Accelerated partial breast irradiation (APBI) with multicatheters after lumpectomy for breast cancer (BC) may be an alternative to whole breast irradiation in selected patients. The aim is to show our 5 year experience.
MethodBetween June 2007 and June 2012, 87 BC patients have been evaluated for APBI. Inclusion criteria were: age over 40 years, unifocal tumor, infiltrating ductal or intraductal carcinoma, tumor size smaller than 3cm and no lymph node involvement.
ResultsTreatment was completed in 48 patients and contraindicated in 39. The average age of treated patients was 59 years. Operating time was 126min with 9 implanted catheters in each patient. No complications were observed during surgery or radiotherapy. Patients were discharged from hospital after 4–6 days. Tumor size was 11mm. Of these, 35 were infiltrating ductal and 13 intraductal carcinomas. 44 patients received adjuvant treatment. Mean follow-up was 20 months with no evidence of local or distant recurrence. The cosmetic outcome was good or excellent in 63% of cases.
ConclusionsAPBI with multicatheter placed after lumpectomy for BC is feasible and safe but requires a strict selection of patients. Moreover, it may have certain advantages over other APBI techniques and over standard radiation therapy.
La irradiación parcial acelerada de la mama (IPAM) con multicatéteres tras cirugía conservadora del cáncer de mama puede ser una alternativa a la radioterapia externa adyuvante convencional para un grupo seleccionado de pacientes. El objetivo es describir nuestra experiencia en los últimos 5 años.
MétodosEntre junio de 2007 y junio de 2012 fueron evaluados 87 pacientes con cáncer de mama para IPAM. Los criterios de inclusión fueron: edad mayor de 40 años, tumor unifocal, histología de carcinoma ductal infiltrante o intraductal, tamaño menor de 3 centímetros y ausencia de afectación ganglionar. Se valoraron….
ResultadosLa IPAM se completó en 48 pacientes y se contraindicó en 39. La edad media de las pacientes tratadas fue de 59 años. La mediana del tiempo quirúrgico fue de 126 minutos (rango), con una media de 9 catéteres implantados por paciente. No se registraron complicaciones durante la intervención ni en la radioterapia. La mediana de la estancia hospitalaria fue de 4,6 días (rango). El tamaño tumoral medio fue de 11 milímetros. En 35 casos se trataba de carcinomas ductales infiltrantes y en 13 de carcinomas intraductales. Cuarenta y cuatro pacientes recibieron tratamiento adyuvante. Con una mediana de seguimiento de 20 meses (rango) no se ha observado recidiva local ni a distancia. El resultado estético fue bueno o excelente en el 63% de casos.
ConclusionesLa IPAM con multicatéteres colocados en el mismo acto operatorio de la cirugía conservadora del cáncer de mama es una técnica segura y fiable pero exige una meticulosa selección de pacientes. Puede presentar ventajas respecto a otras técnicas de IPAM y respecto a la radiación convencional.
Conservative surgery associated with adjuvant radiotherapy for breast cancer treatment has been performed for 40 years, providing the same survival rates as simple mastectomy.1,2 Standard adjuvant external radiotherapy includes radiation of the entire breast and a “boost” in the lumpectomy bed for 30 daily sessions administered over the course of 6 weeks. It is known that most local recurrences after lumpectomy are located in the surgical bed or immediate vecinity.3 This fact would justify the therapeutic use of accelerated partial-breast irradiation (APBI) even as the sole radiation technique in selected patients. Limiting the volume of breast tissue to be irradiated makes it possible to shorten the treatment to 5 days, reduce the toxicity of radiotherapy4 and achieve excellent cosmetic results,5 without affecting the local control of recurrence or survival.6
There are different techniques for administering APBI,7 which can be summarized in three large groups: brachytherapy,8 intraoperative radiotherapy9 and techniques that apply an external beam of radiation.10
The aim of this study is to describe our experience over the last five years with the application of brachytherapy with multiple catheters placed during the same surgical procedure as the breast cancer extirpation.
MethodsBetween June 2007 and June 2012, 87 consecutive patients with early breast cancer were evaluated prospectively for APBI. In our series, we placed catheters in the same surgical procedure in which the tumor was removed and not in a second operation days or weeks after the first, which is another option.
