Complete resection with clear margins in locally advanced pelvic visceral tumours, primary or recurrent, occasionally requires total pelvic exenteration (TPE).
MethodsWe reviewed the results of EFA in 34 consecutive patients operated on between June 2006 and December 2013.
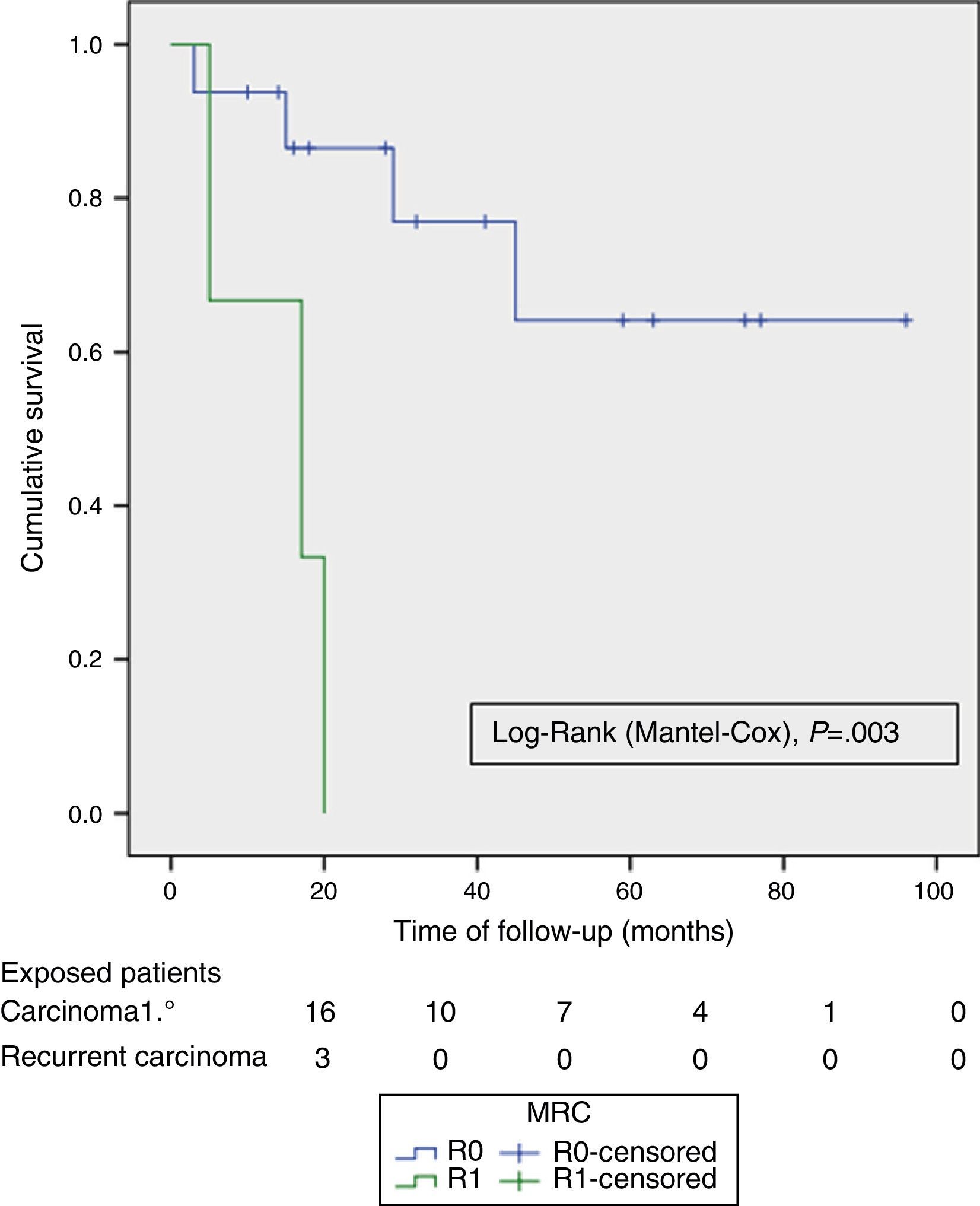
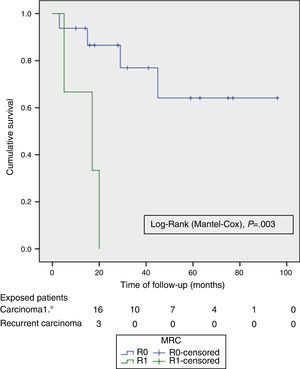
ResultsMedian age was 62 (40–82) years; 24 (70%) were male. The tumour origin most frequent was advanced primary rectal tumour (APRT), with 19 cases (55.9%) and most common type of exenteration was supraelevator (61.8%). R0 resection was achieved in 24 (70.6%) patients and in 16 (85%) of the APRT. Fifteen (79%) patients had pT4 APRT, and 4 (20%) pN +. Reconstruction of the bowel and bladder was performed with two stomas in 17 cases (50%), colorectal anastomosis and Bricker in 11 (32.3%) and wet double barrelled colostomy in 6 (17.6%). There was no postoperative mortality; 23 (67.5%) patients had complications, and 5 (14.6%) required a postoperative reoperation to solve them. Median follow-up was 23 (13–45) months. Overall survival (OS) and disease free survival (DFS) at 2 years were 67% and 58% respectively, and the median OS and DFS was 59 months (95% CI 26–110) and 39 months (95% CI 14–64), respectively. The DFS of R0 was significantly better (P=.003) than R1.
