The loss of the modulator role of the endothelium could be involved in the pathogenesis of diabetic vascular complications. Transition metal compounds, such as tungsten and vanadium, have been proposed as possible agents in the treatment of diabetes by simulating the effects of insulin. The mesenteric vascular bed intervenes in vascular resistance and is a source of vasoactive compounds, such as prostanoids. The aim of this work was to study the effects of sodium tungstate and vanadyl sulphate treatments on the metabolic parameters and the release of prostanoids of the mesenteric vascular bed in an experimental model of Streptozotocin-induced diabetes. In diabetic rats, a significant increase was observed in plasma levels of glucose, triglycerides and total cholesterol. On the other hand, there was a significant reduction in the release of vasodilator prostanoids, such as prostacyclin and prostaglandin E2 and vasoconstrictor thromboxane A2 through the mesenteric vascular bed. Both sodium tungstate and vanadyl sulphate normalised glycaemia, triglyceridaemia and cholesterolaemia in rats diabetics. On the other hand, only treatment with sodium tungstate reversed the reduction in the release of vasodilator prostanoids, improving in diabetic animals the prostacyclin/thromboxane ratio, an indicator of vascular dysfunction. In conclusion, unlike vanadyl sulphate, sodium tungstate is shown to be more effective in controlling metabolic changes and the production of vasodilator prostanoids observed in experimental diabetes induced by streptozotocin.
La pérdida del rol modulador del endotelio podría estar implicada en la patogénesis de las complicaciones vasculares diabéticas. Los compuestos de metales de transición tales como wolframio y vanadio se han propuesto como posibles agentes en el tratamiento de la diabetes al simular los efectos de la insulina. El lecho vascular mesentérico interviene en la resistencia vascular y constituye una fuente de compuestos vasoactivos como los prostanoides. El objetivo de este trabajo fue estudiar los efectos de los tratamientos con tungstato de sodio y sulfato de vanadilo sobre los parámetros metabólicos y la liberación de prostanoides del lecho vascular mesentérico en un modelo experimental de diabetes inducida por estreptozotocina. En ratas diabéticas se observó un aumento significativo de los niveles plasmáticos de glucosa, triglicéridos y colesterol total. Por su parte, se observó una reducción significativa en la liberación de los prostanoides vasodilatadores como la prostaciclina y la prostaglandina E2 y del vasoconstrictor tromboxano A2 por el lecho vascular mesentérico. Tanto el tungstato de sodio como el sulfato de vanadilo normalizaron la glucemia, la trigliceridemia y la colesterolemia en las ratas diabéticas. Por otra parte, solo el tratamiento con tungstato de sodio revirtió la reducción en la liberación de prostanoides vasodilatadores, mejorando en los animales diabéticos la relación prostaciclina/tromboxano, un indicador de disfunción vascular. En conclusión, a diferencia del sulfato de vanadilo, el tungstato de sodio demuestra ser más eficaz para controlar las alteraciones metabólicas y de la producción de prostanoides vasodilatadores observadas en la diabetes experimental inducida por estreptozotocina.
Vascular complications are a major cause of morbidity and mortality in diabetes mellitus. Although the relationship is not fully understood, loss of the modulatory role of the endothelium could be involved in the pathogenesis of diabetic vascular complications.1
One of the various vascular disorders caused by diabetes mellitus is altered arachidonic acid metabolism. Members of the prostanoid family, comprising prostaglandins (PG) and thromboxanes (TX), are formed from arachidonic acid in the cyclooxygenase pathway. These vasoactive substances exhibit a wide range of biological actions, including regulation of vasomotor tone.2
The mesenteric vascular bed, which is made up of resistance vessels, intervenes in vascular resistance and is a source of prostanoids.3 In diabetes, peripheral arteries are at high risk of blockages, limiting blood flow to distal tissues.4 PG such as prostacyclin (PGI2) and PGE2 are vasodilator prostanoids that maintain adequate blood flow to peripheral tissues. In this sense, an altered pattern of prostanoid release has been observed in mesenteric vessels in an experimental model of diabetes.5
Transition metal compounds, such as tungsten and vanadium, act by mimicking the effects of insulin, lowering plasma glucose levels, and have therefore been proposed as possible agents in the treatment of diabetes mellitus.6,7 Sodium tungstate and vanadyl sulfate have also been reported to have an effect on vascular prostanoid production in fructose-overloaded rats. Sodium tungstate prevented a reduction in the release of vasodilators (PGI2 and PGE2) from the mesenteric bed of fructose-overloaded rats, while vanadyl sulfate had no effect on the release of such prostanoids.8
Based on the data outlined above, the objective of this study was to analyse the effects of sodium tungstate and vanadyl sulfate treatments on metabolic parameters and the release of prostanoids from the mesenteric vascular bed in rats with streptozotocin (STZ)-induced diabetes.
