La nutrición y la salud cardiovascular: una mirada desde el presente hacia el futuro
Más datosCardiovascular disease (CVD) is an important challenge for clinicians, researchers and governments to reduce the impact on the global health burden and socioeconomic costs. Moreover, far from diminishing, cardiometabolic risk factors leading to CVD development are on the rise. In order to stop the CVD pandemic, it is not enough to merely attempt to control traditional risk factors. In this regard, chronobiology, the science that studies biological rhythms, has become an important field in research in the last years. Circadian disruption or chronodisruption, defined as a relevant disturbance of the internal temporal order of physiological circadian rhythms significantly increases the risk of CVD. In this article we review some of the evidence that has made chronobiology one of the most emerging scenarios to take into account in routine clinical practice in which a translation of all this evidence should be mandatory.
La enfermedad cardiovascular (ECV) es un desafío importante para los profesionales sanitarios, los investigadores y los gobiernos debido a que es necesario reducir su impacto en la carga de salud global y los costes socioeconómicos. Además, lejos de disminuir, los factores de riesgo cardiometabólicos que conducen a ECV siguen creciendo. Para detener la pandemia de ECV no basta con simplemente intentar controlar los factores de riesgo tradicionales. En este sentido, la cronobiología, ciencia que estudia los ritmos biológicos, se ha convertido en un importante campo de investigación en los últimos años. La disrupción circadiana o cronodisrupción, definida como una alteración del orden temporal interno de los ritmos circadianos fisiológicos, aumenta significativamente el riesgo de ECV. En este artículo revisamos algunas de las evidencias científicas que han hecho de la cronobiología uno de los escenarios emergentes a tener más en cuenta en la práctica clínica habitual, con el objetivo de realizar una traslación de esta evidencia al trabajo diario llevado a cabo en la prevención cardiovascular.
Cardiovascular diseases (CVD) are the most important cause of morbidity and mortality worldwide.1,2 This pathology is an important challenge for clinicians, researchers and governments to reduce the impact on the global health burden and socioeconomic costs. Moreover, far from diminishing, cardiometabolic risk factors leading to CVD development are on the rise.3–5 In this regard, an excellent example of this last statement is type 2 diabetes, which has attained the status of a global pandemic. Thus, the International Diabetes Federation (IDF) has recently published that 537 million adults live with diabetes worldwide, a rise of 16% (74 million) since the previous IDF estimates in 2019. In fact, one in ten (10.5%) adults around the world are currently living with diabetes and the total number is predicted to rise to 643 million (11.3%) by 2030 and to 783 million (12.2%) by 2045.
In order to stop the CVD pandemic, it is not enough to merely attempt to control traditional risk factors. Residual cardiovascular risk remains a problem even if reasonable control of the risk factors, such as low-density lipoprotein cholesterol, is achieved. Furthermore, despite the efforts of healthcare professionals, researchers and institutions, CVD control is not achieved and it continues to appear in patients who already had an event and seemed to have adequate metabolic control. Therefore, the search for new strategies for this disease is a priority in most cardiovascular research programs. In this regard, chronobiology, the science that studies biological rhythms, has become an important field in research in the last years. Circadian disruption or chronodisruption, defined as a relevant disturbance of the internal temporal order of physiological and behavioral circadian rhythms, either due to the influence of diet, sleep disorders, individual chronotype or shift work significantly increases the risk of metabolic disorders and CVD, but also of many pathologies as cancer, neurodegenerative diseases, and mental disorders.6,7