ConclusionsTPE is a potentially curative procedure for advanced pelvic visceral malignancies with similar morbi-mortality than other extended excisional surgery.
La resección completa con márgenes libres en los tumores viscerales pélvicos localmente avanzados, primarios o recurrentes, requiere ocasionalmente de una exenteración pélvica total (EPT).
MétodosRevisamos los resultados obtenidos con la EPT en 34 pacientes consecutivos operados entre junio de 2006 y diciembre de 2013.
ResultadosLa mediana de edad fue de 62 (40-82) años; 24 (70%) eran varones. El origen tumoral más frecuente fue el avanzado y primitivo de recto (TAPR), con 19 casos (55,9%) y el tipo de exenteración, la supraelevadora (61,8%). Se logró una resección R0 en 24 (70,6%) pacientes y en 16 (85%) de los TAPR. Quince (79%) pacientes con TAPR tenían pT4, y 4 (20%) pN+. La continuidad intestinal y urinaria se realizó con dos estomas en 17 casos (50%), Bricker y anastomosis colorectal en 11 (32,3%) y colostomía húmeda “double barreled” en 6 (17,6%). No hubo mortalidad postoperatoria; 23 (67,5%) pacientes tuvieron complicaciones y 5 (14,6%) requirieron una reoperación en el postoperatorio. La mediana de seguimiento fue de 23 (13-45) meses. La supervivencia global (SG) y libre de enfermedad (SLE) a los 2 años fueron del 67% y 58% respectivamente, y la mediana de SG y SLE fue de 59 meses (IC 95% 26 a 110) y de 39 meses (IC 95% 14 a 64), respectivamente. La SLE de las R0 fue significativamente mejor (p = 0,003) que las R1.
ConclusionesEPT es un procedimiento potencialmente curativo para las neoplasias viscerales avanzadas de la pelvis con una morbimortalidad similar a otras cirugías exeréticas mayores.
The term pelvic exenteration or evisceration refers to the complete en bloc resection of at least two contiguous organic structures from the pelvis as needed to obtain negative surgical margins in cases of advanced neoplasms of the pelvic organs. In total pelvic exenteration (TPE), all the organs in the true pelvis in men and women are removed. In women, exenteration may also be anterior (rectum-sparing) or posterior (bladder-sparing). The TPE and the posterior exenteration may be supralevator or infralevator, i.e., with or without preservation of the levator ani and the anorectal stump.1 Exenterations may extend to vascular, lymphatic, muscle and even osseous structures (composite pelvic resection/exenteration).2
TPE was described by Brunschwig in 1948 as a palliative procedure for the treatment of advanced cervical cancer.3 An improvement to that procedure involved the use of part of the ileum as urinary diversion, as described by Bricker in 1950. Later on, TPE was described as a treatment for advanced rectal cancer and, in 1981, its use for locoregional recurrence of rectal cancer was first published.4
Advanced primary rectal tumours (APRTs) account for 5%–20% of rectal cancers and, without treatment, median survival is less than 1 year, with a 5-year survival rate of only 5%.5 Even after a potentially curative resection, 2%–30% of rectal cancer patients experience a locoregional recurrence (LRR); in the absence of re-resection, this results in a mean survival of 7–8 months. Although chemoradiotherapy (CRT) may control or alleviate local symptoms for some time and prolong survival by 10–17 months, radical resection is the only curative option.5
The treatment of advanced cervical and endometrial cancer includes CRT; however, 25% of patients will experience non-metastatic local recurrence. The 5-year survival rate of patients with recurrent cervical cancer treated with TPE is 45% (25%–55%), which warrants this approach in well-selected cases.6 The long-term outcomes of TPE for other pelvic tumours (sarcomas, non-differentiated urological tumours, etc.) are the hardest to systematise due to their rare frequency.
TPE has been historically associated with a high post-operative morbidity and mortality. However, the latest published results indicate that TPE is an increasingly safe procedure thanks to advancements in imaging tests, a careful selection of patients, multidisciplinary involvement and improved surgical techniques and postoperative care.4 Nevertheless, there are few current TPE-related references in Spain.
The purpose of this paper was to analyse the morbidity–mortality and the overall survival and disease-free survival in our patients treated with TPE.
