Recent research has revealed that clonal hematopoiesis of indeterminate potential (CHIP) characterised by the acquisition of somatic mutations in hematopoietic stem cells, is not only a common age-related disorder and a premalignant condition, but it is also associated with the development of atherosclerotic vascular diseases. Mutations in DNMT3A, TET2 and ASXL1 were each individually associated with coronary heart disease, stroke and coronary calcification. Therefore, CHIP emerges as a new risk factor for atherosclerotic vascular pathologies and its detection may be relevant as a new therapeutic target in order to modify the natural course of the disease.
Investigaciones realizadas en los últimos años han demostrado que la hematopoyesis clonal de potencial indeterminado (CHIP), resultado de mutaciones somáticas en células madre, no es solo un proceso relacionado con la edad y un estado premaligno, sino una condición que predispone al desarrollo de enfermedades cardiovasculares. La presencia de mutaciones DNMT3A, TET-2 y ASXL1 en células hematopoyéticas se ha asociado con un riesgo elevado de cardiopatía e ictus isquémicos, así como con elevada calcificación coronaria. CHIP emerge como un nuevo factor de riesgo de enfermedad aterosclerótica y su detección puede tener importantes implicaciones terapéuticas para modificar la historia natural de la enfermedad.
Cardiovascular disease, the leading global cause of mortality, is caused by atherothrombotic vascular occlusion, which is responsible for such relevant clinical pictures as unstable angina, myocardial infarction, sudden death and stroke. Atherothrombosis is a disease characterised by the accumulation of lipids and chronic inflammation in the tunica intima of medium and large arteries, and comprises two differentiated stages: the first takes place over several decades and involves the accumulation of lipids, macrophages and other inflammatory cells, and extracellular matrix (ECM) in the intima and subendothelial space of the vessel wall, generating atheromatous plaque, a lesion that is characteristic of the early stages of atherosclerosis. This process often does not cause cardiovascular complications because blood flow is preserved through so-called expansive (eccentric) remodelling of the vessel wall, thereby preserving the lumen. The second phase occurs in only a small percentage of patients (less than 5%) and involves the rupture or erosion of the vessel wall and formation of an occlusive thrombosis that leads to ischaemia and necrosis of the affected organ. Autopsy findings in patients who have died as a consequence of an acute atherothrombotic event have revealed that the “culpable lesion” is not characterised by significant stenosis of the lumen (as was indicated in previous decades), but by alterations in the composition of the atheromatous plaque that resulted in its vulnerability. Atheromatous plaques lead to clinical manifestations due to rupture and exposure of procoagulant material to contact with circulating blood, triggering the clotting process (atherothrombosis). Plaques that rupture, known as vulnerable plaques, are characterised by a thin fibrous layer, large necrotic nucleus and inflammatory infiltrate. Inflammatory activity predisposes the plaque to rupture mediated by proteolytic enzymes such as metalloproteases that break down the ECM. Nowadays, a plaque's composition, rather than the degree of stenosis, is considered to be the main determiner of the appearance of atherothrombotic clinical syndromes.1,2
The haematopoietic component of atherosclerosisTaking into account the role inflammation plays in atherosclerosis, the involvement of a myeloid component is logical in all stages of this process, from the initial stages to progression and, finally, to the disease's thrombotic sequelae.