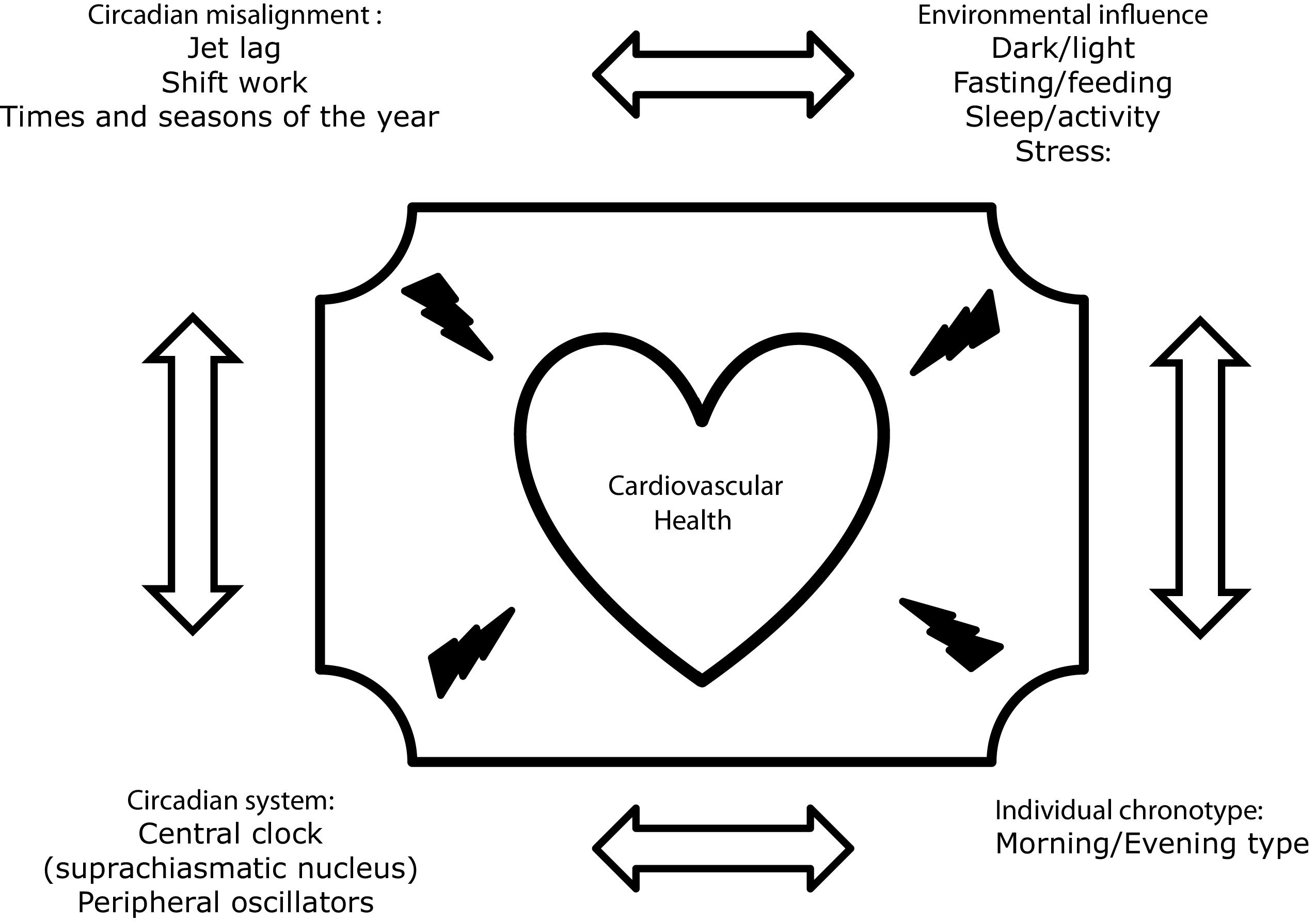
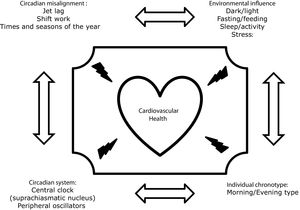
Chronodisruption can be induced by any impairment of the functioning of the inputs of the circadian system, such as alterations in the light–dark, eating–fasting and activity–rest cycles, Fig. 1. In this regard, it has been demonstrated that nutrition is one of the most important.8,9 In animal models, diets rich in fat produce deleterious effects on rodents’ circadian system organization by blunting feeding/fasting cycles. In addition, several studies performed on experimental animals have demonstrated that when animals eat at the “wrong time,” they become obese.10–12 In humans, there is also much evidence showing that the disruption of our circadian rhythms is related to metabolic abnormalities or that there are important interactions between our clock genes and our environment, which determines changes in our metabolism. Thus, our group demonstrated that chronic consumption of a healthy diet might play a contributing role in triggering glucose metabolism by interacting with the rs1801260 SNP at CLOCK gene locus in metabolic syndrome patients (MetS).13 On the same gene, we observed that chronic consumption of a low-fat diet improves cardiometabolic risk factors according to the CLOCK gene in patients with coronary heart disease.14 In the same context, we had demonstrated that a Period 2 genetic variant interacts with plasma saturated fatty acids to modify plasma lipid concentrations in adults with MetS.15
Following the evidence about chronobiology and nutrition, we also must take into account that although eating is fundamental to survival, the timing of eating can synchronize different organs and tissues that are related to physiological functions such as food digestion, absorption, or metabolism, such as the stomach, gut, brain, liver, pancreas, or adipose tissue. Studies performed in experimental animal models suggest that food intake is a major external synchronizer of peripheral clocks. Therefore, the timing of eating may be decisive in fat accumulation and mobilization and affect the effectiveness of weight loss treatments. In this setting, an elegant study evaluated the role of food timing in weight-loss effectiveness in a sample of 420 individuals who followed a 20-week weight-loss treatment. They found that late lunch eaters lost less weight and displayed a slower weight-loss rate during the 20 weeks of treatment than early eaters. Surprisingly, both groups were similar in energy intake, dietary composition, estimated energy expenditure, appetite hormones, and sleep duration. Nevertheless, late eaters were more evening types, had less energetic breakfasts and skipped breakfast more frequently than early eaters.16 Subsequently, the same group demonstrated that higher dietary consumption after waking up and lower consumption close to bedtime are associated with lower BMI, but the relationship differs by chronotype. Furthermore, the data demonstrate a clear relationship between the timing of carbohydrates and protein intake and obesity.17 Finally, it has been demonstrated that the timing of food intake is related to changes in salivary microbiota18 or changes in body fat composition.19
