This document is an update to the clinical practice recommendations for the management of cardiovascular risk factors in diabetes mellitus. The consensus is made by members of the Cardiovascular Risk Group of the Spanish Diabetes Society. We have proposed and updated interventions on lifestyle, pharmacological treatment indicated to achieve therapeutic objectives according to the levels of HbA1c, degree of obesity, hypertension, hyperlipidemia, heart failure, platelet antiagregation, renal insufficiency, and diabetes in the elderly, as well as new biomarkers of interest in the evaluation of cardiovascular risk in individuals with diabetes mellitus. The work is an update of the interventions and therapeutic objectives in addition, it is noted the need for the inclusion of specialists in Endocrinology, Metabolism and Nutrition in Cardiac Rehabilitation Units for the control and monitoring of this population.
El presente documento es una actualización de las recomendaciones de práctica clínica para el manejo de los factores de riesgo cardiovascular en la diabetes mellitus. Este consenso está elaborado por los miembros del Grupo de Riesgo Cardiovascular de la Sociedad Española de Diabetes (SED). Se han propuesto y actualizado las intervenciones sobre el estilo de vida, tratamiento farmacológico indicado para alcanzar los objetivos terapéuticos según los niveles de HbA1c, grado de obesidad, hipertensión arterial, hiperlipemia, insuficiencia cardiaca, antiagregación plaquetaria, insuficiencia renal y diabetes en el anciano, así como nuevos biomarcadores de interés en la evaluación del riesgo cardiovascular. El trabajo es una actualización de las intervenciones y objetivos terapéuticos; además, se señala la necesidad de la inclusión de los especialistas en Endocrinología, Metabolismo y Nutrición en las Unidades de Rehabilitación Cardiaca para el control y seguimiento de esta población.
Atherosclerotic cardiovascular disease (CVD) includes acute coronary syndrome (ACS), established chronic coronary heart disease (acute myocardial infarction [AMI], stable or unstable angina and coronary revascularisation), ischaemic stroke, transient ischaemic attack and peripheral arterial disease. CVD is the main cause of morbidity and mortality in individuals with diabetes, and is the disease which contributes most to the direct and indirect costs of diabetes.1,2 A cardiovascular risk factor (CVRF) is understood to be a biological characteristic, condition and/or lifestyle change which increases the medium- or long-term likelihood of suffering from, or of dying from any cause of a CVD in individuals who have a CVRF.3 To assess this risk, tables/equations of probabilities have been created based on prospective population studies. The following are considered classic CVRFs: age, gender, smoking, diabetes, total cholesterol, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C) and blood pressure (BP). Other so-called non-classical CVRFs (family history, obesity, fat distribution, triglyceride levels, stress and socio-economic level) may serve to modulate the calculated risk.4
In medicine, consensus documents/recommendations/clinical guidelines are important elements to direct prevention, diagnostic and treatment strategies for different diseases. They are a reference for professionals and medical societies, and they should be updated on a regular basis.1,5,6 The aim of recommendations is to provide consistency in routine clinical practice. In this study, we reviewed the most relevant aspects of the last two years regarding CVRFs in diabetes and, in accordance with regular practice,5,7–9 we updated the recommendations of the Diabetes and Cardiovascular Risk Working Group of the Sociedad Española de Diabetes (Spanish Diabetes Society, SED), primarily highlighting the new cardiovascular risk (CVR) classification of the population with diabetes, as well as the emergence of new treatments that help not only to improve HBA1c control, but also have a protective cardiovascular (CV) effect.1 The primary lipid target in prevention is the lowering of LDL-C with statins. These drugs continue to be the first choice for prevention of CVD and CV mortality in diabetic patients. The indications of the first biological drug with significant lipid-lowering potency on plasma LDL-C levels of lipoprotein a [Lp(a)] with raised HDL-C and reduced triglycerides with no alteration in glucose metabolism and no associated myopathy, are discussed. The use of statins in kidney failure is also updated. Finally, the importance of the Endocrinology and Nutrition specialist in Cardiac Rehabilitation Units is emphasised.
Lifestyle changeDietAn improved and adapted lifestyle in patients with diabetes is essential to help control the condition and to reduce their CV risk.1 However, such efforts should not delay pharmacological treatment, which should be started simultaneously and be adjusted based on the response to lifestyle change, always bearing in mind that lifestyle change is more cost-effective than pharmacological treatments. One of the most relevant studies, due to its design, duration and the population studied, was the Look AHEAD study which advocated the importance of taking action on lifestyle.10–12 Lifestyle change is important, especially in individuals with a higher body mass index (BMI), waist circumference and waist-to-hip ratio, due to its beneficial effect on insulin resistance, among other factors. Early detection of malnutrition and/or the risk of suffering from it, especially in elderly people with diabetes, are also important, due to the increased risk of presenting with sarcopenia and associated frailty.13,14 It is essential to determine the patient's calorie intake, and to recommend a diet which facilitates a loss of 5–10% of body weight. The Mediterranean diet should be considered as the paradigm of a cardioprotective diet and is therefore the recommended diet, particularly in our field.15 The Mediterranean diet is characterised by a high intake of fruit and vegetables, cereals and pulses, nuts and olive oil, all of optimal quality, moderate consumption of fish, poultry and eggs, and a low intake of dairy products, red meat, processed meat and sugar, in addition to a moderate consumption of wine during meals. In general, this has been shown to be effective not only in preventing CVD, but also in controlling complications associated with diabetes.16,17 The intake of fibre in the diet is important (14g/1000kcal) and of whole-grain foods (half of all grain intake), fibre between 30–45g/day. Advising against consumption of sugary drinks, reducing the intake of saturated fats to <7% of the total calories ingested and avoiding the intake of trans fats is recommended.18 Part of the saturated fat intake should be replaced by polyunsaturated and monounsaturated fatty acids, emphasising that what is important is not the quantity of fat consumed—as long as it is not greater than 35% of the total calorie intake—but its quality. Replacing saturated fat with monounsaturated fat, especially that derived from virgin olive oil, is recommended. Proper hydration is very important, with water and herbal teas representing the best sources of fluids, not forgetting that sweetened drinks “do not replace” water and should be limited.
The systematic supplementation of antioxidants (vitamins E and C, selenium, magnesium, chromium and carotenes, among others) is not recommended due to the lack of tests on its clinical efficacy and the concern about its long-term safety. However, it is important to plan meals (choice of foods and portions/exchanges) to include the daily recommended amount of all micronutrients. The glycaemic load and/or the glycaemic index of carbohydrates in the diet can be taken into account, and those with a high glycaemic index should be avoided.19,20 Controlling calorie intake and its distribution throughout the day to prevent weight gain and postprandial hyperglycaemia is crucial.