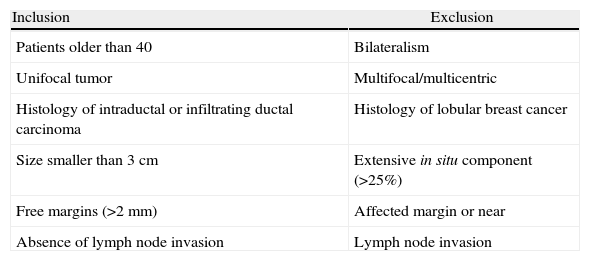
The inclusion and exclusion criteria11 are detailed in Table 1. The preoperative study included mammogram and ultrasound and in all patients there was a previous diagnosis of malignancy by core needle biopsy. In 75 cases (86%), MRI was also performed.
Inclusion and Exclusion Criteria.
| Inclusion | Exclusion |
| Patients older than 40 | Bilateralism |
| Unifocal tumor | Multifocal/multicentric |
| Histology of intraductal or infiltrating ductal carcinoma | Histology of lobular breast cancer |
| Size smaller than 3cm | Extensive in situ component (>25%) |
| Free margins (>2mm) | Affected margin or near |
| Absence of lymph node invasion | Lymph node invasion |
Treatment included the following steps:
- 1.
General anesthesia and conservative surgery: lumpectomy and selective biopsy of the sentinel lymph node.
- 2.
Intraoperative frozen biopsy of the edges of the lesion and the sentinel lymph node. When there were microcalcifications, intraoperative mammography of the surgical specimen was performed.
- 3.
The surgical bed was marked with titanium clips and a drainage tube was inserted.
- 4.
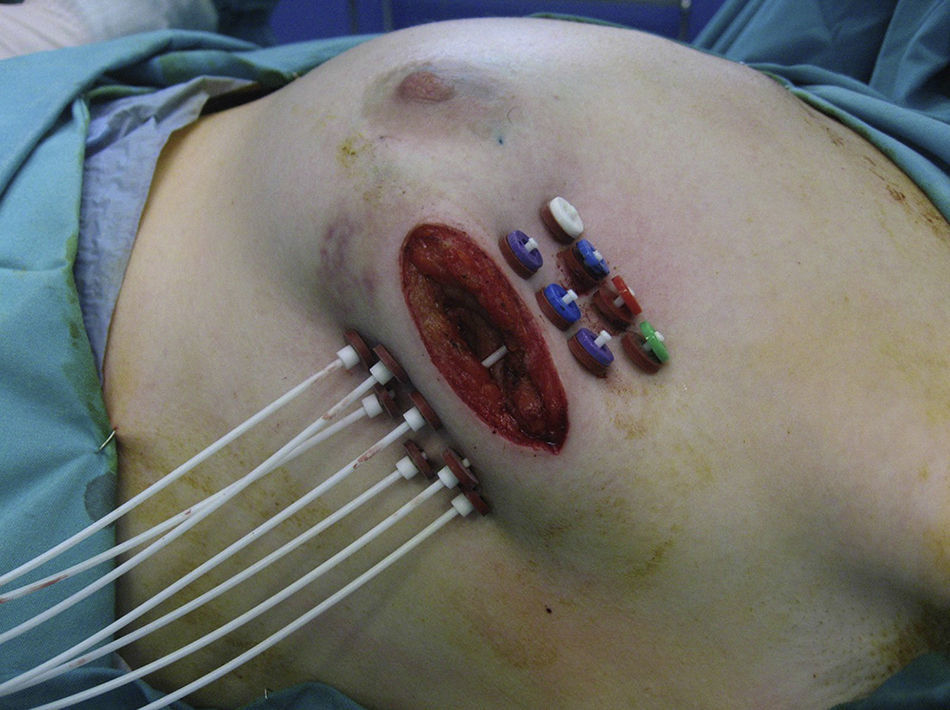
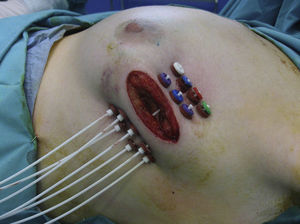
If the results of the intraoperative biopsy were favorable, the radiation oncologist placed catheters in the tumor bed in 2 planes, with a separation of 1.5cm between catheters and at least 7mm from the skin with the free-hand technique (Fig. 1).