Methods and materialsThe experiments were approved in advance by the local ethics committee on animal research (Comité Institucional para el Cuidado y Uso de Animales de Laboratorio [CICUAL], Faculty of Pharmacy and Biochemistry, Universidad de Buenos Aires, Res. No. 2259). All animals included in the experimental protocols were fed and housed according to CICUAL guidelines.
A total of 42 male Wistar rats, weighing 180–240g at the beginning of the experiment, were used. The animals were divided into 6 groups (n=7 per group): control (C); diabetic (D); tungstate-treated (CT); vanadyl-treated (CV); diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV). Groups CT and DT received sodium tungstate, 2g/l, while groups CV and DV received vanadyl sulfate, 125mg/l, both administered in their drinking water.
Experimental diabetes was induced with a single intraperitoneal (i.p.) injection of STZ, 55mg/kg in citrate buffer (groups D, DT and DV). The animals in groups C, CT and CV received buffer only. One week later, blood glucose levels were measured in blood samples taken from the caudal artery (Accutrend Glucose Meter, Roche Diagnostics, Mannheim, Germany). Animals with blood glucose levels higher than 300mg/dl were considered diabetic, and treatment with sodium tungstate and vanadyl sulfate were then commenced. Thirty days after STZ or buffer administration, the animals were euthanised by decapitation. Prior to euthanasia, blood samples were taken from the retro-orbital sinus under anaesthesia (ketamine, 80mg/kg PRO-SER SA, and xylazine, 12mg/kg PRO-SER SA) to measure blood glucose levels (Accutrend Glucose Meter, Roche Diagnostics, Mannheim, Germany), total cholesterol (Wiener Colestat enzimático, Wiener Lab, Rosario, Argentina) and triglycerides (TG Color GPO/PAP AA, enzymatic method, Wiener Lab, Rosario, Argentina) using spectrophotometric methods (Automatic Analyzer Abbott Spectrum CCX).
After euthanasia, the mesenteric vascular bed of all animals from all groups was removed and dissected. It was then transferred to Petri dishes containing Krebs solution with the following composition: NaCl, 118mM; KCl, 4.7mM; MgSO4, 1.2mM; NaH2PO4, 1.0mM; CaCl2, 2.6mM; NaHCO3, 25.0mM; glucose, 11.1mM. The tissues were incubated in this solution for 60min at 37°C. In order to measure the released prostanoids, at the end of the incubation period, the media were acidified to pH 3.50 with 1M formic acid and extracted three times with two volumes of chloroform. The chloroform extracts were pooled and evaporated to dryness.
Prostanoids were measured by reversed-phase HPLC with a C18 column (BBS Hypersil C18, Thermo Electron Co., Bellefonte, PA, USA), using 1.7Mm H3PO4 67.2:acetonitrile 32.8V/V as the mobile phase, a flow rate of 1mlmin−1 and measuring UV absorbance at 218nm. The samples were resuspended in 0.15ml of the mobile phase and injected into the HPLC system. Authentic standards of prostanoids 6-keto PG F1α (stable metabolite of PGI2), PGE2 and TXB2 (stable metabolite of TXA2) (Sigma Chemical Co., Saint Louis, MO, USA) were run with the samples and a comparative analysis was performed to determine the quantity of prostanoids present in the samples. All values were corrected for recovery loss as determined by parallel standards. Results were expressed as ng of prostanoid per mg of wet tissue weight.
Statistical analysisAll data are expressed as mean±SEM. Intergroup comparisons were made by one-way analysis of variance (ANOVA). The Tukey post-test was applied. A p-value<0.05 was considered statistically significant.
ResultsFinal body weight values showed that the weight of STZ-induced diabetic rats decreased significantly compared to rats in group C (295±6 vs. 372±6; p<0.01). In diabetic animals, vanadyl treatment produced a significant decrease in body weight (183±11 vs. D, p<0.01), while tungstate treatment increased this parameter (394±23 vs. D, p<0.01) to values similar to those observed in group C. Neither of the treatments significantly changed the body weight of control animals.