Materials and MethodsWe conducted a retrospective review of patients who underwent a TPE from June 2006 to December 2013, after a search for female (ICD-9 68.8) and male (ICD-9 57.71) pelvic evisceration codes in the hospital archive and database.
The medical records of patients undergoing TPE were reviewed and the following data were registered in a database: demographic variables, date of surgery, days of postoperative stay, diagnosis or type of tumour (colorectal, genitourinary or other, primary or recurrent), type of TPE (supra- or infralevator, extended or not to other organs or structures), UICC resection type (R0, R1, R2), form of reconstruction (urostomy and colostomy, wet colostomy or urostomy and colorectal/anal anastomosis), management of the residual pelvic cavity, findings and pathological staging, delivery of preoperative or postoperative radiochemotherapy, postoperative (Clavien–Dindo7) and late (beyond 30 days) complications and patient condition (disease-free, date of recurrence and/or death) by May 2014.
All these patients were assessed by the corresponding multidisciplinary tumour committees and, even though most surgeries were shared, the colorectal surgery division was in charge of all patients undergoing TPE.
We analysed the data with the SPSS 21.0 programme (SPSS, Chicago, Illinois, USA). Overall survival (OS) and disease-free survival (DFS) were calculated from the resection date to the date of death or lost to follow-up and the onset of first recurrence, respectively. The Kaplan–Meier method was used to analyse survival. The log-rank test was used for the univariate analysis of the survival curves. We considered P values <.05 as statistically significant differences.
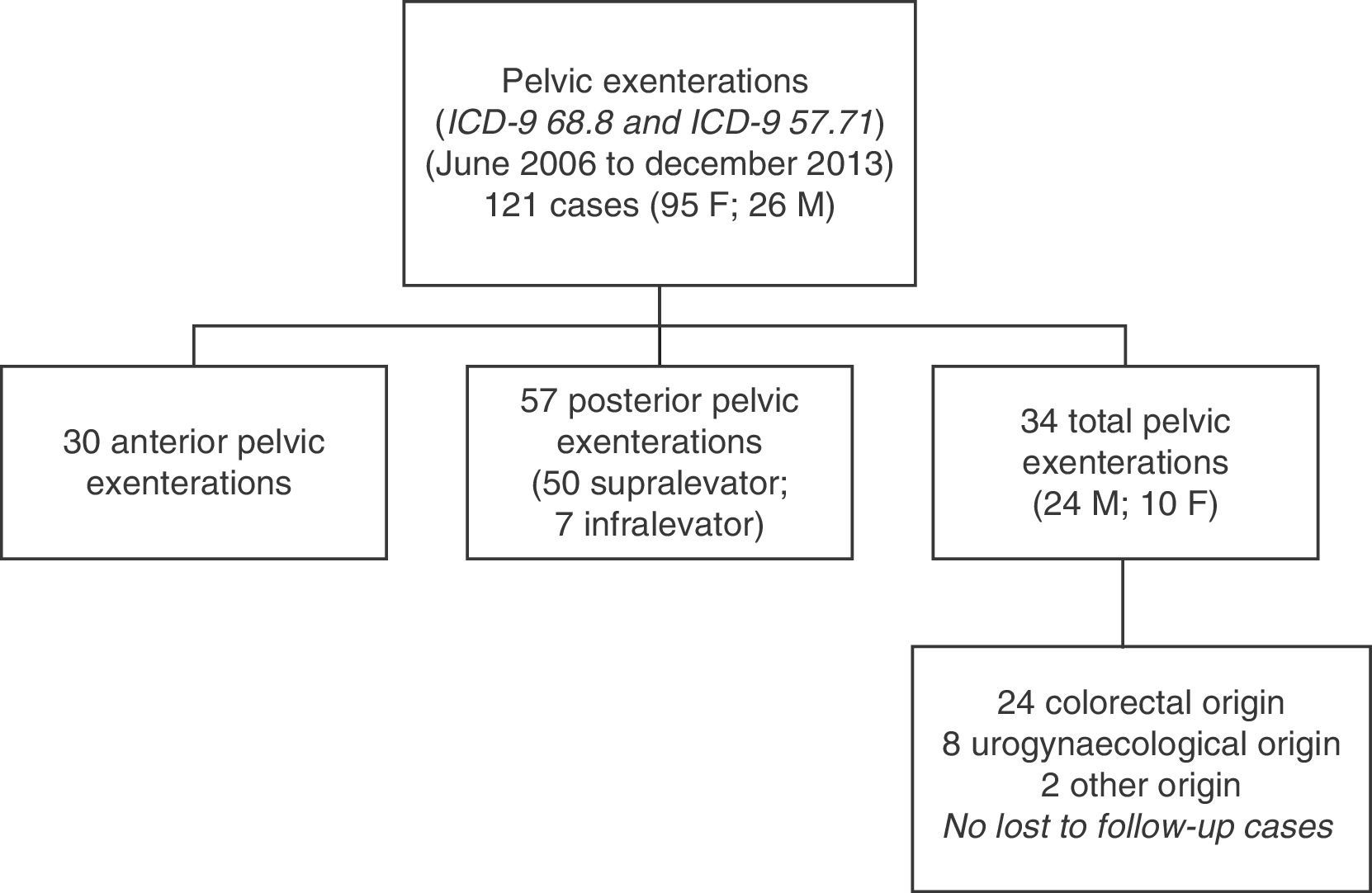
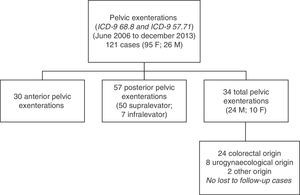
ResultsA total of 121 pelvic exenterations or eviscerations were performed between the indicated dates: 30 were anterior exenterations for urogynaecological disease, 57 were posterior exenterations for tumours in the rectum or the rectosigmoid junction with involvement of the uterus or vagina or vice versa, and 34 were TPEs, which account for the group of patients we analysed in this paper. Fig. 1 shows the population and location of tumours undergoing TPE with complete follow-up.