The discovery of T-lymphocytes in human atheromatous plaques and subsequent identification of all of the cell types in the innate and acquired immune system have confirmed the immune system's involvement. Recent advances implicate various subpopulations of monocytes, the most abundant myeloid cells in atheromatous plaque, as well as an increase in haematopoietic activity around atheromatous plaques.3–6 Myeloid cells contribute to initiating thrombosis, forming enzymes that break down the ECN and producing tissue factor, the main initiator of the blood clotting process. Finally, neutrophils can locally aggravate thrombosis by creating neutrophil extracellular traps (NETs) that promote the growth and persistence of the thrombus by inducing procoagulant factors and amplifying inflammation by activating inflammasome and generating IL-1β. In 2004, a fibrous material released by neutrophils in the presence of lipopolysaccharide was described, that is composed primarily of DNA released through chromatin decondensation, histones and proteolytic enzymes (myeloperoxidase, elastase, etc.) that traps and destroys bacteria, and named NET. Subsequent studies showed that NET is present in numerous systemic pathological processes with a significant inflammatory component, such as sepsis, autoimmune diseases, cancer, trauma and various thrombotic diseases, as well as in atherosclerotic lesions.7,8 NETs promote thrombosis through a number of mechanisms: platelet activation and aggregation, activations of the intrinsic and extrinsic coagulation pathways, increased thrombin generation and defective fibrinolytic activity.7,8