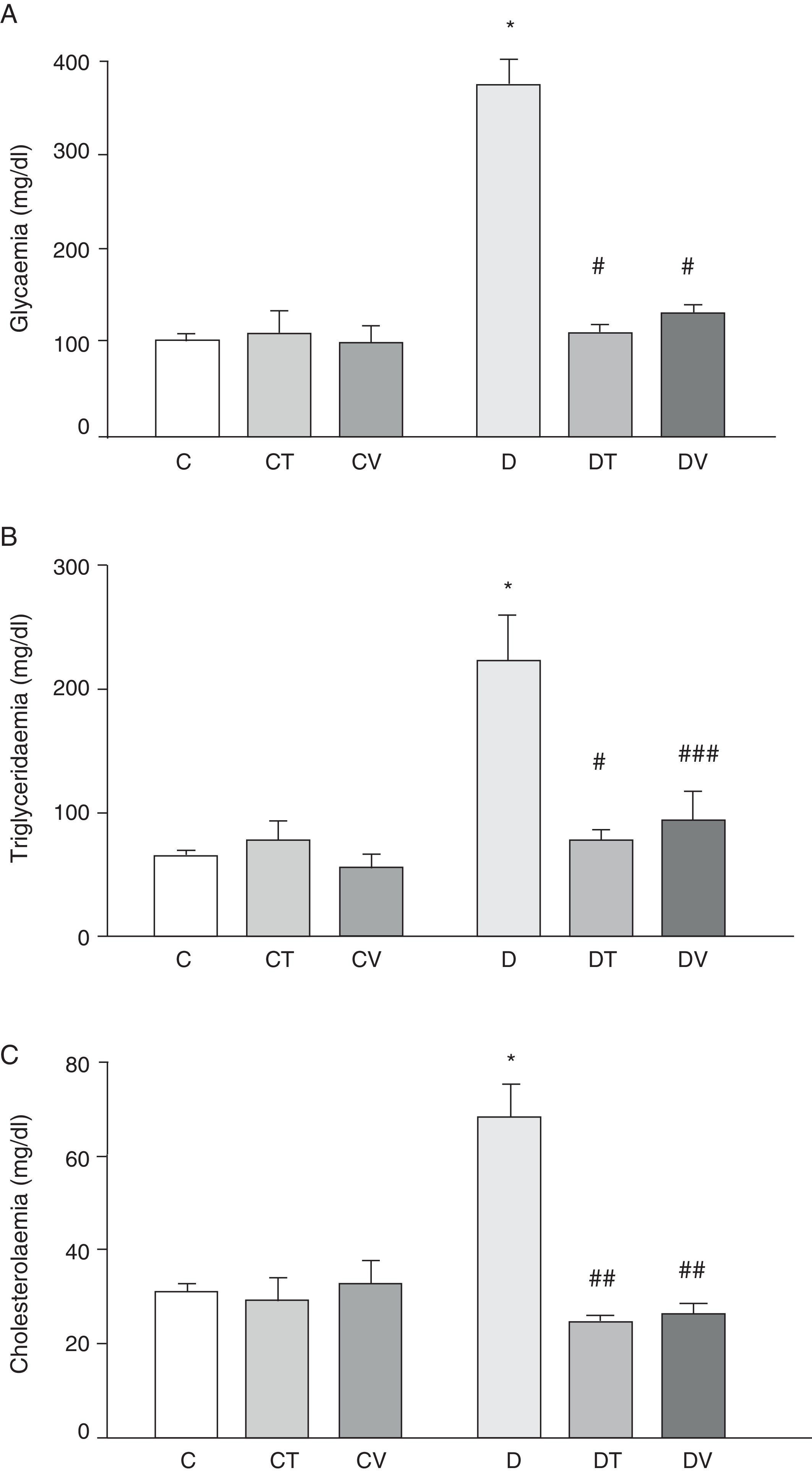
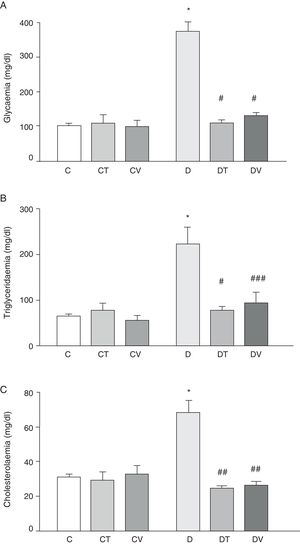
Fig. 1 shows plasma data: glycaemia, triglyceridaemia and cholesterolaemia. STZ-induced diabetes increased the values of these parameters significantly (p<0.001 vs. C). Both treatments (tungstate and vanadyl) were able to normalise plasma glucose (p<0.001 vs. D) and triglyceride (p<0.001 and p<0.05 vs. D, respectively) levels, while reversing the increase in cholesterol levels (p<0.01 vs. D). It must be noted that neither tungstate nor vanadyl affected plasma glucose, triglyceride and total cholesterol levels in control animals.
Metabolic parameters: (A) glycaemia (mg/dl), (B) triglyceridaemia (mg/dl) and (C) cholesterolaemia (mg/dl) in control (C), tungstate-treated (CT), vanadyl-treated (CV), diabetic (D), diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV) rats. Results are expressed as mean±SEM. *p<0.001 vs. C; #p<0.001 vs. D; ##p<0.01 vs. D; ###p<0.05 vs. D.