- 5.
Hospital discharge on the second or third postoperative day after withdrawal of the surgical drainage, with brachytherapy catheters.
- 6.
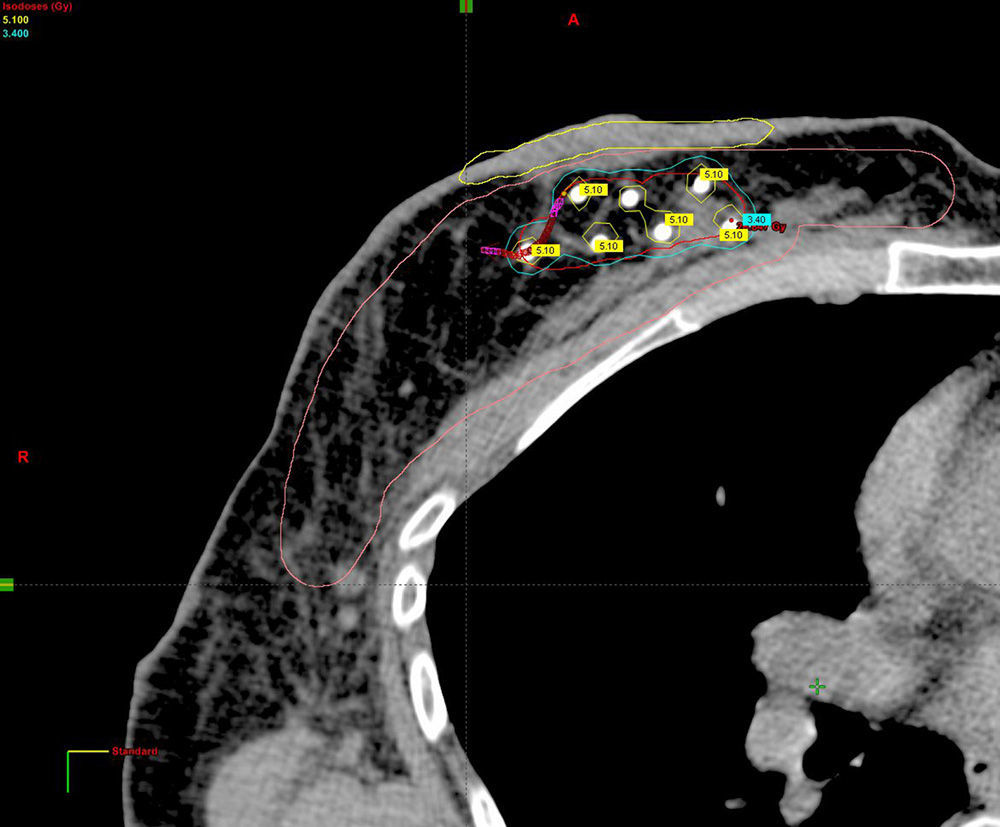
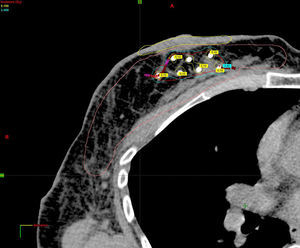
Planning of 3D dosimetry using simulation computed tomography after final histology results were received (Fig. 2).
- 7.