Given their possible interaction with drugs, it is becoming increasingly necessary to assess and determine different dietary products and functional foods, due to their exponential growth in the marketplace, the growing interest of the population for their consumption and commercial pressure. There is evidence that certain dietary products reduce the concentration of cholesterol, especially phytosterols (2g daily reduce LDL-C by 7–10%) or some of oriental inspiration, such as red yeast rice (which has a similar action to statins due to its monacolin K content.21–25 However, we know that phytosterols interact with ezetimibe, reducing its efficacy, and monacolin K interacts with statins, increasing their side effects.
Recommendations- •
Follow a heart-healthy diet.
- •
Follow a Mediterranean diet low in carbohydrates.
- •
Increase consumption of extra virgin olive oil and nuts.
The importance of physical exercise was already indicated in 1992 by the American Heart Association (AHA), which included physical inactivity as a CVRF, also associating it with an increased risk of diabetes mellitus (DM) type 2 (DM2).26,27 Nowadays, a sedentary lifestyle is considered one of the main CVRFs. Regular physical exercise increases glucose uptake, reduces the risk of DM2 and prevents hypertension (HTN). In fact, physical exercise is associated with a mean reduction of 6–7mmHg in the systolic and diastolic BP of hypertensive patients. Another of its benefits is increased HDL-C levels. Regular physical exercise is a very effective measure to prevent DM2 and to improve metabolic disorders, including lipid profile and insulin resistance.28,29
It is important to assess the type of activity and physical exercise, as well as the frequency, duration and intensity of the exercise, especially in elderly individuals.30,31 The risk of CV complications during exercise is extremely low (5–17 deaths/million/year, according to intensity of the exercise) in apparently healthy adults. Therefore, a preliminary cardiology exam would not be necessary in low-risk diabetic patients. A preliminary cardiology and ergometric exam would be appropriate in diabetics with a sedentary lifestyle who are intending to start a demanding training plan. It is recommended that patients with diabetes do at least 150min/week of aerobic physical activity of moderate intensity (50–70% of the maximum heart rate), spread over at least three days of the week, avoiding going for more than two consecutive days without activity.27–32 Diabetic patients with a history of unrecognised hypoglycaemia and previous episodes of severe hypoglycaemia, treated with insulin/oral sulphonylureas should be warned to monitor their capillary glycaemia not only before and during exercise, but also afterwards, in order to prevent and recognise delayed hypoglycaemia associated with physical activity.
Recommendations- •
Do regular aerobic physical exercise (>150min/week).
- •
Moderate aerobic physical activity (50–70% of maximum heart rate spread over at least three days of the week).
- •
Avoid more than two consecutive days without physical exercise.
In recent years, the link between diabetes and high rates of anxiety and depression has been revealed. This could adversely affect treatment adherence, as well as contribute to the development of complications secondary to the condition.32 Not only is it important to assess the mood, mental health and psychological well-being of the patient, but it is also important to understand the socio-cultural environment, which is becoming increasingly significant in the comprehensive assessment of diabetics. It is also important to be able to administer appropriate personalised treatment. Nowadays, socio-cultural level is considered to be another CVRF which is increasingly being attributed greater weight in the development of diabetes and CVR.
As well as being associated with greater CVR, psychosocial factors, low socio-economic level, social isolation, depression or hostility and occupational or family stress worsen the prognosis of patients with established coronary heart disease and significantly hinder the control of classic CVRFs.33–35 Lack of treatment adherence represents a significant barrier in the secondary prevention of CVD. The consequence of poor treatment adherence include an increase in severe CV complications, as a result of not achieving the therapeutic targets, with the consequent increase in mortality, reduction in quality of life of surviving patients, a greater care burden and an increase in health costs resulting from complications and hospital admissions. Reduced co-payment, automatic reminders, mail-order pharmacies, advice from a healthcare professional and fixed-dose combination therapies are measures which improve treatment adherence.35 The polypill for the secondary prevention of CVD is the first fixed-dose combination therapy approved in Europe as a substitution treatment for adult patients appropriately controlled by its individual ingredients administered separately at equivalent therapeutic doses.36–38
Recommendations- •
Analysis of the capacity to carry out activities of daily living.
- •
Assessment of the socio-cultural environment.
- •
Use of the polypill once treatment has been established.
As part of habits and lifestyle, it is becoming increasingly important to analyse the role of circadian rhythms, to determine the patient's sleep, as well as to assess and/or analyse their sleep. Currently, sleeping for at least 7h is recommended.1 It is important to rule out the existence of a sleep disorder, such as sleep apnoea and, when applicable, to treat it in order to improve the patient's quality of life and control the CVRFs.39,40
Recommendations- •
Seven hours of sleep a night.
Smoking is the modifiable CVRF with the most impact on CVD prevention. Patients with DM2 who smoke have a significant increase in total CVR, mortality, stroke and AMI compared to patients with DM2 who do not smoke.41,42 In clinical practice, we should advise all patients to stop smoking. Likewise, avoiding exposure to tobacco smoke (passive smoker) is advised as this also significantly increases the risk of CVD. If patients agree to try and stop smoking, they should be referred to specific tobacco addiction units and/or treatment should be prescribed for it as a routine component in the comprehensive care of diabetic patients. Modified-risk tobacco products are not a risk-free option in this strategy. Evidence on this hypothetically reduced risk is very limited.1 Although to a lesser extent than conventional cigarettes, the vapour from electronic cigarettes contains potentially toxic substances (generated by heat) which are by-products of the solvent released in the vapour and/or the trace constituents of the flavoured additives. There are studies which indicate a harmful vascular effect.43,44
Recommendations- •
Stop smoking.
Numerous studies have demonstrated that an increase in body fat leads to increased CVR. The relative risk of diabetes in males with a BMI of 35kg/m2 is 40 times higher than those with a BMI of 23kg/m2. Small weight loss of between 5 and 10% equates to better control of both clinical and metabolic parameters as well as psychological parameters, and all without the need for pharmacological support, only through changes in lifestyle and dietary modifications.45,46
Bariatric surgery (BS), as was already reported in 2009,2 may be considered in adults with DM2 and BMI≥35kg/m2, especially if DM2 or associated comorbidities are difficult to control with a heart-healthy lifestyle and/or drug treatment. It is essential to bear in mind, when assessing the costs, that individuals with DM2 who undergo BS need additional lifelong “lifestyle” change and continuous and close medical monitoring.47 BS has shown the almost or complete normalisation of blood sugar levels in 40–95% of patients with DM2, depending on the length of time they have had diabetes, the surgical procedure chosen, levels of C-peptide and the criteria used for referral.48–50 BS improves CVRFs and CV episodes in DM2 in the long-term, as well as the remission of DM.50–52 In patients with DM2 and a BMI of 30–35kg/m2, BS continues to present glycaemic benefits. As a result, it has recently been indicated that metabolic surgery should be considered in adults with DM2 and with a BMI between 30.0 and 34.9kg/m2. If hyperglycaemia is not properly controlled, despite adequate drug treatment with oral antidiabetic drugs or injectable antidiabetic drugs and insulin in monotherapy or in combination therapy,1 this indication could be assessed in patients who are class II overweight (BMI of 27–30kg/m2).