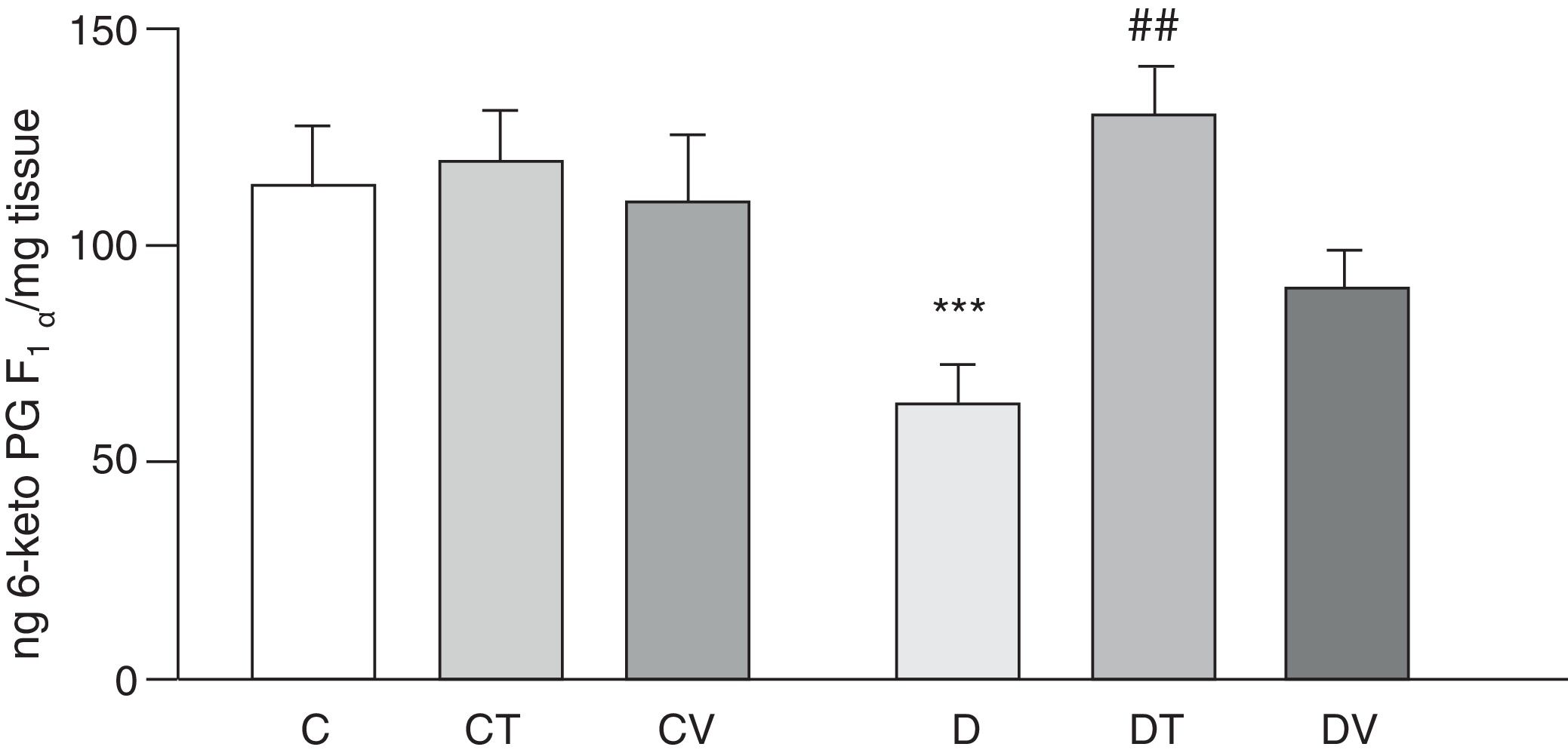
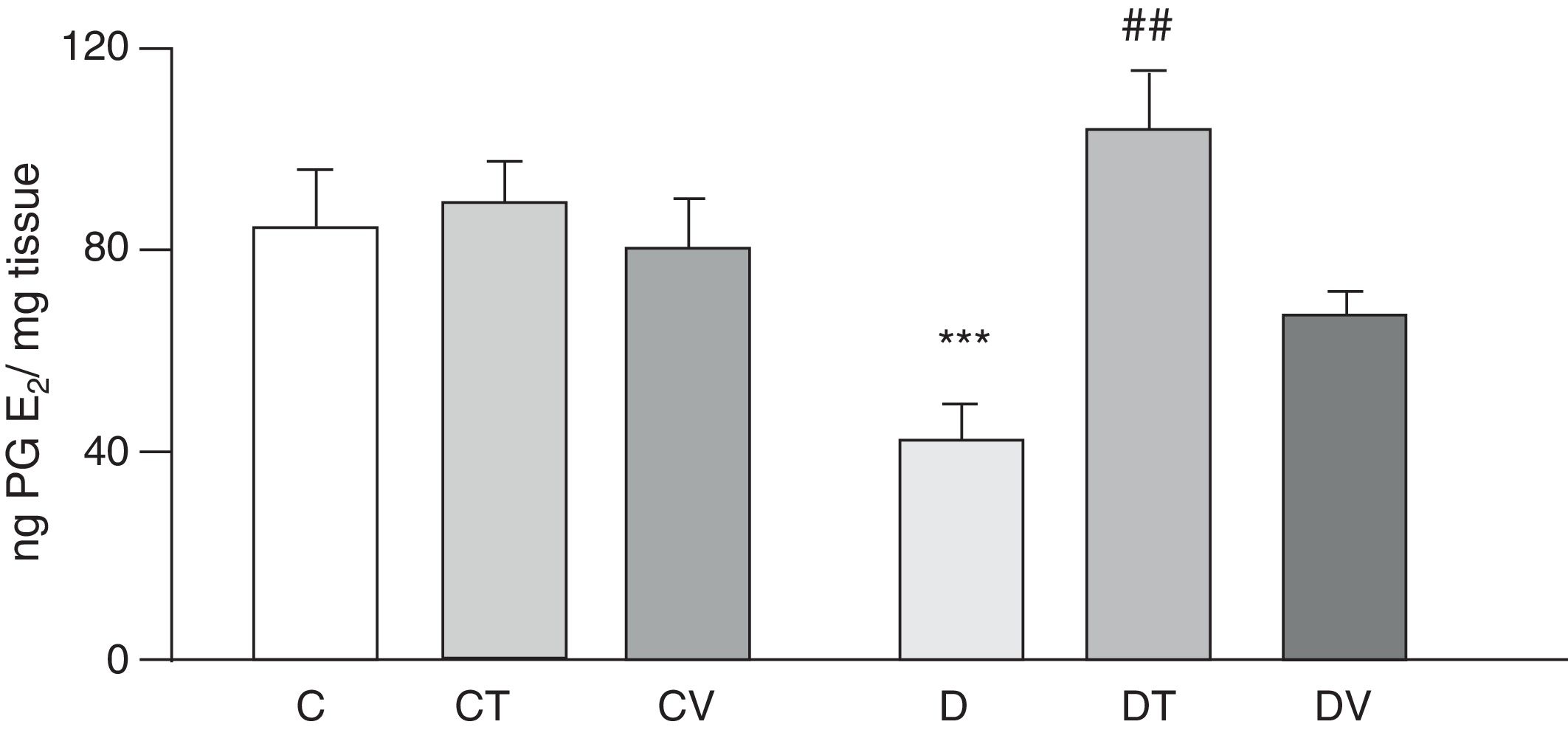
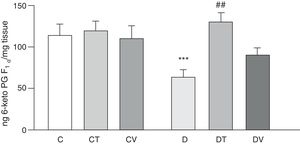
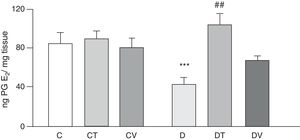
Figs. 2 and 3 show the effects at 30 days of STZ treatment in the absence and presence of tungstate and vanadyl treatments on baseline release of vasodilator prostanoids 6-keto PG F1α (stable metabolite of PGI2) and PGE2, respectively, from mesenteric beds into the incubation medium. In diabetic rats, a significant decrease in the release of both prostanoids is observed compared to group C (p<0.05 vs. C), with no differences observed in groups CT or CV. Sodium tungstate treatment in diabetic rats was able to significantly reverse the reduction of both prostanoids (p<0.05 vs. D), an effect which was not observed with vanadyl sulfate treatment.
Release of 6-keto PG F1α from the mesenteric bed. Release of 6-keto PG F1α (ngmgtissue−1) from control (C), tungstate-treated (CT), vanadyl-treated (CV), diabetic (D), diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV) rats. Results are expressed as mean±SEM. ***p<0.05 vs. C; ##p<0.01 vs. D.
Release of PGE2 from the mesenteric bed. Release of PGE2 (ngmgtissue−1) from the mesenteric bed of control (C), tungstate-treated (CT), vanadyl-treated (CV), diabetic (D), diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV) rats. Results are expressed as mean±SEM. ***p<0.05 vs. C; ##p<0.01 vs. D.