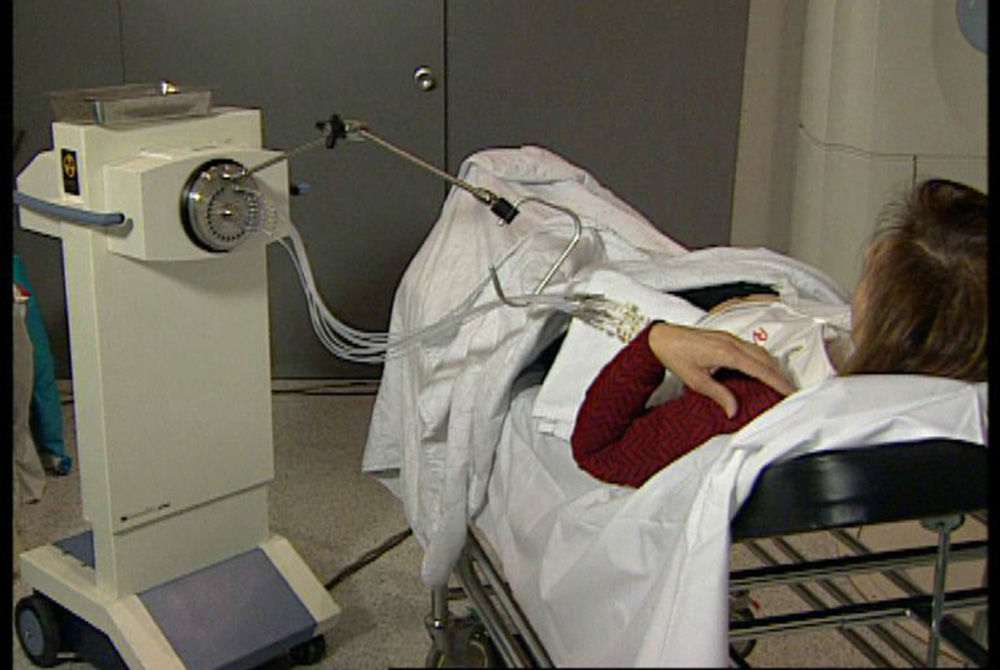
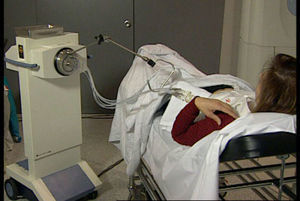
Administration of APBI in 2 daily sessions of 3.4Gy for 5 days until completing 34Gy in 10 sessions (Fig. 3) using a source of Ir192.
- 8.
Withdrawal of catheters after the last session of brachytherapy and postoperative review after one month.
- 9.
Antibiotic prophylaxis with amoxicillin-clavulanate: one intravenous dose of 2g during surgery and oral treatment with 500mg every 8h until the withdrawal of catheters. Analgesia with paracetamol every 8h and metamizole as needed by the patient.
- 10.
Monitoring included physical examination, chest radiography and work-up: every 3 months during the first year, every 4 months during the second year, every six months between the second and fifth years, and annually after the fifth year. Six months after the procedure, mammogram and ultrasound were ordered, which are done annually thereafter. Once a year, liver ultrasound is also usually done in patients who had infiltrating tumors. Depending on the hormone treatment, some patients undergo annual gynecological review and densitometry.
We have performed a descriptive study of complications, cosmetic outcome and recurrence. To evaluate the esthetic results, we have asked our patients for their opinion and we followed the Harvard criteria,12 which assesses this result as excellent, good, fair or poor.
Statistical AnalysisThe results are expressed as mean with standard deviation (SD) and as medians with ranges.
ResultsThe protocol for breast conserving surgery associated with multicatheter APBI was completed in 48 patients and was contraindicated in 39. Catheters were not inserted in 12 patients because the indication was rejected before surgery either due to the result of preoperative tests, intraoperative biopsy findings or technical problems. In 6 patients, the catheters that were inserted during the surgical procedure were removed in the immediate postoperative period when the definitive histopathological diagnosis was known. In 21 of the patients who did not meet strict criteria for exclusive APBI, these catheters were also used to administer the “boost” for 2 days in two daily sessions, representing a nominal dose of 13.6Gy.
Three weeks later, the remaining radiation was administered as hypofractioned external-beam radiation therapy for another 3 weeks, shortening the total length of treatment by 3 weeks compared to conventional external-beam radiotherapy.
Reasons that contraindicated exclusive APBI are summarized in Table 2.
Contraindications for Exclusive APBI.
| Affected sentinel lymph node (11) |
| Extensive in situ component (10) |
| Affected margin or close proximity (5) |
| Technical problems: wound tension, tumors too superficial (5) |
| Multifocal/multicentric tumors (3) |
| Benign histology (2) |
| Lobular carcinoma (1) |
| Bilateral tumor (1) |
| Anatomic pathology criteria for poor prognosis (1) |
The average age of our patients was 59 (SD). Average operative time was 123min (range 72–234). An average of 9 catheters was placed per patient (SD 1.4).