Recommendations- •
Overweight or obesity class I: diet and exercise, 5–10% loss.
- •
Drugs with indication in obesity and diabetes: metformin and association of GLP-1 with SGLT2 inhibitors (dapagliflozin approved).
- •
Bariatric surgery indicated in adults with DM2 and a BMI>35kg/m2.
- •
It can be assessed in obese class I individuals and class II overweight individuals with a BMI of 27–30kg/m2 with poorly controlled DM2 associated with CVRF.
HbA1c levels of ≥6.5% have been established as a diagnostic criterion of DM, provided that the diagnosis is carried out in a laboratory which uses a standardised method in accordance with the National Glycohemoglobin Standardization Program (NGSP), certified and standardised for the Diabetes Control and Complications Trial (DCCT), the International Diabetes Federation (IDF) and the European Association for the Study of Diabetes (EASD), and that the patient does not have anaemia or haemoglobinopathy which alters HbA1c levels.53,54
Several studies have confirmed the importance of glycaemic control in DM.55,56 We know that a 0.9% reduction of HbA1c reduces CV episodes by around 10–15%. The reduction of HbA1c to values close to 7% reduces micro- and macroangiopathic complications.55 The HbA1c target should be personalised based on factors such as age, life expectancy, comorbidity, duration of diabetes, risk of hypoglycaemia or the adverse consequences of hypoglycaemia, the patient's motivation and treatment adherence. An HbA1c level of ≤6.5% is considered optimal if it can be achieved in a safe and cost-effective manner; a reasonable goal would be HbA1c∼7%. Personalising the degree of glycaemic control is recommended; therefore, in young patients with no other CVRFs and with no complications, some stricter HbA1c targets should be considered, using more potent drugs to reach lower HbA1c levels (∼6.5%), provided that they are achieved without significant hypoglycaemic episodes or other adverse effects. In patients with CVD—especially elderly patients, a history of severe hypoglycaemia, limited life expectancy, advanced microvascular disease or macrovascular complications—with long-standing diabetes, the HbA1c target should be less demanding, between 7% and 8%, or even higher.1,57
Patients with DM type 1 (DM1) should be treated with intensive insulin therapy, either with multiple doses of insulin (3–4 daily doses) or with a continuous subcutaneous infusion of insulin, mainly with insulin analogues, which are associated with a lower risk of hypoglycaemia; the cost-effectiveness of these should be assessed in patients with DM2, bearing in mind the age and factors related to the risk and severity of the hypoglycaemia. The most recently introduced long-acting insulin analogues—glargine U300 and degludec U100 and U200—have more prolonged and stable pharmacokinetic and pharmacodynamic characteristics than glargine U100 and detemir.58 There is evidence that analogues (glargine and detemir) have a similar degree of control and number of hypoglycaemic episodes, and are associated with a lower rate of hypoglycaemic episodes than intermediate-acting NPH insulin. We should also point out that Glar U300 is associated with a lower rate of hypoglycaemia than Glar U100, and that the long-acting analogue degludec causes fewer hypoglycaemic episodes than the analogue Glar U100.58,59 In patients with DM2 with a high risk of CV events, degludec was not inferior to glargine in terms of the incidence of CV events.59
It must be borne in mind that premixed insulin provides less dosing flexibility and it has been associated with a higher frequency of hypoglycaemic events when compared with baseline and bolus-baseline regimens.1 It is necessary to optimise hypoglycaemia treatment in patients with DM1 and DM2 at an early stage to achieve CVD prevention in the mid-to-long term.
Until recently, metformin was the only oral hypoglycaemic agent in which an association with a lower risk of CVD had been proven. The results of the Empagliflozin Cardiovascular Event Trial (EMPA-REG) in patients with DM2 with high CVR demonstrated that the sodium–glucose co-transporter 2 (SGLT2) inhibitor, empagliflozin, is associated with a significant reduction in CV episodes.60–62 SGLT2 inhibitors block the sodium–glucose transporter in the proximal nephron, which increases renal glycosuria and reduces glycaemia and, therefore, HbA1c.63 They are also associated with weight loss (∼2kg) and a reduction in BP (around 2–4mmHg), without the risk of hypoglycaemia being increased. SGLT2 inhibitors have become a recent indication in high-risk patients with DM2 and established macrovascular complications; however, not all SGLT2 inhibitors have the same effects, nor is this indication listed.64–66 If SGLT2 inhibitors are reference drugs in patients with heart failure (HF) and DM2, the mechanisms of this cardioprotective effect are still to be clarified and their side effects must be controlled, especially the risk of ketoacidosis, as well as the increased risk of non-traumatic lower limb amputations associated with canagliflozin.66–68
Subsequently, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) study also demonstrated that patients with DM2 and high CVR may benefit from adding liraglutide to their regular treatment.68 The results are similar to those produced by empagliflozin in the EMPA-REG OUTCOME trial, but in the EMPA-REG trial the benefits were evident from the start of the clinical trial. Based on the CV results of the EMPA-REG OUTCOME and LEADER studies, assessing the use of these drugs in patients with a high CVR is recommended, even if metformin continues to be the initial drug of choice in patients with DM2.1
More recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) also showed the superiority of semaglutide versus placebo in the protection of CV episodes.69–72 Knowing the indications for drug interactions in the summary of product characteristics is recommended. Recently, the association of SGLT2 inhibitors (dapagliflozin) with the glucagon-like peptide 1 (GLP-1) receptor agonists has been accepted.73
In general, studies with saxagliptin (SAVOR-TIMI), sitagliptin (TECOS), lixisenatide (ELIXA), alogliptin (EXAMINE) and the recent Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL) indicate that the primary composite endpoint does not differ from that of the placebo group, confirming the non-CV inferiority of these treatments and their safety.74–80
RecommendationsAn HbA1c level of ≤6.5% is optimal if it can be achieved in a safe and cost-effective manner.An HbA1c level of 7–8%, or even > 8%, in patients with CVD, especially in elderly patients, those with a history of severe hypoglycaemia, limited life expectancy, advanced microvascular disease or macrovascular complications, with long-standing diabetes.
The first-line drug of choice is metformin; the use of SGLT2 inhibitors can be assessed, in particular empagliflozin, or GLP-1 inhibitors, liraglutide, in patients with established CVD and sub-optimal control as a second drug option and/or associated with metformin from the start.