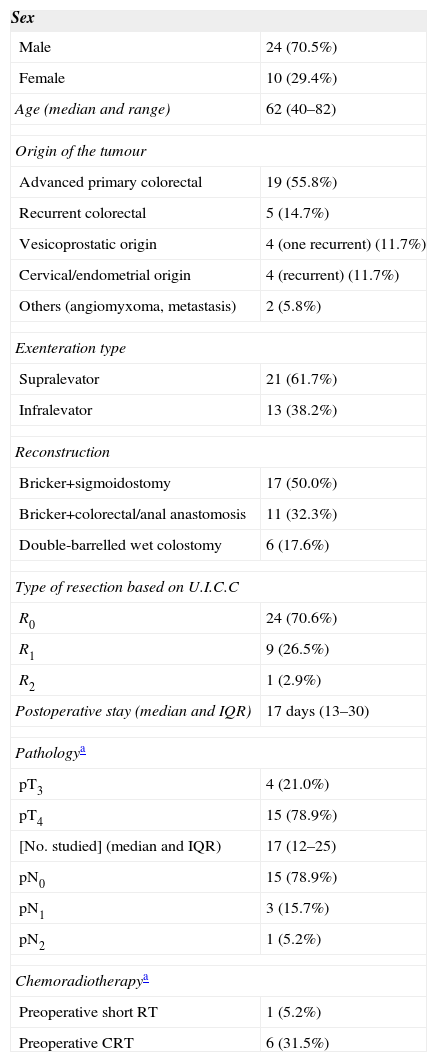
The demographic data and part of the clinical data analysed in our patients are shown in Table 1. As shown, the series is composed primarily of men; the median age was 62 years (range: 40–82) and the origin of tumours most frequently requiring TPE was APRT. All cancers of gynaecological origin were recurrent cervical or endometrial tumours.
Demographical and Clinical Data of Patients With Total Pelvic Exenteration.
| Sex | |
|---|---|
| Male | 24 (70.5%) |
| Female | 10 (29.4%) |
| Age (median and range) | 62 (40–82) |
| Origin of the tumour | |
| Advanced primary colorectal | 19 (55.8%) |
| Recurrent colorectal | 5 (14.7%) |
| Vesicoprostatic origin | 4 (one recurrent) (11.7%) |
| Cervical/endometrial origin | 4 (recurrent) (11.7%) |
| Others (angiomyxoma, metastasis) | 2 (5.8%) |
| Exenteration type | |
| Supralevator | 21 (61.7%) |
| Infralevator | 13 (38.2%) |
| Reconstruction | |
| Bricker+sigmoidostomy | 17 (50.0%) |
| Bricker+colorectal/anal anastomosis | 11 (32.3%) |
| Double-barrelled wet colostomy | 6 (17.6%) |
| Type of resection based on U.I.C.C | |
| R0 | 24 (70.6%) |
| R1 | 9 (26.5%) |
| R2 | 1 (2.9%) |
| Postoperative stay (median and IQR) | 17 days (13–30) |
| Pathologya | |
| pT3 | 4 (21.0%) |
| pT4 | 15 (78.9%) |
| [No. studied] (median and IQR) | 17 (12–25) |
| pN0 | 15 (78.9%) |
| pN1 | 3 (15.7%) |
| pN2 | 1 (5.2%) |
| Chemoradiotherapya | |
| Preoperative short RT | 1 (5.2%) |
| Preoperative CRT | 6 (31.5%) |
Most cases of TPE were supralevator. An R0 resection was achieved in 70% of the series (85% in APRTs); this required extending the monoblock resection and including loops of ileum, the cecum in three cases, the coccyx in one case and a vulvectomy and complete vaginectomy in two other cases.