It is interesting to note that recent studies have been analysing the role of anti-inflammatory strategies that, by modulating innate immunity (e.g. canakinumab, an IL-1β antagonist, CANTOS study), may prevent the development of atherosclerosis.9 Acquired immunity has also been found to play a role, as helper T-cells produce proinflammatory cytokines such as tumour necrosis factor-alpha, IL-1β and interferon-γ, with powerful proatherosclerotic actions.10
The concept of clonal haematopoiesis and its relevance in cardiovascular diseasesRecent research using range of genomic techniques has revealed that clinical haematopoiesis, characterised by the acquisition of somatic mutations, is not only a common age-related disorder and a premalignant condition, but it is also associated with the development of cardiovascular diseases.11
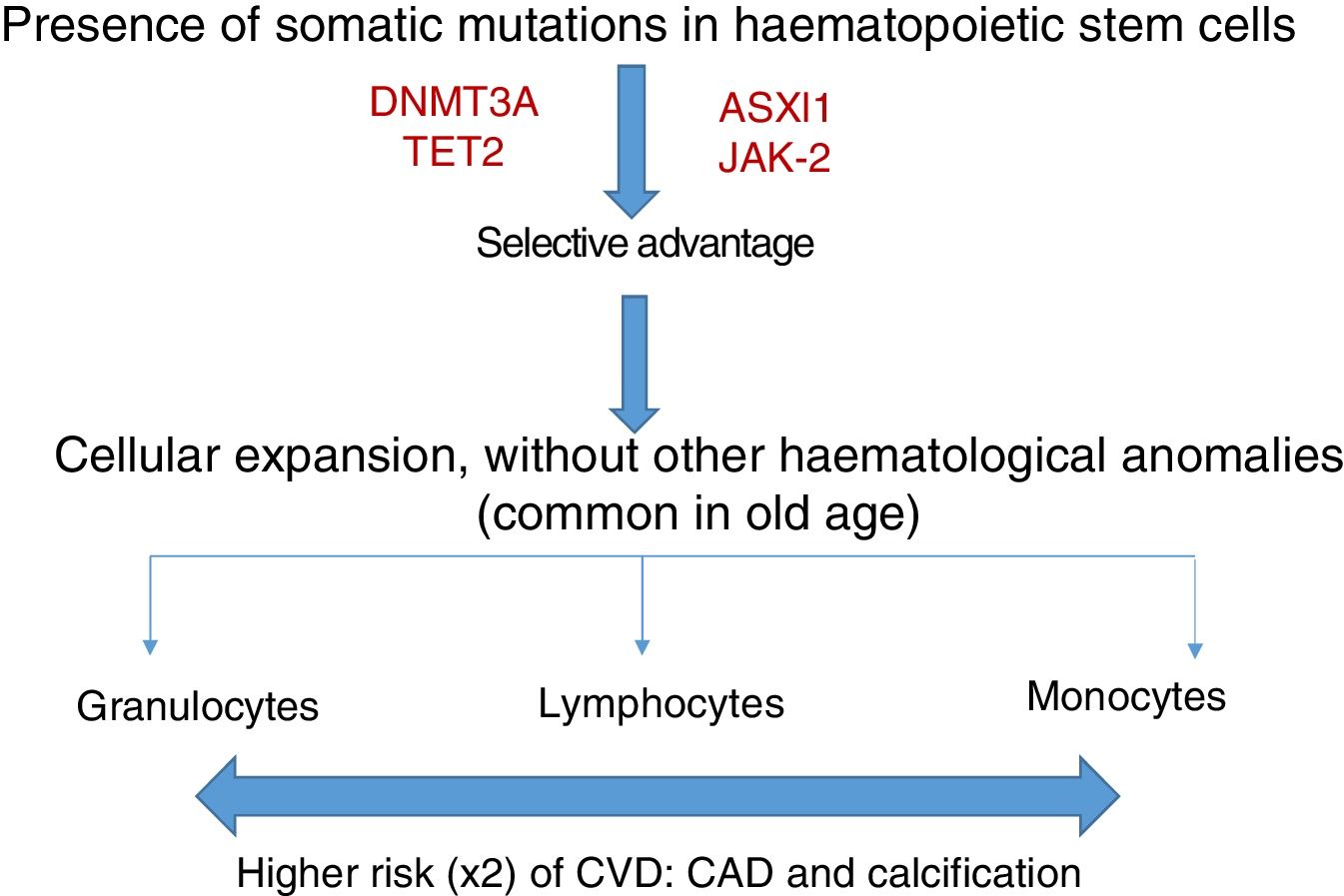
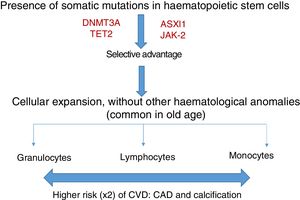
Somatic mutations are rare before 40 years of age, but are present in 10–20% of subjects in their eighties; the majority consist of a single mutation, with DNMT3A being the most common, followed by TET2 and ASLX1. Other less common mutations are TP53, JAK-2, SF3B1 and BCOR.12,13 Somatic mutations in clonal haematopoiesis persist over time and are considered a premalignant condition with similarities to other clonal haematopoietic disorders, such as monoclonal gammopathy of undetermined significance (MGUS), monoclonal B-cell lymphocytosis, myelodysplastic syndromes or paroxysmal nocturnal haemoglobinuria.14 The vast majority of mutations in clonal haematopoiesis affect genes involved in epigenetic regulation, for example DNMT3A and TET2 regulate DNA methylation.11