Blood pressure: control of hypertension in DM2The European Guidelines on cardiovascular disease prevention81 remain the same with no relevant new data provided. Measuring the BP of diabetic patients at each visit is recommended. If elevated values are detected, the appropriate screening should be performed by means SBPM or ABPM. The control target should be personalised. In general, a systolic BP (SBP) of <140mmHg is advised. Lower SBP values, below 130mmHg, may be appropriate in younger individuals and in patients with microalbuminuria. The diastolic BP (DBP) target in diabetic patients is <90mmHg, although the limit is not well established, with 80–90mmHg being accepted according to age and associated comorbidities.
In patients with a confirmed BP of ≥140/90mmHg, in addition to lifestyle changes and weight loss if they are overweight, the Dietary Approaches to Stop Hypertension (DASH)82 is advised. This recommends reducing sodium to less than 2.3g/day (100mmol/day) and increasing potassium intake ≈4.7g/day (120mmol/day) provided there is no abnormal urinary potassium excretion. Potassium intake should be reduced to <4.7g/day (120mmol/day) to prevent adverse cardiac effects (arrhythmias) due to hyperkalaemia.83,84 Following a reduction in alcohol consumption and an increase in physical activity, drug therapy should be started if the BP targets are not achieved.
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, calcium channel blockers and thiazide diuretics are the preferred options for first-line treatment.83–85 The selection of medicines should be based on factors such as albuminuria, CVD, HF or post-myocardial infarction status, as well as the race/ethnicity of the patient, possible metabolic side effects, treatment adherence and the cost.
Patients with diabetes and HTN should be treated pharmacologically with an ACE inhibitor or an ARB, due to their greater protective effect against the onset or progression of nephropathy. ACE inhibitors and ARBs may delay the progression of nephropathy and diabetic retinopathy, which is why they are particularly indicated in patients with DM.86–88 In the event of intolerance to one of them, one may be replaced by the other. To reach the BP targets, the combination of two or more drugs at their maximum dose is generally required. It is important to administer one or more antihypertensive drugs before going to bed, in order to prevent nocturnal HTN, as nocturnal BP is a more powerful predictor of a CV episode than daytime BP.89 The use of an ACE inhibitor with an ARB in combination therapy is not recommended, in particular in patients with diabetic nephropathy, due to the risk of exacerbating kidney failure and facilitating hyperkalaemia. In cases in which it is considered necessary, it should be carried out under close monitoring of kidney function, electrolyte balance and BP. The combination of aliskiren with an ACE inhibitor or an ARB in patients with impaired kidney function or diabetes is contraindicated. Candesartan and valsartan are still authorised for the treatment of HF in combination with an ACE inhibitor only in patients who cannot use mineralocorticoid receptor antagonists.
If ACE inhibitors, ARBs or diuretics are used, kidney function and serum potassium levels must be monitored. When BP is >140/90mmHg, a diuretic may be added to the ACE inhibitors or ARBs and, if it remains elevated, the use of a calcium blocker should be evaluated. If poor control continues, consider adding a beta-blocker.
BP control and prevention of related morbidity and mortality can clearly be improved.1,90,91 However, the understanding, treatment and control of HTN are persistently low worldwide.92,93 One of the greatest challenges is to prevent therapeutic inertia (leaving diabetic patients with BP values ≥140/90mmHg), as this would result in an unacceptable burden in terms of human lives, sequelae and socio-economic costs. In patients with BP>140/90mmHg, as well as insisting on lifestyle changes, drug treatment should be started (it may be started with two drugs in a single presentation if >150/100mmHg) and readjusted if necessary, avoiding therapeutic inertia, in addition to lifestyle measures.1,94
Recently, the joint guideline of the American College of Cardiology (ACC), the AHA and related societies (Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. Hypertension 2017 November 13) modified the diagnostic criteria of HTN (>130 and/or 80mmHg) and control targets, and recommends lifestyle changes for all subjects above these values and antihypertensive drug treatment for values ≥140 and/or 90mmHg or levels ≥130 and/or 80mmHg if the CVR>10% in 10 years, according to the risk equation of this guideline. This risk estimate takes into account components such as diabetes. This means that diabetic patients would be candidates to receive drug treatment with BP levels ≥130 and/or 80mmHg.95 Pending the position of European societies involved in CVD prevention, this working group of the SED recommends maintaining the current European recommendations on CVD prevention.
Recommendations- •
BP<140/90mmHg.
- •
In the event of nephropathy: ACE inhibitor/ARB as first choice.
- •
Assess adding SGLT2 inhibitors as an oral hypoglycaemic agent.
Hypercholesterolaemia is a key pathogenic factor in the development and in the progression of vascular injury.95 It is important to ascertain the lipid profile of patients with DM2 at diagnosis and on an annual basis, in order to evaluate and suggest therapeutic targets more accurately.1 To improve the lipid profile of patients with diabetes, changing lifestyle, reducing the consumption of saturated fats, trans fats and cholesterol, increasing the consumption of omega-3 fatty acids (especially in individuals who do not eat fish regularly), viscous fibre and plant stanols/sterols, weight loss (if applicable) and increasing physical activity is recommended.96,97
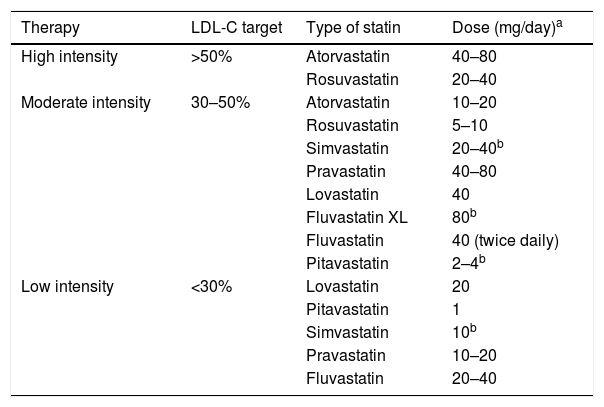
In the recommendations of the different National Cholesterol Education Program—Adult Treatment Panel (NCEP-ATP) reports, the therapeutic target was the LDL-C levels. Subsequently, the American College of Cardiology/American Heart Association (ACC/AHA) guideline, published in 2013,98,99 gave rise to multiple considerations with the different perspectives of several scientific societies due to the new paradigm of treatment with statins, abandoning the LDL-C therapeutic targets.100 The ACC/AHA guideline indicates that diabetics with LDL-C levels between 70 and 190mg/dl should start moderate-intensity statin therapy, basing it not on the LDL-C levels as a target, but on the tolerance to statins and the fixed-dose effect in accordance with the CVR (Table 1). Recently, the guideline issued by the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) on the management of dyslipidaemia and CVD prevention101 classified LDL-C levels in accordance with the CVR, based on the studies which indicate that the greatest CV benefit is obtained with the lowest LDL-C levels. The low incidence of coronary heart disease in subjects with low LDL-C values, subjects with genetic defects of the protein inhibited by ezetimibe (Niemann-Pick C1 Like 1 [NPC1L1] protein), together with the results of the IMPROVE-IT study support the indication of ezetimibe in combination with statins when these do not achieve the LDL-C target.101,102
Statin therapy according to the percentage reduction target of LDL-C.