Most frequently, the reconstruction of the urinary and digestive tracts was made with a double stoma (Bricker–Wallance [sic: Wallace] II and sigmoidostomy), followed by Bricker–Wallance [sic: Wallace] II and colorectal anastomosis (with protective stoma in three cases and without a diverting stoma in eight cases) and the least frequent one was the double-barrelled wet colostomy.
The management or treatment of the resulting pelvic cavity after a TPE was not homogeneous. Whenever possible, a pedicled omentum flap was used to fill the pelvis, either in isolation or combined with biological or absorbable meshes, and in three cases we decided to fill the voided pelvis with breast prosthesis. In five cases, a pedicled myocutaneous flap of the anterior abdominal rectal muscle was made for the reconstruction of the vagina or the pelviperineal wound resulting from the infralevator TPE.
Table 1 lists the most significant pathological findings referred to APRTs. Of the 19 cases, 15 (79%) had pT4, tumour infiltration of the structures included in the specimen, and in 4 (21%) cases the adhesion to the structures was of inflammatory or fibrous nature, without a true tumour invasion. Based on the WHO classification, there were mucinous adenocarcinomas in six cases and a signet ring cell adenocarcinoma in one case (patient with long-standing ulcerative colitis). A median of 17 adenopathies (IQR 12–25) was obtained in the specimens studied; in most of them there was no lymphatic tumour invasion. In the histological analysis of the specimens, three prostate cancers and one bladder cancer not previously diagnosed were incidentally found.
The use of CRT, even in cases of APRT, was not very uniform. Only seven (36%) tumours of colorectal origin included the preoperative use of long-term (with chemotherapy) or short-term radiotherapy due to the presence of extensive mucinous tumours, with genitourinary, perineal or vulvar fistulisation, or in the case of severely debilitated patients. Postoperative adjuvant radio- or chemotherapy was delivered in 26% of these cases.
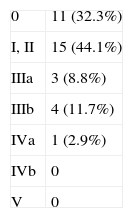
Table 2 shows the series’ postoperative morbidity pooled with the Clavien–Dindo classification.7 Mortality was null and 67.5% of patients had a complication, which resulted in a median hospital stay of 17 days (IQR 13–30). The most common complication was prolonged ileus. Five patients (14.6%) required a reoperation during the postoperative period for different reasons (dehiscence of the colorectal anastomosis, urinoma due to ureteroileal fistula, femoral–femoral bypass due to occlusion and incarcerated inguinal hernia). Five other patients (14.6%) had to be operated on due to complications arising during follow-up (nephrectomy after a complicated nephrostomy, removal of breast prosthesis due to persistent infection, and complications related to the preservation of anorectal stumps in the supralevator TPE).
Morbidity and Mortality in the Total Pelvic Exenteration Series, Grouped According to Clavien–Dindo Classification,7 as Well as the Number of Late Reoperations.
| 0 | 11 (32.3%) | |
| I, II | 15 (44.1%) | |
| IIIa | 3 (8.8%) | |
| IIIb | 4 (11.7%) | |
| IVa | 1 (2.9%) | |
| IVb | 0 | |
| V | 0 |
Late reoperations: 5 (14.6%).
With a median follow-up of 23 months (IQR 13–45) after a TPE, 21 (62%) patients are alive without evidence of disease, 12 (35%) have died of disease progression or intercurrent disease (one case) and one (3%) patient is alive with recurrent disease.