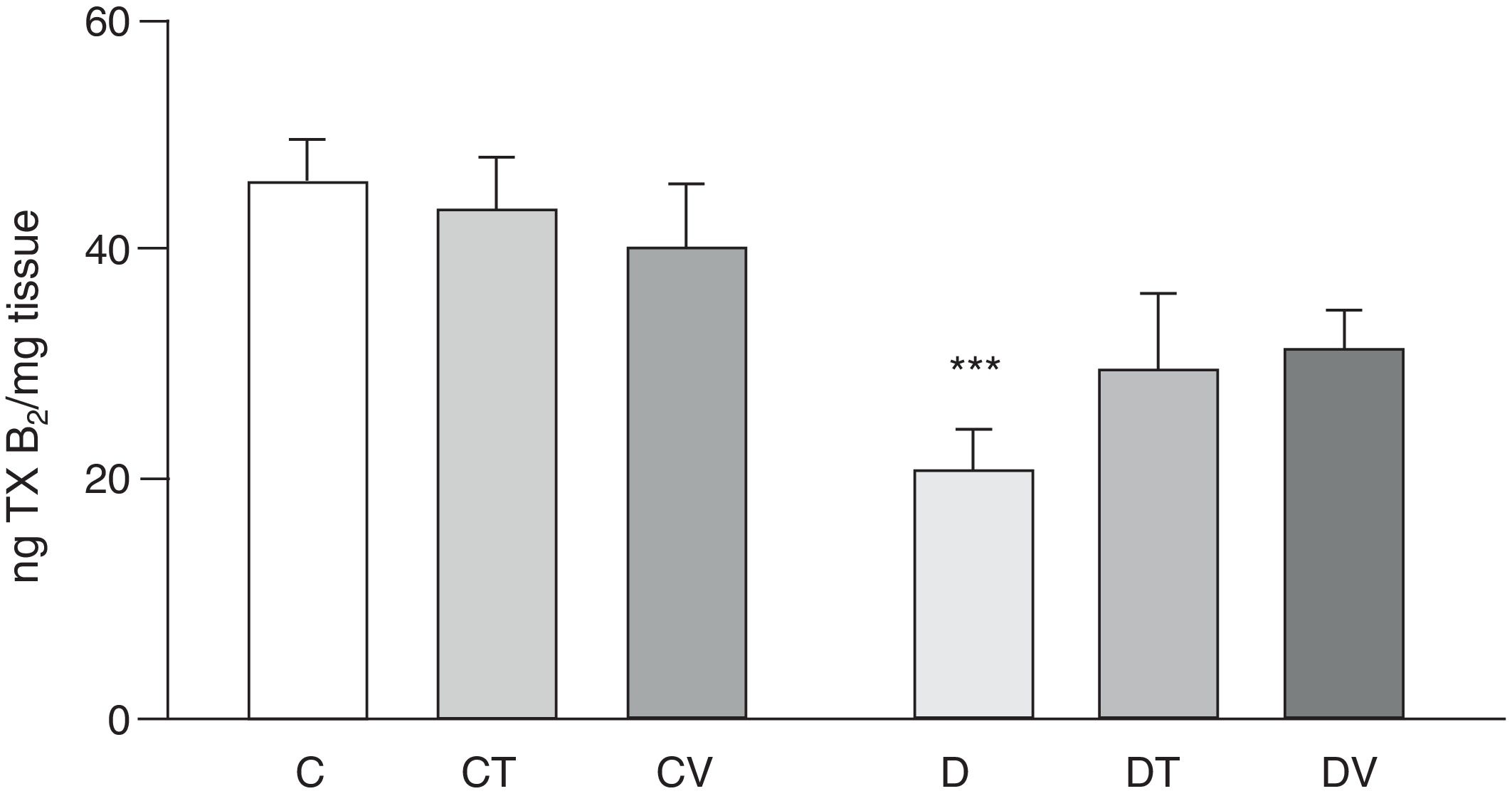
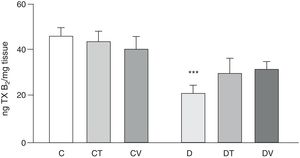
Effects on the release of the vasoconstrictor TXB2 (stable metabolite of TXA2) from the mesenteric vascular bed are shown in Fig. 4. In comparison with group C, rats with STZ-induced diabetes showed a significant decrease in its release (p<0.05 vs. C), which was not modified with tungstate or vanadyl treatment in diabetic animals. Neither of the treatments with these transition metals modified production of this prostanoid in control animals.
Release of TXB2 from the mesenteric bed. Release of TXB2 (ngmgtissue−1) from the mesenteric bed of control (C), tungstate-treated (CT), vanadyl-treated (CV), diabetic (D), diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV) rats. Results are expressed as mean±SEM. ***p<0.05 vs. C.
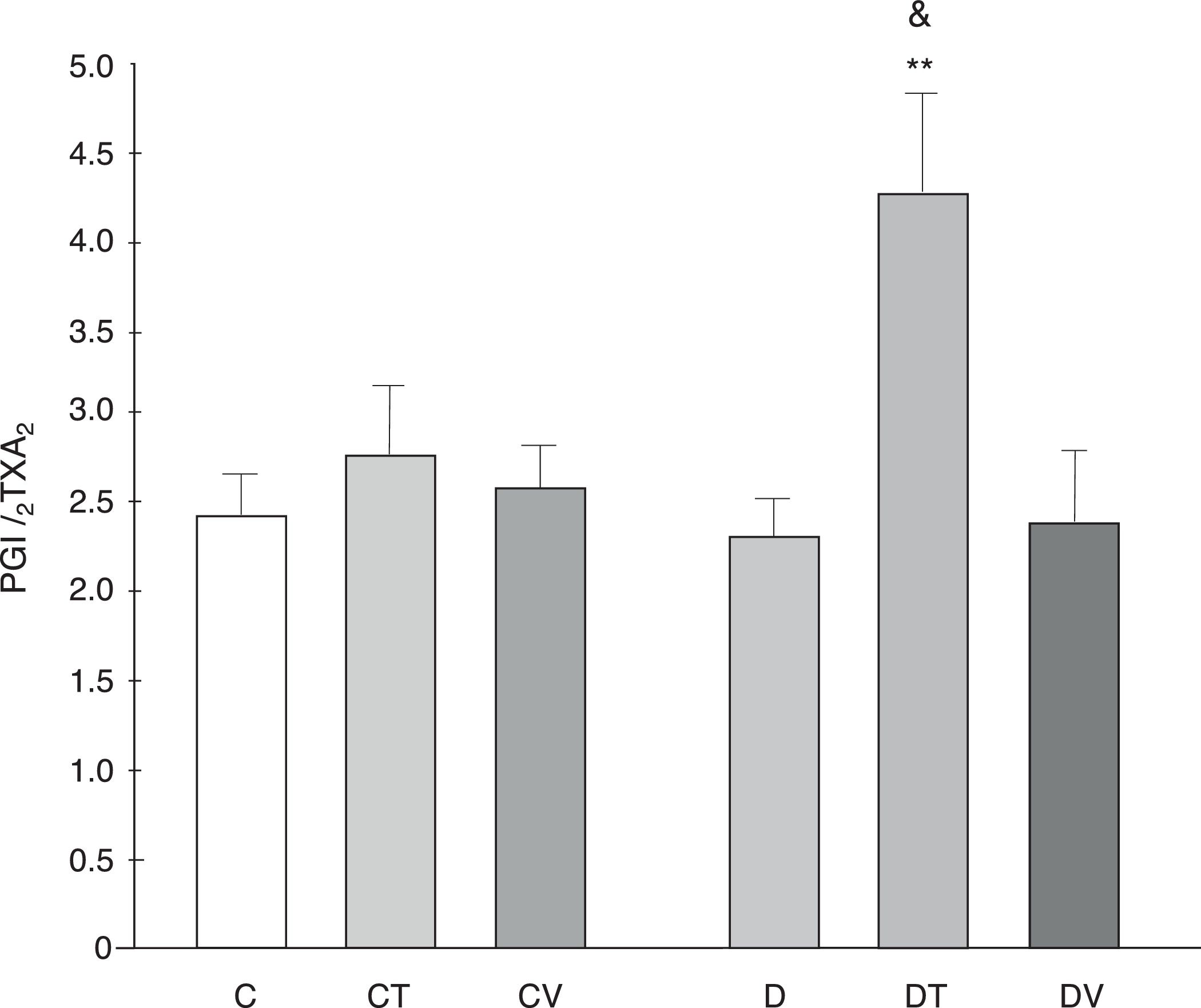
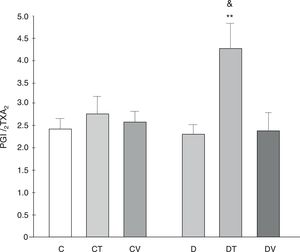
The PGI2/TXA2 release ratio (measured as their stable metabolites) was not modified by diabetes, although tungstate treatment did increase it significantly (p<0.01 vs. C, Fig. 5).
PGI2/TXA2 release ratio from the mesenteric bed. PGI2/TXA2 release ratio from the mesenteric bed of control (C), tungstate-treated (CT), vanadyl-treated (CV), diabetic (D), diabetic and tungstate-treated (DT) and diabetic and vanadyl-treated (DV) rats. Results are expressed as mean±SEM. **p<0.01 vs. C; &p<0.05 vs. CT.
This study shows that both sodium tungstate and vanadyl sulfate, administered orally, reverse the onset of metabolic alterations, but only sodium tungstate was able to maintain normal release of vasodilator prostanoids, such as PGI2 and PGE2, altered by the diabetic state, from the vascular bed.