| Therapy | LDL-C target | Type of statin | Dose (mg/day)a |
|---|---|---|---|
| High intensity | >50% | Atorvastatin | 40–80 |
| Rosuvastatin | 20–40 | ||
| Moderate intensity | 30–50% | Atorvastatin | 10–20 |
| Rosuvastatin | 5–10 | ||
| Simvastatin | 20–40b | ||
| Pravastatin | 40–80 | ||
| Lovastatin | 40 | ||
| Fluvastatin XL | 80b | ||
| Fluvastatin | 40 (twice daily) | ||
| Pitavastatin | 2–4b | ||
| Low intensity | <30% | Lovastatin | 20 |
| Pitavastatin | 1 | ||
| Simvastatin | 10b | ||
| Pravastatin | 10–20 | ||
| Fluvastatin | 20–40 |
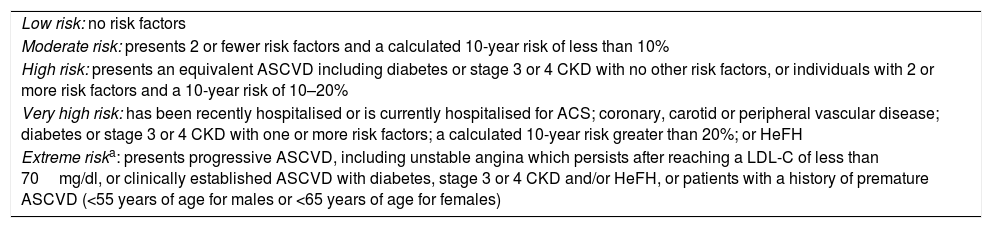
According to the AACE/ACE 2017, patients with DM may be classified as high risk, very high risk or extreme risk101 (Tables 2 and 3). Currently, DM is considered to be high risk.1 However, if a patient has one or more of the other classic CVRFs in addition to DM, the optimal LDL-C value must be <70mg as the patient is at very high risk. If, in addition to DM, the patient has clinically established CVD, then he or she is considered to be at extreme risk and must have LDL-C levels below 55mg/dl.101
New risk classification according to the American Association of Clinical Endocrinologists and the American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease, 2017.
| Low risk: no risk factors |
| Moderate risk: presents 2 or fewer risk factors and a calculated 10-year risk of less than 10% |
| High risk: presents an equivalent ASCVD including diabetes or stage 3 or 4 CKD with no other risk factors, or individuals with 2 or more risk factors and a 10-year risk of 10–20% |
| Very high risk: has been recently hospitalised or is currently hospitalised for ACS; coronary, carotid or peripheral vascular disease; diabetes or stage 3 or 4 CKD with one or more risk factors; a calculated 10-year risk greater than 20%; or HeFH |
| Extreme riska: presents progressive ASCVD, including unstable angina which persists after reaching a LDL-C of less than 70mg/dl, or clinically established ASCVD with diabetes, stage 3 or 4 CKD and/or HeFH, or patients with a history of premature ASCVD (<55 years of age for males or <65 years of age for females) |
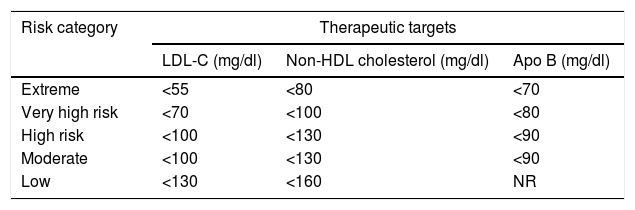
This new category was included based on evidence from clinical trials and was supported by meta-analyses which reveal that the additional reduction of LDL-C yields better outcomes in individuals with ACS. The IMPROVE-IT trial demonstrated that the lowest rates of cardiovascular events in patients with ACS were achieved when LDL-C levels were lowered to 53mg/dl using the combination of ezetimibe with statins
New therapeutic targets according to criteria from the American Association of Clinical Endocrinologists and the American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease, 2017.
| Risk category | Therapeutic targets | ||
|---|---|---|---|
| LDL-C (mg/dl) | Non-HDL cholesterol (mg/dl) | Apo B (mg/dl) | |
| Extreme | <55 | <80 | <70 |
| Very high risk | <70 | <100 | <80 |
| High risk | <100 | <130 | <90 |
| Moderate | <100 | <130 | <90 |
| Low | <130 | <160 | NR |
Statins continue to be the cornerstone of lipid-lowering therapy for the reduction of CV episodes,1,103–105 and their use is contraindicated in pregnancy. Following statins at the maximum tolerated dose, if the primary LDL-C target is not reached, then ezetimibe can be considered. Although recent studies indicate that statins confer a risk of developing diabetes,106,107 with the exception of pitavastatin, there is a modest increase in DM2 risk. This incidence increases with age, with the duration of lipid-lowering treatment and with the presence of metabolic syndrome components.1,106 Treatment with high-dose statins may be associated with a higher risk of DM than therapy with low or moderate doses. The JUPITER study in primary prevention revealed that the CV benefits and the benefits of reduced mortality after treatment with statins were greater than the risk of developing diabetes108,109; although most statins at high doses are associated with a greater incidence of diabetes and may minimally exacerbate glycaemic control, the effects on CVD prevention and CV mortality compensate for this.
In all patients with CVD, if the LDL-C targets are not met, ezetimibe should also be taken. In the event that the targets indicated with maximum tolerated doses of statins plus ezetimibe are not met, or if there is intolerance to statins, treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors should be contemplated. PCSK9 inhibitors are administered subcutaneously every 14 or 28 days, and evolocumab and alirocumab are currently available.110 PCSK9 inhibitors have an increased capacity for reducing LDL-C, which may be between 50% and more than 70%, regardless of whether it is used in monotherapy or as a combination therapy with statins or other lipid-lowering drugs.111,112 Furthermore, they have a low incidence of adverse effects, reduce triglycerides and lipoprotein(a) levels and raise HDL-C levels. In addition to reducing CVR, they have reversed atheromatous plaque.111–115 At present, the funded use of PCSK9 inhibitors in patients with diabetes would be contemplated only in patients with established CVD (coronary heart disease, ischaemic stroke and peripheral arterial disease) whose LDL-C is greater than 100mg/dl despite maximum tolerated doses of statins and ezetimibe.