In the last years, it is has been known that an individuals’ preferred time of the day for an activity/rest cycle has a significant influence on the metabolic response. This is known as individual chronotype and individuals can be classified as a morning, intermediate, or evening type. A growing number of studies have examined the relationship between chronotype and general health.20 Thus, a recent prospective study of 385292 UK biobank participants studied the association of combined sleep behaviors and genetic susceptibility with CVD incidence. During a median of 8.5 years of follow-up, they documented 7280 incident CVD cases, including 4667 CHD and 2650 stroke cases. Nearly 10% of cardiovascular events in this cohort could be attributed to poor sleep patterns. Moreover, participants with poor sleep patterns and high genetic risk showed the highest risk of CHD and stroke.21 In line with these findings, a recent cross-sectional study evaluated associations of chronotype with overall cardiovascular health, health behaviors, and cardiometabolic risk factors among 506 women (mean age=37±16y, 62% racial/ethnic minority) in the American Heart Association Go Red for Women Strategically-Focused Research Network cohort at Columbia University (New York City, NY, USA). They found that evening chronotype is associated with poor cardiovascular health and adverse health behaviors.22 In other cohorts, the same findings were found. Thus, Garaulet et al. studied the potential associations between individual chronotype and cardiometabolic outcomes in young adults of two independent populations from Europe and America. They found that evening chronotypes have increased cardiometabolic risk and lipid alterations compared to neither nor morning chronotypes.23 The same group has demonstrated previously important interactions between clock genes and the individual chronotype.24,25 Finally, we have explored whether individual chronotypes were associated with cardiometabolic risk in patients in secondary prevention from the CORDIOPREV study. Metabolic Syndrome (MetS) was determined at baseline, and metabolic and inflammation markers were measured at baseline and yearly during the 4 years of follow-up. We found that evening types had higher triglycerides, C-reactive protein and homocysteine and lower HDL-C than morning-types. In addition, evening-types had a higher prevalence of MetS. Evening chronotypes were more sedentary, displayed less and delayed physical activity and ate and slept later. In addition, evening-types had lower amplitude, greater fragmentation, lower robustness and less stable circadian pattern, all related to a less healthy circadian pattern.26
Concluding remarks and future perspectivesIn routine clinical practice, no one has any doubt that lifestyle interventions are the initial strategies for the prevention and treatment of cardiovascular diseases. However, it is time to move beyond. The discovery of the molecular basis of the circadian rhythm by Jeffrey Hall, Michael Rosbash, and Michael Young was a critical knowledge to the field of medicine to such an extent that this finding was recognized by the Nobel Prize in Physiology or Medicine, 2017.27 They placed the study of chronobiology as a starting point in future intervention strategies on people's health. In the past, the clinical practice did not pay much attention to the circadian rhythm, which can synchronize different organs and tissues and significantly influence metabolic responses. Its disruption is related to numerous metabolic disorders as we have previously mentioned. Until now, diagnoses were made based on the physical examination, laboratory, or imaging tests, but all were done in cross-sectional time points. In the last years, as we discussed earlier, there has been increasing evidence about the influence of chronobiology on individual health, which should be considered (Table 1). Based on this evidence, new disciplines are emerging, such as chrononutrition in which not only is taken into account what is eaten but also when it is eaten, how often and with what regularity or the chronotherapy which has been shown to improve drug efficacy effectively and to reduce drug toxicity given that circadian changes in pharmacokinetics and pharmacodynamics (drug target) are two essential sources of time-varying drug effects.