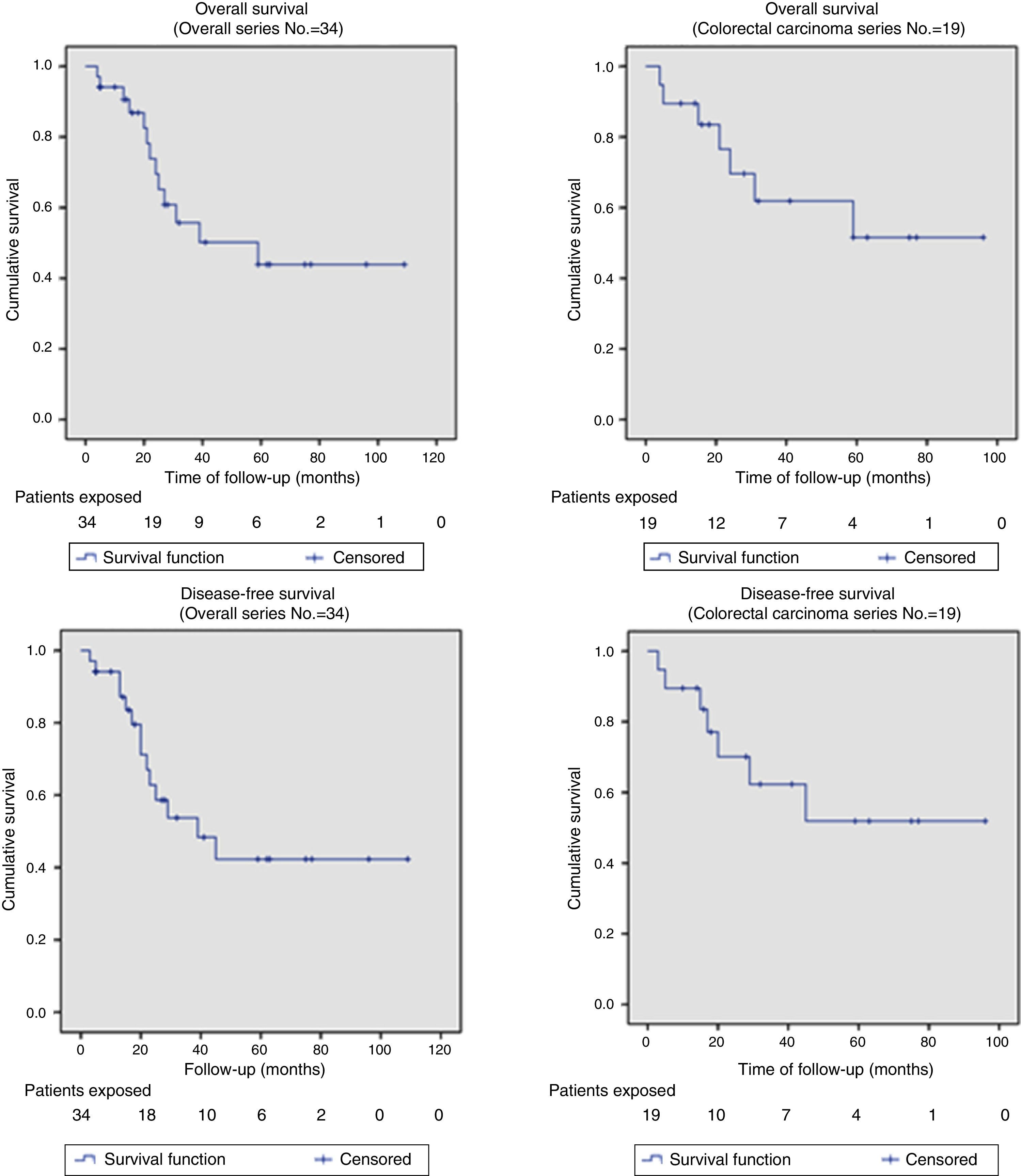
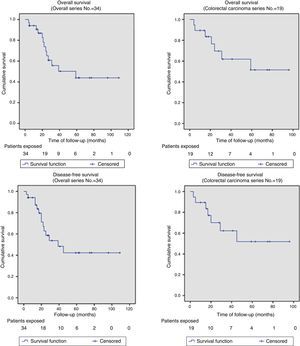
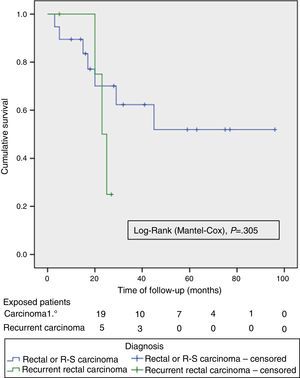
Out of the series’ total number of patients, the 2-year OS and DFS were 67% and 58%, respectively. The median OS and DFS in the series were 59 months (95% CI: 26–110) and 39 months (95% CI: 14–64), respectively. Given that the event (relapse or disease-related death) has not occurred in 50% of patients with APRT, the median OS and DFS cannot be calculated for this group (Fig. 2 shows that more than 50% were alive and free from disease at the end of the study); otherwise, the 2-year OS and DFS for this group of patients were 69.6% and 62.3%, respectively (Fig. 2).