The remaining cholesterol, determined as total cholesterol—(HDL-C+LDL-C), is considered to be one of the main risk factors of atherosclerosis and CV episodes and an indirect marker of hypertriglyceridaemia. Fibrates may help to improve elevated triglyceride levels, and a protective role of diabetic retinopathy and of other microangiopathic complications has been indicated.116 However, we should not forget that the primary target of CVD prevention is LDL-C, and that the evidence of a potential CV benefit from treatment with fibrates after statins comes from the post hoc analysis of randomised studies.117 There are studies in progress to directly test this hypothesis. When a patient with DM2 requires combination therapy of a statin combined with a fibrate to reduce the residual risk attributable to atherogenic dyslipidaemia, the only fibrate recommended is fenofibrate. Gemfibrozil is contraindicated.118–120
Recommendations- •
First therapeutic option: statins (see Table 4).
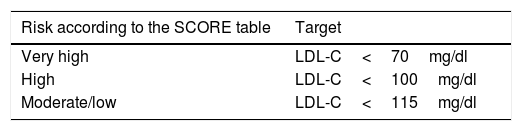
Table 4.LDL cholesterol (LDL-C) therapeutic target of statin treatment according to cardiovascular risk in the diabetic population.
Risk according to the SCORE table Target Very high LDL-C<70mg/dl High LDL-C<100mg/dl Moderate/low LDL-C<115mg/dl Diabetics with LDL-C 70–190: start moderate-intensity therapy.
High-intensity statin therapy should be started depending on the cardiovascular risk determined by the SCORE table and calibrated in our REGICOR tool.
In individuals with type 2 diabetes aged <40 or >75 years, assess the risk-benefit and the patient's preferences.
- •
If targets are not achieved, add ezetimibe.
- •
In case of intolerance to statins and/or not reaching targets with statins at maximum tolerated dose and ezetimibe, start treatment with PCSK9 inhibitors.
Both in DM1 and in DM2 with high CVR (>10% at 10 years), the benefit of treatment with acetylsalicylic acid (ASA) (75–162mg/day) as a primary prevention strategy is not clearly demonstrated. It is recommended in diabetic males >50 years of age or females >60 years of age who have at least one major CVRF, such as a family history of CVD, HTN, smoking, dyslipidaemia or microalbuminuria. ASA should not be recommended for primary prevention of CVD in adults with diabetes and a low risk of CVD (10-year risk<5%), such as males <50 years of age and females <60 years of age with no other CVRFs, as the potential effects of bleeding probably offset the benefit.121–123 The use of ASA in secondary prevention (75–162mg/day) is advised.1
For patients with CVD and a documented allergy to ASA, clopidogrel (75mg/day) should be used. Clinical practice guidelines recommend dual antiplatelet therapy for a period of one year for patients with ACS who have suffered an AMI.124,125 Once this period of time has elapsed, the patient continues the treatment with only one antiplatelet agent, normally ASA. Up to one year after an ACS, combination therapy of ASA (75–162mg/day) and clopidogrel (75mg/day) is reasonable. It is recommended to use a P2Y12 receptor inhibitor for one year for patients with DM and ACS, and, for those subjected to a percutaneous coronary intervention (PCI), the duration depends on the type of stent. For patients with a PCI due to ACS, prasugrel or ticagrelor should preferably be administered.125–128
In patients with known CVD, treatment with an ACE inhibitor, statins (if they are not contraindicated) to reduce the risk of CV episodes and ASA must be taken into account. In patients with a previous AMI, beta-blockers should be continued for at least two years after the acute episode. The use of beta-blockers after the infarction is very important to prevent sudden death.129 The use of ACE inhibitors and beta-blockers is recommended for at least two years after an AMI. Currently, heart surgery should be the therapeutic option for the majority of individuals with DM2 with a known multi-vessel disease.130,131 In DM with stabilised HF, metformin may be used if kidney function is >30ml/min/1.73m2, but it should be withdrawn in unstable or hospitalised patients,1 and, if there is no contraindication, the use of selective SGLT2 inhibitors should be evaluated due to their beneficial effects on CVR and on CV mortality.132
Recommendations- •
Acetylsalicylic acid (ASA) in primary prevention: low doses (100mg/day) if CVR>10% at 10 years.
- •
ASA in secondary prevention: low doses (100mg/day).
- •
The use of ACE inhibitors and the use of beta-blockers is recommended for at least two years after an AMI.
- •
Selective SGLT2 inhibitors due to their beneficial effects on CVR and on CV mortality.
Diabetic nephropathy is a kidney condition which occurs as a result of poor chronic control of the disease, and microalbuminuria (albumin: creatinine ratio >30mg/g) is its initial clinical manifestation (incipient nephropathy). The stage of the nephropathy is not only related to its progression, but also to the patients’ CV risk. Therefore, the early detection of patients with chronic kidney disease (CKD) and its treatment are important as they reduce CV morbidity and the speed of progression of kidney disease, thereby lowering costs for the healthcare system.133,134 Diabetic nephropathy is very often accompanied by HTN and reduced kidney function.87
Metformin is the first indication in diabetics, and, in recent years, the experience in its use has increased considerably in such a way that, based on observational studies, its use is considered reasonably safe in patients with a glomerular filtration rate (GFR) greater than 30ml/min/1.73m2. Its use in patients with GFR<30ml/min continues to be contraindicated due to the risk of lactic acidosis. The GFR should be assessed before the start of treatment and at least once a year. When a fixed-dose combination of drugs which contain metformin is used in patients with reduced kidney function, the restrictions and the efficacy with regard to the other active substance used in the combination should also be considered; in general, it is not recommended in patients with moderate kidney failure.
Regarding glinides, repaglinide is the secretagogue that has traditionally been indicated in CKD and it may be used in dialysis. DPP-4 inhibitors have shown beneficial and safe effects in glycaemic control for diabetic patients with CKD without causing any additional adverse effect, although, with the exception of linagliptin, they require dosage adjustments according to the degree of CKD.134–138 Pioglitazone can also be used in patients with CKD who do not have any contraindication to the drug; attention should be paid to possible salt and water retention, which may be a drawback in advanced stages of the disease.134 GLP-1 receptor agonists may be used with no alterations of the dose in category G2, G3a or G3b CKD. However, they are all contraindicated in categories G4 and G5 (estimated GFR below 30ml/min/1.73m2).139
Although BP should be kept at values below 140/90mmHg, BP below 130/80mmHg is recommended when albuminuria is present (albumin: creatinine ratio [ACR] >30mg/g). Treatment should be personalised in order to prevent symptoms of hypotension. Albuminuria should be reduced to <300mg/g by using renin–angiotensin aldosterone system inhibitors (ACE inhibitors, ARBs), with microalbuminuria being considered an independent CVR marker, as well as a marker of diabetic nephropathy, in order to slow down the progression of the nephropathy.137 Haemoglobin levels should be kept at 11g/dl or higher, using erythropoietin to control anaemia.139
In general, in primary prevention it is recommended to treat patients with CKD (categories G3–G5 not on dialysis) with statins or a statin plus ezetimibe. As it has been demonstrated that the pharmacological reduction of hyperlipidaemia safely reduces CV episodes in CKD,140–143 the KDIGO guidelines recommend a moderate-intensity therapy, with a fixed dose and with no additional measures. In order to personalise treatment, it is important to remember that there are statins which do not require adjustment, such as atorvastatin, at any stage of kidney failure. For stage 4–5 kidney failure, fluvastatin (dose to be used between 20–40mg), lovastatin (10–20mg), pravastatin (10–20mg), simvastatin (5–20mg) and pitavastatin (1–2mg) should be considered. In stage 3, the dose of rosuvastatin to be used is 5–20mg/day, and, in stage 4–5, the maximum dose of rosuvastatin is 10mg.8,134,136
Ezetimibe does not require a dosage adjustment, and its therapeutic efficacy has been proven. In case of intolerance to statins and/or not reaching targets with the maximum tolerated dose, PCSK9 inhibitors do not require adjustment in moderate–severe CKD. The KDIGO guidelines recommend statins in these patients, but, given the risk of interactions, they suggest using low doses and increasing them cautiously, especially when ciclosporin is administered. If switching from tacrolimus to ciclosporin, the statin dose should be reduced.