The surgical technique was segmental resection with sentinel node biopsy in 41 cases. Six patients underwent oncoplastic techniques: five round-block and one crescent pattern. In 6 patients, no axillary surgery was performed because they presented intraductal carcinomas less than 2cm in size, and in one patient standard axillary clearance was done due to failure to identify the sentinel lymph node.
Mean hospital stay was 4 days (range 2–14). Although there was no contraindication for hospital discharge, 7 patients chose to remain hospitalized for part or all of the APBI treatment, all of whom were women that resided in other regions. Two patients were reoperated because the edges were affected; the catheters were reinserted and the treatment plan was successfully completed. Another patient had complete necrosis of the nipple-areola complex in the immediate postoperative period, before initiating APBI, but she was also able to successfully complete the treatment and the subsequent cosmetic result was very good. One patient presented seroma in the postoperative period, which was drained by ultrasound-guided fine needle aspiration (FNA). We observed no bleeding or infection during the operation or in the postoperative period. There were also no complications during radiotherapy.
Mean tumor size was 11mm (SD: 4). The histological type of breast cancer was infiltrating ductal in 35 cases and intraductal in 13. The sentinel lymph node was negative except in one case with isolated tumor cells.
Eleven patients received chemotherapy starting at the third week after the removal of catheters. In 44 cases, hormone treatment was recommended.
As for the cosmetic result, 66% of our patients felt the appearance of their breast was either good or excellent. In no cases were there any significant esthetic repercussions or late complications secondary to radiation, such as dermatitis, lung fibrosis or heart disease.
With an average follow-up of 22 months (range 5–64), no local or distant recurrences have been observed.
DiscussionThe transition from full to partial irradiation of the breast is comparable to the evolution in surgery 40 years ago when the change was made from mastectomies to conserving surgeries,13 or the one that occurred 20 years ago with the introduction of the sentinel lymph node technique to replace axillary clearance in many cases. This translates to less aggressive therapies for selected patients with early breast tumors and good prognosis. The ideal is to personalize treatment to the maximum based on the characteristics of the patient and tumor.
The experience published to date about patients treated with APBI is increasing from year to year with the aim of maintaining the local control of the disease, while simultaneously reducing radiotherapy time.6 APBI has thus become consolidated as an alternative to the standard 6-week treatment.
The two most widely used methods of brachytherapy in breast cancer are catheters and balloons, either of which can be placed during the operation or in the postoperative period.
The MammoSite8 balloon is an intracavitary device that is inserted into the lumpectomy bed. Its use has become popular, especially in the U.S., since it was approved by the FDA in 2002. More than 60,000 patients have been treated,14 with 10-year follow-ups that show similar results for local control of recurrence and survival to those of conventional external radiotherapy of the whole breast.15 More recently, other types of intracavitary devices have been introduced on the market, such as SAVI, ClearPath or Xoft Axxent, all of which are hybrids between balloons and catheters.
To administer intraoperative radiation therapy, several accelerators have been developed for this purpose, such as TARGIT, HAM, etc.13 The group that has accumulated the most experience is the European Institute of Oncology in Milan, which has treated more than 2000 patients since 1999 with the ELIOT accelerator.9 This group administers 21Gy directly to the surgical bed before closing the cavity and receiving the final results of the pathology tests.
Recently, thanks to the development of more accurate linear accelerators, APBI techniques have been used with external-beam radiation therapy, such as three-dimensional conformal radiotherapy (3D-CRT)10 and modulated intensity radiotherapy.
Among the different APBI techniques, interstitial brachytherapy with catheters has been used more extensively.7 It has been used for the past 15 years16 with good results in terms of local control of recurrence, and there are already series with follow-ups of up to 10 years.14 It was originally developed to administer the boost on the lumpectomy scar after conventional external-beam radiotherapy7 and was later indicated as the only form of irradiation (exclusive APBI) in selected patients with early tumors. Catheters are placed with a template in the shape of a grid, or with the free hand technique, which requires more manual dexterity.