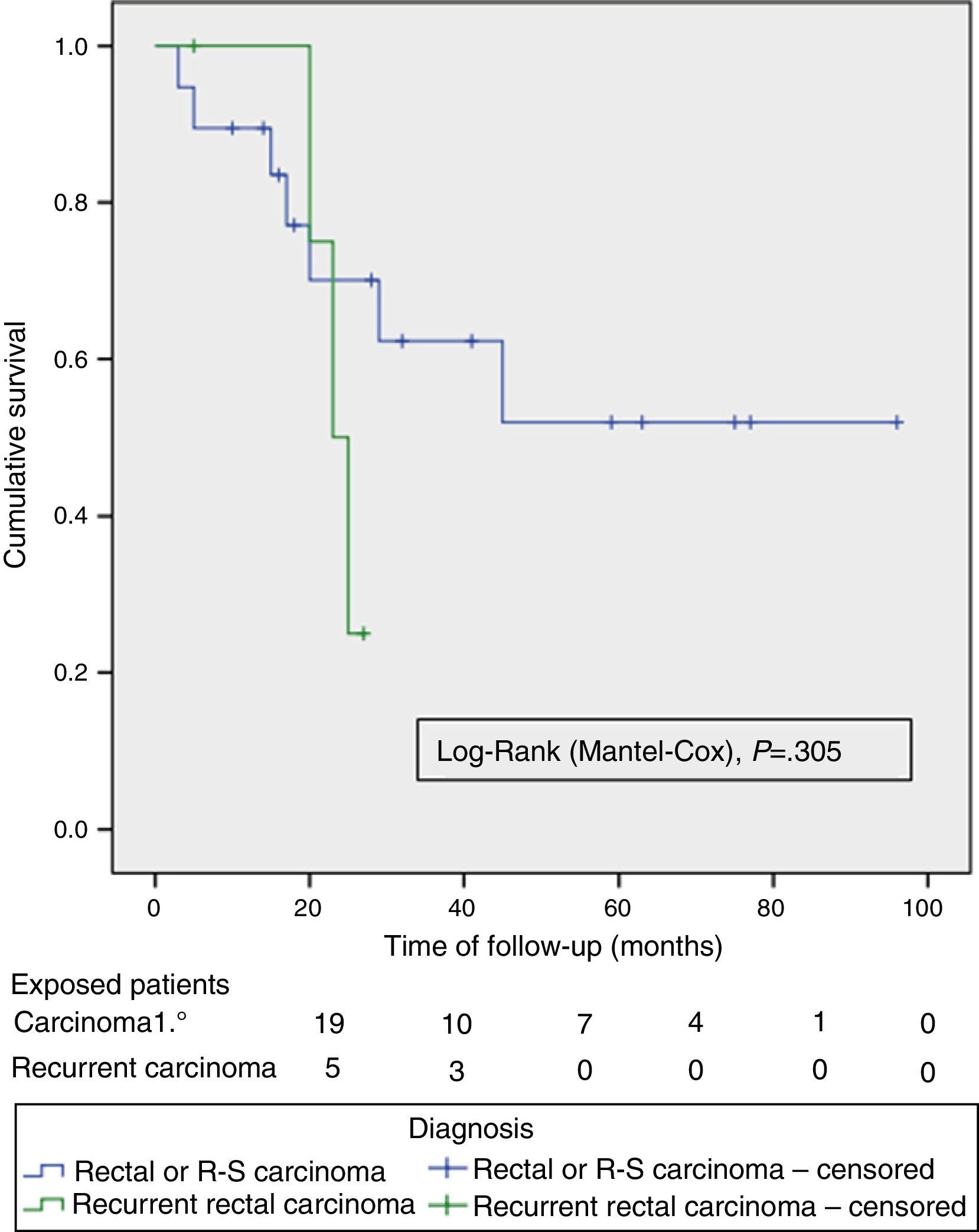
The OS and DFS curves were compared between APRTs and LRRs, and between R0/R1 resections by log-rank test. We only found a significant difference in the DFS between R0 and R1 resections. The small number of patients with recurrent tumours and R1 resections could explain these results (Figs. 3 and 4).
Just as in other contemporary series,4,8–10 we perform TPEs primarily to treat APRTs. A systematic review on TPEs for APRTs4 reports a 5-year survival of 52% (range 31%–77%) and a median survival of 35.5 months (range: 14–93 months). These figures are lower for TPE due to recurrent rectal cancer, with a 5-year survival of 18% (range: 0%–37%) and a median survival of 18 months (range: 8–38 months), indicating the worst prognosis for this subset of patients.
The preoperative imaging tests and the choice of cases allowed us to perform 70% of R0 resections in the overall series, and 85% in the case of APRTs, percentages similar to those from other recent publications.4,8–10 This provides evidence of the differences between performing a TPE for APRTs versus LRR with respect to a potentially curative resection with free margins. This translates into the significant DFS-related differences between R0 and R1 resections that we found.