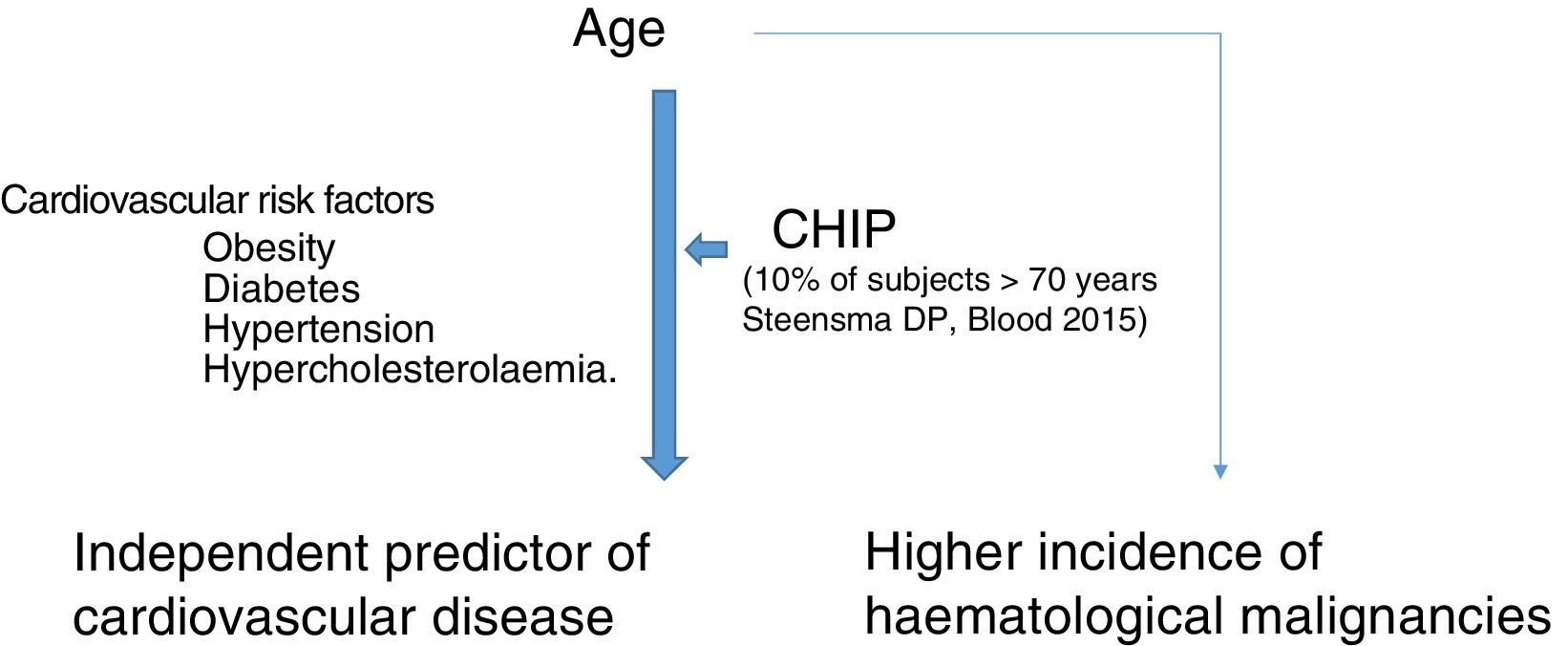
Clonal haematopoiesis of indeterminate potential (CHIP) is therefore characterised by the expansion of haematopoietic clones bearing somatic mutations that confer a selective advantage, with clonal expansion of granulocytic, lymphocytic and monocytic cell types. It has previously been associated with advanced age and an increased risk of haematological malignancies12,14 (Fig. 1). Recent studies indicate that the presence of CHIP is associated with accelerated atherosclerosis in mice and elevated risk of coronary disease in humans.15,16 The connection between CHIP and clinical cardiovascular disease was recently demonstrated by Jaiswal et al.,16 in broad series of patients from prospective (BioImage and MDC) and retrospective (ATVB and PROMIS) studies of 4726 patients with coronary artery disease and 3529 healthy controls. Mutations were observed in ASXL1, TET2, DNMT3A and JAK-2 that were associated with an elevated risk of ischaemic heart disease (HR 2.0), ischaemic stroke (HR 2.6) and coronary artery calcification (Fig. 2). It is interesting to note that the cardiovascular risk attributable to CHIP was comparable to or greater in magnitude than that of traditional cardiovascular risk factors such as hypertension, hypercholesterolaemia and smoking. The association between CHIP and cardiovascular disease may be due to not-yet-identified variables or share physiological aspects with the senescence process, such as vascular inflammation. In this sense, the fact that TET2 down-regulates IL-6 indicates that somatic mutations in TET2 may promote inflammatory processes.
