital (Javier Jiménez Miramón); Granollers General Hospital (Dídac Ribé i Serrat); La Paz Hospital (Isabel Prieto Nieto); León Hospital Complex (Tomas González de Francisco, Amor Turienzo Frade); Dr. Peset Hospital (Teresa Torres Sánchez, Eva Martí Martínez); Rafael Méndez University General Hospital (Sergio Rodrigo del Valle Ruiz); Reina Sofía General Hospital (Pedro Parra Baños); San Pedro de Alcántara Hospital (Francisco Romero Aceituno); Torrevieja Health Hospital (UTE) (Alessandro Garcea); Santa María de Lérida Hospital (Ricard Batlle Solé); Virgen del Puerto Hospital (Alberto Pérez García); Segovia Hospital (Guillermo Ais Conde); L’Hospitalet General Hospital (Luis Ortiz de Zárate); Reus Hospital (Jesús Sánchez Pérez); Valencia Institute of Oncology (IVO, for its initials in Spanish) (Rafael Estevan Estevan); Viladecans Hospital (Albert Sueiras Gil); Cruces Hospital (Jose María García González); Ramón y Cajal Hospital (Javier Die Trill); Manises Hospital (Amparo Solana Bueno); University Hospital de la Ribera Alzira l (Francisco Javier Blanco González); Nuestra Señora del Rosell Hospital (Ana M. Lage Laredo); Mérida (José Luis Domínguez Tristancho); Foundation Alcorcón Hospital (Paula Dujovne Lindenbaum).
Please cite this article as: Ortiz H, Codina A, en representación del Grupo Colaborador del Proyecto Vikingo. Resultados del proyecto docente y auditado del cáncer de recto de la Asociación Española de Cirujanos. Seis años desde su inicio. Cir Esp. 2013;91:496–503.
The names of the members of the Viking Project Collaborative Group (years 2006–2011) are listed in Appendix 1.
The present study was presented as a talk given at the 29th National Congress of Surgery, Madrid, 2012. Partial results were published in Colorectal Dis. 2013;15(5):544–51. doi:10.1111/codi.12141.