Most APBI studies with multicatheters have been published by hospitals in the U.S. Of special interest is the prospective multicenter phase II study by the American Radiation Therapy Oncology Group, RTOG 95-174,6 for its long follow-up period (seven years). This study treated 100 patients in three years, analyzing toxicity as well as local and distant recurrence. The inclusion criteria of this study differs from ours, since there was no age limit; it only included infiltrating ductal carcinomas and included patients with up to 3 affected axillary lymph nodes. The sentinel lymph node technique was not only one; instead, axillary sampling with extirpation of at least 6 lymph nodes was used. They reported local recurrence figures of 4% and an overall survival of 93%.
In 2007, Ott et al.5 published the longest series, with 274 patients from the German-Austrian multicenter study. This study included patients older than 35; lymph node disease was accepted only in the form of micrometastases, but only patients with hormone-dependent tumors were admitted. The catheter placement was not performed in the same surgery but after a mean period of 57 days after tumor excision. With a mean follow up of 32 months, local control rates (99.2%) and overall survival (98.5%) were excellent. In 94% of these patients, the cosmetic result was also good or excellent.
Our group published its results for brachytherapy with multicatheters and high-dose rate radiation therapy in 26 patients treated over 7 years but with catheters placed 4 weeks after surgery.17 The rates for local control of recurrence and 6-year survival were 96%.
At present, there is a growing number of published series with follow-up periods approaching 10 years that compare APBI with multicatheters and conventional external-beam radiation therapy. No differences have been detected between the two techniques in terms of control of local recurrence and survival.18
APBI with multicatheters may have advantages over other APBI techniques. When compared with the MammoSite balloon, brachytherapy catheters are cheaper, the radiation dose is less homogenous19 and it appears to interfere less with wound healing by not needing such a large space for insertion of the balloon, with the consequent risk of seroma, wound dehiscence and infection. With regards to intraoperative radiotherapy,9 the clear disadvantage of this type of partial irradiation is that it is performed without knowing the final result of the histopathological study. This can result in insufficient treatment if the resection margins were affected or the need for completing radiotherapy on lymphatic drainage areas if there was lymph node involvement. As for the finer techniques of external-beam radiation such as 3D-CRT10 and modulated intensity radiation therapy, these approaches are, a priori, very attractive since they are not invasive and theoretically administer a selective, homogeneous radiation dose with little toxicity for adjacent structures. The reality is that it requires very sophisticated linear accelerators to conform the radiation dose from multiple angles. This means that, to date, few patients with very small tumors in certain locations benefit from these techniques, and the published series are short.
Therefore, we can conclude that APBI is a feasible and safe technique, but it requires careful selection of the patients to be treated. Intraoperative placement of catheters has the advantage that the identification of the surgical bed is far more precise and direct. It is performed in a single surgical time that is just about 30min longer than conventional surgery. Its main advantage over conventional external-beam radiation therapy is the shortening of the radiotherapy period from 6 weeks to 1. Despite the short follow-up and the limited number of patients, our impression is that cosmetic results are somewhat better after breast conserving surgery and that it appears to have less toxicity than standard radiotherapy. APBI with multicatheters may also present further advantages over other techniques of accelerated partial irradiation due to its cost, simplicity and dosimetry.
Nonetheless, a greater number of patients and longer follow-ups are necessary, along with the results of the phase III multicenter studies that are underway, such as the NSABP B-39/ROTG0413 or GEC-ESTRO APBI,19 in order to be able to affirm that the results for local control of recurrence and survival are equivalent to standard radiotherapy.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Rodríguez-Spiteri Sagredo N, Martínez Regueira F, Olartecoechea Linaje B, Arredondo Chaves J, Cambeiro Vázquez M, Pina Insausti LJ, et al. Irradiación parcial acelerada con multicatéteres en la cirugía conservadora por cáncer de mama. Cir Esp. 2013;91:490–495.
Part of the information of this manuscript has been presented as a poster in the 16th Meeting of the Breast Pathology Group of the Spanish Association of Surgeons (Pamplona, May 2010) and at the 32nd Congress of the European Society of Surgical Oncology (Valencia, September 2011).