The treatment of hypertriglyceridaemia in CKD should be based on lifestyle changes. Fibrates are not recommended to reduce CVR and their use is advised against if the glomerular filtration rate is <15ml/min.134 Fibrates and omega-3 fatty acids could be considered in patients with significantly elevated fasting triglyceride levels (>500mg/dl) to prevent the risk of pancreatitis.144,145 Regarding omega-3 fatty acids, there is evidence that they reduce triglyceride levels, but not CV episodes and mortality. In general, omega-3 fatty acids are safe, although they can increase bleeding in subjects treated with ASA/clopidogrel. If triglycerides are not controlled with statins or fibrates, omega-3 can be added. This is a safe and well-tolerated combination. Lastly, it is important to note that the KDIGO guidelines recommend the use of antiplatelet agents for CKD.142,144
Recommendations- •
Metformin is considered reasonably safe in patients with a GFR greater than 30ml/min/1.73m2.
- •
In general, in primary prevention it is recommended to treat patients with CKD (categories G3–G5, not on dialysis) with statins or a statin plus ezetimibe.
- •
BP at values below 140/90mmHg; BP below 130/80mmHg is recommended when albuminuria is present.
- •
Fibrates and omega-3 fatty acids with significantly elevated fasting triglyceride levels (>500mg/dl).
- •
Fibrates are not recommended to reduce CVR and their use is advised against if the glomerular filtration rate is <15ml/min.
- •
Antiplatelet agents for CKD.
Diabetes in the elderly tends to follow an asymptomatic course and clinical manifestation in the elderly is often insidious and atypical. The characteristics of elderly patients with diabetes present certain particularities, such as increased clinical heterogeneity, cognitive impairment, depression or falls and a greater risk of morbidity and mortality, which determines the diagnosis and the approach to the disease. Elderly people with diabetes have a greater comorbidity burden than non-diabetics, and they also present a higher risk of depression and functional incapacity.146–148 In patients with DM aged over 65 years, annual screening should be carried out for the early detection of mild cognitive impairment or dementia.
There is no agreement among the different guidelines regarding the HbA1c target in elderly patients, with HbA1c levels ranging between 7% and 9%.146 The European Diabetes Working Party for Older People 2011149 recommends an HbA1c target of 7–7.5% for elderly patients with no complications and 7.6–8.5% for frail elderly patients. Recently, the ADA/EASD recommended that the glycaemic targets be less ambitious in elderly people with a short life expectancy, increased morbidity, on multiple medications or at high risk of hypoglycaemic episodes, considering HbA1c levels of between 7.6% and 8.5% to be acceptable.146 Nutritional assessment is recommended in order to rule out the risk of malnutrition which promotes the development of sarcopenia, a condition which is associated with functional impairment, a risk of falls and institutionalisation in elderly people, with special attention given to elderly patients with DM due to the risk of hypoglycaemia and falls.
Metformin in elderly patients continues to be the first therapeutic option, and sulphonylureas are inexpensive drugs with extensive experience in their use. Their greatest drawback is hypoglycaemic episodes, especially with glibenclamide, whose use is advised against in elderly people. If the indication of sulphonylureas is specified, the use of gliclazide or glimepiride is preferable.150 DPP-4 inhibitors are well-tolerated and do not promote hypoglycaemia in risk patients. Some authors indicate them as a second option. As mentioned previously, insulin analogues have less risk of hypoglycaemia than human insulin. SGLT2 inhibitors are indicated in high-risk patients with DM2 and HF, but they are not recommended in elderly patients with advanced kidney disease, and GLP-1 receptor agonists are not recommended in advanced kidney failure.
Overall control of CVRFs is important in elderly patients with diabetes. In fact, this elicits a greater reduction of morbidity and mortality than glycaemic control itself. Anti-hypertensive treatment is beneficial, even in very elderly individuals. In frail elderly people, a BP of 150/90mmHg is accepted. Levels <120/70mmHg should be avoided.151–153 The weight of evidence in favour of the use of statins increases in the same way as it does for younger patients in secondary prevention. In elderly patients, antiplatelet agents are indicated in secondary prevention, while in primary prevention their use is more controversial and should be personalised. In summary, the ADA recommendations for older adults with diabetes are consistent with the guidelines developed by the American Geriatrics Society (AGS). Both organisations recommend that functional and cognitively intact older adults who have a significant life expectancy receive similar CVD risk-reduction strategies as younger adults.1
Recommendations- •
HbA1c target in elderly patients: HbA1c levels range between 7% and 9%.
- •
In frail elderly people, a BP of 150/90mmHg is accepted. Levels <120/70mmHg should be avoided.
- •
Functional and cognitively intact older adults who have a significant life expectancy should receive similar CVD risk-reduction strategies to those of younger adults.
DM and coronary heart disease are the most significant risk factors in triggering HF. In patients with DM, the most common independent risk factors of developing HF are coronary heart disease and HTN. The incidence of HF is 2.5 times higher in diabetic patients than in the general population. The presence of HF in the diabetic population has a worse prognosis and doubles the risk of needing to be hospitalised or of dying from HF compared to the general population.