Clinical evidence linking chronodisruption with cardiovascular health.
| Reference | Population | Conclusions |
|---|---|---|
| Garcia-Rios A, et al.13 | 475 MetS subjects participating in the CORDIOPREV clinical trial | Chronic consumption of a healthy diet may play a contributing role in triggering glucose metabolism by interacting with the rs1801260 SNP at CLOCK gene locus in MetS patients. |
| Garaulet M, et al.16 | 420 individuals who followed a 20-week weight-loss treatment | Timing of food intake predicts weight loss effectiveness. |
| Xiao Q, et al.17 | 872 middle-to-older-aged adults by six 24-h dietary recalls in 1 year | Higher dietary consumption after waking up and lower consumption close to bedtime associate with lower BMI, but the relationship differs by chronotype. Furthermore, the data demonstrate a clear relationship between the timing of carbohydrate and protein intake and obesity. |
| Fan M, et al.21 | Sleep patterns, genetic susceptibility, and incident CVD: a prospective study of 385292 UK biobank participants. | A healthy sleep pattern was associated with reduced risks of CVD, CHD, and stroke among participants with low, intermediate, or high genetic risk. |
| Makaren N, et al.22 | 506 women in the AHA Go Red for Women Strategically-Focused Research Network cohort at Columbia University | Evening chronotype was related to poor CVH, likely driven by its influence on health behaviors. |
| Aguilar-Galarza A, et al.23 | 2 223 young adults (18–29 years old), 525 from Spain (Europe) and 1 698 from Mexico (America) | Evening chronotype associates with increased triglyceride levels in young adults in two independent populations. |
| Romero-Cabrera JL, et al.26 | 857 patients in secondary prevention from the CORDIOPREV study. | Evening-types with CVD had higher cardiometabolic risk and less robust circadian-related rhythms than morning-types, regardless of the nutritional intervention. |
In the field of clinical chronobiology, there are still significant limitations. On the one hand, there are no standardized measurement methods in routine clinical practice. The development of wearable sensor devices and mathematical models for big data processing will aid in accurately quantifying circadian disruption. Such techniques are important in precision medicine to detect healthy lifestyles and diagnose and treat a variety of diseases. Strategies to restore circadian rhythm using wearable devices can lead to new therapeutic interventions. On the other hand, most human evidence was restricted to cross-sectional studies with a small, simple size and few longitudinal cohorts simultaneously collecting data on chronotype or individual preferences. Finally, there are very few studies with a nutritional intervention and long follow-up periods and very little evidence about the potential mechanisms of how the circadian system in daily rhythms influences metabolic response, which could provide translational evidence for the treatment or prevention of CVD. Therefore, delving into these mechanisms, how omics approaches (genomic, transcriptomic, proteomic, metabolomic) on specific cardiovascular functions (heart, vasculature or endothelial function) may help identify biomarkers of circadian disruption or sub-set susceptibility associated with cardiovascular risk and may offer in-depth mechanistic insights.
However, huge fortresses are being built around this new science. First of all, the evidence is and is growing. In addition, this evidence is growing in a field such as cardiovascular health, where traditional strategies are not succeeding in stopping the growing appearance of disease and the growing boom that exists in all the risk factors that lead to it, such as diabetes, obesity or metabolic syndrome. Second, although there is no standardization in the measurement methods, there are already more and more devices and an increasing population that uses them, which extract millions of data from an individual throughout the day on temperature, position, activity, sleep (rhythmicity and quality), heart rate or exercise/movement (as well as its intensity). It is not possible that in the era of big data, so much information cannot be used to extract clinical conclusions which can be used for personalized interventions as an example in nutrition. Therefore, continuous measurement of the regulatory factors such as light, temperature, melatonin, and mealtime can be used in conjunction with the rapid development of information and communication technology to treat many human diseases.
In conclusion, in addition to the traditional diet and exercise recommendations, it will be necessary to improve circadian alignment with the personalized behavioral and environment through optimal feeding choices and habits, sleep–wake habits, physical activity and exposure to light which could reduce the cardiovascular risk. In this regard, this is no time to wait for illness; it is necessary to anticipate it and predict it to prevent it from occurring, applying new individual intervention strategies that must be supported by the use of millions of physiological and chronobiological data collected from the patient, as well as using their preferences and work circumstances in order to adapt these individual circumstances in the health promotion and disease prevention.
Future remarks: In addition to the traditional diet and exercise recommendations, it will be necessary to improve circadian alignment with the personalized behavioral and environment through optimal feeding choices and habits, sleep–wake habits, physical activity and exposure to light which could reduce the cardiovascular risk.
Authors’ contributionAGR and JMO have contributed equally in the search for information, writing and development of the article.
Declarations inherent to the submission of the manuscript and verificationThe work described has not been previously published nor is it under evaluation for publication in any other journal. Its publication is authorized by all the authors and expressly or tacitly by the responsible authorities of the institution where the work was carried out.
FundingNone.
Conflict of interestNone.