One possible explanation for the lack of effects by vanadyl sulfate is the selected dose. Nevertheless, the dose used matches that used by other authors,6,9–16 and furthermore, this dose produces equivalent effects on lipid and glucose metabolism to vanadyl sulfate, as shown in this and a previously published paper.8 In one fructose-overload model, a smaller dose of vanadyl sulfate (100mg/l) was used, which also had no effect on prostanoid production. In this paper, even increasing the previously used dose from 100 to 125mg/l, without highlighting the effects on lipid and glucose metabolism, was not able to alter prostanoid production. However, efforts have been made to avoid the use of larger doses of vanadyl sulfate in order to prevent toxicity.11
With regard to body weight, it can be observed that vanadyl sulfate treatment significantly reduced body weight in diabetic animals. This effect could be due to a smaller food intake rather than an additional toxic effect.17 This reduced food intake was due to an anorexigenic effect of vanadyl on the central nervous system, as described previously.18
In our STZ-induced diabetes mellitus model, we found high glucose, triglyceride and total cholesterol levels. Sodium tungstate and vanadyl sulfate treatments reversed these alterations. These results partially match those of Nagareddy et al.19, who reported that tungstate treatment in rats with STZ-induced diabetes produced a significant decrease in plasma glucose and triglyceride levels. Ahmed El-Shazly et al.20 found that vanadyl sulfate normalised plasma glucose and triglyceride levels but not total cholesterol levels, although they did discover that the vanadyl(II) thiamine hydrochloride complex was more effective for reversing all 3 parameters in STZ-induced diabetic rats.