To study the causality, experimental studies have been conducted on LDLr−/− mice (that developed spontaneous or diet-induced atherosclerosis) that received bone marrow transplants from mice (TET2−/−). The animals were fed a high-fat diet for 10 weeks, and the expression of genes and proteins expressed by macrophages was analysed, alongside a histological and immunohistochemical study of the atherosclerotic lesion. The results revealed a high degree of atherosclerosis in the aortic arch, as well as the production of cytokines (IL-1β, inflammasome) in the TET2−/− mice, establishing a mechanism whereby the TET2 mutations or deletion might contribute to increasing cardiovascular risk.15,16
Clonal haematopoiesis of indeterminate potential: a new therapeutic target in atherosclerosis?Far from being an irreversible process associated with ageing, it is now thought that atherosclerosis can be halted or even reversed.1 In this sense, statins have a beneficial effect on plaque composition, more so than on the degree of stenosis. Moreover, the protective action that careful control of cardiovascular risk factors and healthy lifestyles confer on the atherosclerotic process could be mediated through their action on haematopoiesis.17 Finally, it has been shown that vitamin C restores TET2 activity in some types of leukaemia and down-regulates expression of apolipoprotein (a) via TET2, which may explain some of its antiatherogenic actions.18
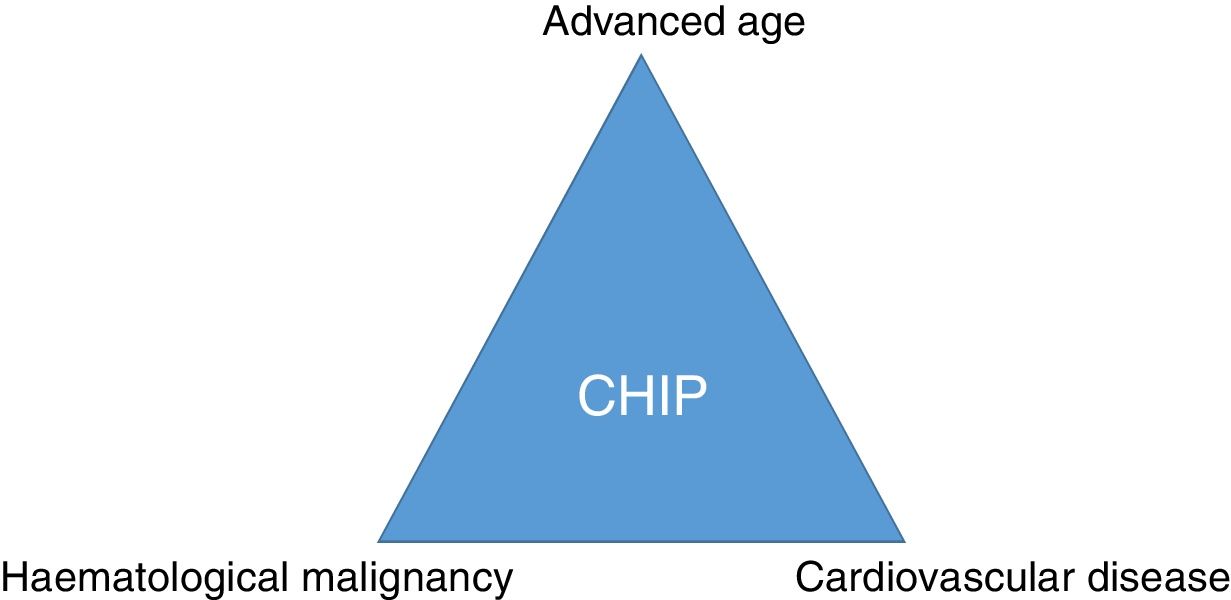
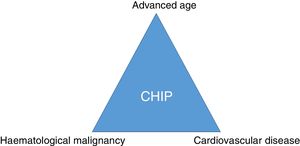
ConclusionSomatic mutations in genes described in patients with haematological malignancies can frequently be detected in the blood of elderly healthy subjects. CHIP is associated with coronary artery disease and degree of atherosclerosis and may imply a link between cancer and cardiovascular disease (Fig. 3). Therefore, CHIP emerges as a new risk factor for atherosclerotic pathologies and ischaemic vascular diseases, and its detection in clinical practice may be relevant as a new therapeutic target in order to modify the natural course of the disease.
Conflict of interestsNone.
Please cite this article as: Páramo Fernández JA. Aterosclerosis y hematopoyesis clonal: un nuevo factor de riesgo. Clin Invest Arterioscler. 2018;30:133–136.