It has been highlighted that for each 1% reduction in HbA1c, the risk of developing HF decreases by 16%. Recently, three categories of HF have been established based on the left ventricular ejection fraction (LVEF): “reduced EF”, LVEF<40%; “moderate EF”, LVEF 40–49%, and “preserved EF”, LVEF≥50%. To prevent and/or delay HF, as well as to improve survival in diabetics with HF, treatment of HTN, use of statins in patients with a high risk of coronary heart disease, use of ACE inhibitors in patients with asymptomatic LV dysfunction and of beta-blockers in asymptomatic LV dysfunction and a history of AMI is recommended.154 HF treatment has traditionally been lifestyle change and, as a drug treatment of HF with reduced ejection fraction (HFrEF), ACE inhibitors or ARBs are used. If the former are not tolerated, beta-blockers and mineralocorticoid receptor antagonists are used, provided that there is no contraindication. If the patient remains symptomatic, replacing the ACE inhibitor with sacubitril/valsartan is recommended.154 Diuretics are used to improve symptoms and to improve exercise capacity, and also if there are signs and/or symptoms of HF. Promising data for better management of diabetes with oral hypoglycaemic agents have recently been published; SGLT2 inhibitors are the first class of agents to improve CV mortality and the outcomes of HF in diabetics with and without established HF.155 GLP-1 analogues, such as liraglutide and semaglutide, have shown a reduction in CV events without altering hospitalisation. Furthermore, the differential effect of each DPP-4 inhibitor on the risk of HF should be assessed.156–159
Recommendations- •
SGLT2 inhibitors are the first class of agents to improve CV and HF mortality in diabetics with and without established HF.
- •
The differential effect of each DPP-4 inhibitor and the GLP-1 analogues should be assessed.
In light of the above, and given the increasing complexity of diagnosing and therapeutically assessing the metabolic disorders which make up the pathogenic basis of CVD, the inclusion of Endocrinology, Metabolism and Nutrition specialists in cardiac rehabilitation units and vascular disease units is fundamental for the monitoring of patients with DM and coronary heart disease, as well as other related macrovascular diseases. The comprehensive treatment of diabetes was revealed by the STENO-2 strategy, which emphasised the importance of multifactorial intervention in DM2 to achieve a marked and lasting reduction in associated morbidity and mortality. Since then, the approach to diabetes has been multifactorial, highlighting the role of this multidisciplinary approach in terms of better CVD prevention in patients with diabetes.56 The participation of the Endocrinology specialist, with special expertise in the metabolism of hydrocarbons, lipids and nutrition, dietary education, weight loss, physical exercise, smoking, reduction of HbA1c levels, choice of treatment for HTN, obesity, study of hydrocarbon, lipid and protein metabolism, antiplatelet drugs and assessment of insulin resistance, will contribute to improving overall treatment of DM with an increased CV risk and associated comorbidities and to enriching these vascular disease units with their specific knowledge of the specialty,160,161 not only by participating in the care activity, but also by collaborating with teaching and research in the CV risk field.
Recommendations- •
The integration of the Endocrinology, Metabolism (diabetes-lipids) and Nutrition specialist in cardiac rehabilitation units and vascular disease units is fundamental for the monitoring of patients with DM and heart disease, with the use of selective SGLT2 inhibitors due to their beneficial effects.
Changes to restful sleep, alterations to the regulatory genes of the circadian rhythm and hepatic steatosis are being increasingly associated with the risk of CVD and DM. Socio-cultural level is considered to be another CVRF which is increasingly being attributed greater weight in the development of diabetes and CVR. The role of vitamin D should be highlighted: reduced BP has been found in women given a vitamin D supplement, as well as insulin resistance characterised by hyperinsulinism; in obese patients with vitamin D deficiency and a greater risk of DM2, it is important to highlight the role of the gut microbiota, both in the development of DM and in CVR.161–163 We should mention the role of hyperhomocysteinaemia due to its thrombogenic effect, without forgetting that the use of metformin may reduce vitamin B12 levels and reduce the recycling of homocysteine to methionine, thereby raising the plasma levels of homocysteine.164 It is important to define the role of non-traditional markers, i.e. the role of lipoprotein(a) without specific treatment, although PCSK9 inhibitors reduce their levels by 20%, as well as C-reactive protein as an inflammatory marker of CVR, but it is also necessary to confirm and define new biomarkers of CVR,101,165 as well as future therapeutic associations of SGLT2 and ACE inhibitors or ARBs for the delay and slowing down of the progression of kidney failure in diabetics.
Recommendations- •
Vitamin D >30ng/ml (assess drug treatment below 30 according to age, time of year and previous levels).
- •
Treat with vitamin D supplements at levels below 20ng/ml. Monitor vitamin B12 and folic acid in patients with DM at baseline and annually, especially if they are undergoing treatment with metformin.
Controlling CVRFs prevents the development of CVD and/or delays it over time. However, epidemiological studies overwhelmingly show poor control of CVRFs in patients with DM2. It is advisable to use the most cost-effective diagnostic procedure for each patient, as well as to plan a personalised therapeutic strategy. The Mediterranean diet and aerobic exercise are beneficial for diabetic patients, facilitating control of CVRFs and the achievement of therapeutic targets. Based on available evidence, antioxidant or vitamin supplements are not necessary if there are no deficiencies. BS seems to be increasingly efficient, reducing comorbidities and DM complications in the short- and long-term, and reversing the condition in a significant number of patients who have DM2 and are obese. There should be an attempt to achieve greater treatment adherence in patients, as well as greater involvement of the guidelines and more realistic targets, personalising treatment and its targets to adapt them to the characteristics of the patient. An HbA1c level around 6.5% may be personalised according to age and risk of hypoglycaemia, with it being possible to accept HbA1c levels of 8.5%. Metformin is the first-line drug, and SGLT2 inhibitors and glucagon-like peptide 1 receptor agonists may be assessed as a second option in patients with CVR, and even as a first option depending on the CVR. BP must be less than 140/90mmHg, with LDL-C levels according to the CVR; statins are the first option, adding ezetimibe to reach LDL-C targets according to the CVR; if they are not reached due to maximum tolerated doses and/or intolerance to statins, use PCSK9 inhibitors. The use of ASA in secondary prevention (75–162mg/day) is recommended. In diabetic patients with a risk of HF, emphasis is placed on treatment with a SGLT2 inhibitor. Age is not a contraindication for the treatment of CVRFs. It should be personalised in each case. In patients with DM aged over 65 years, annual screening should be carried out for the early detection of mild cognitive impairment or dementia, and treatment adherence should be facilitated. It is essential to assess the incorporation of an Endocrinology and Nutrition specialist into cardiac rehabilitation units: the complexity of the correct diagnostic and therapeutic assessment of metabolic disorders which constitute the pathogenic basis of CVD means that the role of a professional with advanced expertise in the management of such disorders is increasingly necessary to improve the cardiometabolic control of patients with DM2 and to achieve the therapeutic targets of the greatest possible number of CVRFs.
Please cite this article as: Arrieta F, Iglesias P, Pedro-Botet J, Becerra A, Ortega E, Obaya JC, et al. Diabetes mellitus y riesgo cardiovascular. Actualización de las recomendaciones del Grupo de Trabajo de Diabetes y Riesgo Cardiovascular de la Sociedad Española de Diabetes (SED, 2018). Clin Invest Arterioscler. 2018;30:137–153.