On the other hand, 21% of the cases of advanced rectal cancer were staged as pT3, which implies that the adhesion to the genitourinary structures were of inflammatory or fibrous nature, not due to tumour infiltration. It should be noted that three of four patients with pT3 had received preoperative CRT. Even intraoperatively, the uncertainty remains between the risk of causing a tumour spread and the need of performing an extended resection. These findings are consistent with other publications that report 20%–56% of TPE specimens not infiltrated to the genitourinary organs.4,6,11 In the recurrent cases, the main causes of uncertainty derive from the absence of a clear plane in the pelvic lateral walls and the difficulty to differentiate the infiltration from the fibrous adhesion to the coccyx and sacrum. In these cases, the resection extended to anatomically well-defined osseous, vascular and nervous structures achieves an increase in R0 resections.11,12 On the other hand, CRT is not a real or valid option in cases of tumours in previously irradiated pelvises, extensive mucinous tumours, recto-genitourinary fistulas or fistulas outside the perineum or vulva, or severely debilitated patients. This derives from the data related to the fact that only 62% of our patients with APRT received CRT, a figure somewhat lower than in other series, although those series include both APRT and LRR.4,8–11
Only 20% of primary rectal tumours were pN1-2, indicating that these advanced tumours are comparatively less lymphotropic. The heavy presence of mucinous tumours (more expansive than infiltrative) in this series may produce a bias in this direction. Together with obesity and recurrent disease, lymph node involvement has been indicated as the main factor of poor prognosis for TPE in APRTs.8,9 Cervical or endometrial carcinomas have a high rate of lymphatic involvement, which justifies the systematic performance of at least a bilateral obturator lymphadenectomy, with a prognostic implication different from that of colorectal cancer.6
In the last century, the historical early postoperative mortality after TPE was 23%.3,4 Obviously, the advancements in many fields of medicine and surgery have contributed to diminish the morbidity and mortality. The systematic review of Yang et al., on series of TPEs between the year 2000 and 2012 indicates a median mortality of 2.2% (range: 0%–25%), and a morbidity of 57% (range: 37%–100%).4 Of our patients, 67.5% experienced a postoperative complication, 14.6% required a surgical reoperation during their admission and a further 14.6% required a reoperation for late complications. The null mortality and morbidity obtained are similar to those in other current series, with a similar hospital stay as substitute data for overall morbidity.6,8–10
The main source of postoperative morbidity lies in the urinary, vaginal and intestinal diversion or reconstruction and in the management of the resulting empty pelvic cavity.
Although intestinal and genitourinary reconstruction after a TPE is possible in some patients without the need of stomas,13 most patients do not have this possibility (due to resection of the membranous urethra or infralevator exenteration) or have a very fibrous or irradiated pelvis and require a double diversion. Typically, double diversions are performed with two separate stomas. The Bricker ileal conduit is the most widely used procedure as urinary diversion, but it is not free from problems. The incidence of ureteroileal stenosis, hydronephrosis, recurrent pyelonephritis or silent impaired renal function occurs in up to 20% of cases,4,6,13 requiring lifetime follow-up. An alternative is urinary and faecal diversion with the use of a single stoma by double-barrelled wet colostomy, a procedure that is technically different from the not-recommended traditional wet colostomy. With this procedure, the Bellvitge group has published one of the longest series in the literature, with satisfactory and comparable outcomes, in terms of urinary morbidity, to double stoma, Bricker and colostomy, separetely.14 Our experience with six cases is very limited to draw conclusions. The procedure is faster, avoids an ileo-ileal anastomosis and requires a single stoma, facilitating the use of an anterior rectal myocutaneous flap, if required, and may be more acceptable for sick patients; however, handling stomatherapy instruments is not so simple and, additionally, surveillance is required due to the possibility of ascending urinary tract infections.
The pelvis void of organs, and frequently irradiated, is associated with complications such as abscesses, bowel obstruction, intestinal fistula or dehiscence of perineal wounds. The contribution of vascularised tissue, such as omentoplasty or myocutaneous flaps, helps fill the empty cavity and reduce morbidity.15 In general, an omentoplasty is too small to fill the entire pelvis, so biological meshes or other kinds of meshes may be used simultaneously or alternatively to repair the pelvic floor.15 We used a breast prosthesis to fill the pelvis on three occasions and this may be a good option when postoperative radiotherapy is required16 or when a reconstruction of the bowel transit by colorectal anastomosis is required, preventing the small intestine from entering the pelvis.17
Even though 62% of the TPEs that we performed were supralevator, we only reconstructed the bowel transit in half of them, leaving an anorectal stump abandoned and stapled in the rest. This has been the main cause of late reoperations in the series, due to problems of dehiscence, continuous suppuration and fistulisation, and therefore, completing the anorectal excision seems more advisable in case of non-reconstruction, as also mentioned by other authors.18
Today, the morbidity–mortality pattern of TPE is similar to the one of other major surgeries; however, the disfiguring nature of the intervention and the need for a prolonged rehabilitation process give greater prominence to the assessment of the resulting quality of life. In this respect, two studies19,20 indicate that the quality of life of patients with prolonged disease-free survival undergoing TPE for APRT does not differ from that of patients treated with a standard rectal resection or from those who have not required a urinary diversion.
In summary, TPE is a potentially curative procedure for advanced visceral pelvic neoplasms with a currently acceptable morbidity–mortality profile. An adequate patient selection and a multidisciplinary approach are crucial to improve the outcomes.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: Carballo L, Enríquez-Navascués JM, Saralegui Y, Placer C, Timoteo A, Borda N, et al. Exenteración pélvica total en el tratamiento de las neoplasias avanzadas, primarias o recurrentes, de vísceras pélvicas. Cir Esp. 2015;93:174–180.