Regarding the mechanisms involved in the observed actions of these agents, it has been suggested that tungstate treatment may be capable of regenerating a stable and functional β-cell population, which would lead to normal glucose levels.21 In STZ-induced diabetic rats, the main action of tungstate appears to be restoration of hepatic glucose metabolism by increasing the capacity of the liver to utilise glucose through glycolysis and gluconeogenesis. Moreover, tungstate also reduces the hepatic potential for glucose production by reducing expression of the main enzyme in gluconeogenesis, phosphoenolpyruvate carboxykinase.22
However, vanadium compounds can mimic actions of insulin through alternative signalling pathways, which involve the inhibition of phosphotyrosine phosphatases and the interaction between two non-insulin receptor tyrosine kinases.17 It has also been suggested that vanadyl sulfate may regenerate β-cells in rats with experimental diabetes.23 Vanadium treatment has also been reported to stimulate insulin-mediated GLUT4 translocation in STZ-induced diabetic rats.24 However, the lipogenic transcription factor, SREBP-1c, regulator of enzyme gene expression for fatty acid and triglyceride synthesis, is induced by insulin and negatively regulated in STZ-induced diabetic rats. Vanadyl sulfate treatment has therefore been reported to significantly increase SREBP-1c expression in comparison to the diabetic group,20 which could partly explain the effects on triglyceride and cholesterol levels observed in our model.
The progression of vascular complications in diabetes has been associated with a decrease in vascular PGI2/TXA2 ratio.25 As previously reported,5 STZ-induced diabetes for 30 days significantly reduces the release of vasodilator prostanoids PGI2 and PGE2 and the vasoconstrictor TXA2 from the mesenteric vascular bed. In our experiments, only sodium tungstate treatment was able to increase and normalise diabetes-reduced PGI2 and PGE2 levels to values similar to those reported for control rats. However, the treatment was not able to effectively restore TXA2 release in diabetic rats. This differential effect resulted in an increase in PGI2/TXA2 ratio, which is an indicator of good endothelial function.
The mechanisms involved in this effect have not yet been clarified. Nevertheless, increased production of reactive oxygen species (ROS) by hyperglycaemia is recognised as one of the main causes of diabetic complications.26 Extensive studies have also been conducted on the influence of oxidative stress on vascular alterations that occur with type 1 diabetes27, and, in this condition, excessive ROS production may inhibit the production of nitric oxide (NO) and PGI2.28 De la Cruz et al.29 have also demonstrated reduced activity of vascular NO synthase in STZ-induced diabetic animals and reduced plasma nitrite/nitrate levels, used as an indirect indicator of overall NO production. In turn, Heidari et al.30 described the protective effects of sodium tungstate against oxidative stress in diabetic rats, demonstrating significant increases in blood and pancreas levels of thiobarbituric acid reactive substances in diabetic rats compared to control rats, and that sodium tungstate administration was able to significantly decrease these levels. Sodium tungstate administration has also been reported to improve the biomechanical properties of bone by restoring the activity of antioxidant enzymes such as glutathione peroxidase, catalase and superoxide dismutase in the femur of diabetic rats.31 Another study showed the antioxidant properties of vanadyl sulfate. This study demonstrated that catalase, superoxide dismutase, glutathione reductase, glutathione peroxidase, glutathione S-transferase, carbonic anhydrase, glucose-6-phosphate dehydrogenase and lactate dehydrogenase activity was increased in the stomach of STZ-induced diabetic rats. Under these conditions, administration of vanadyl sulfate in drinking water for 60 days was associated with a significant reduction in glutathione reductase, glutathione peroxidase and glutathione S-transferase activity.32
It is a well-known fact, however, that tungstate is an effective inhibitor of xanthine oxidase, an enzyme involved in free radical formation.33 Considered as a whole, these observations may partially explain the alterations in vascular prostanoid release observed in experimental diabetes and their reversal by sodium tungstate. However, vanadyl sulfate treatment did not modify prostanoid release from the mesenteric bed in any of the experimental groups. Misurski et al.34 observed that sodium orthovanadate-mediated vasorelaxation of the perfused vascular bed of Sprague-Dawley rats involved recruitment of both endothelium-derived hyperpolarising factor and endothelium-derived NO and not vasodilator eicosanoids.
Cyclooxygenase (COX) is an enzyme that transforms arachidonic acid into prostaglandin H2, which is then converted into different PG and TX by respective synthases. Of the best characterised isoforms, COX-1 is a constitutive enzyme whose expression is rarely subject to change.35 The isoform COX-2, however, is an inducible enzyme that is subject to complex regulation of its expression and activity. Consequently, diabetes has been shown to be associated with altered activity of vascular COX, leading to altered production of prostanoids.36 Interestingly, the vasodilating effect of arachidonic acid through PGI2 in mesenteric arteries of non-diabetic dogs has been reported to revert to a TXA2-mediated vasoconstricting effect in diabetic animals.37 An increase in COX-2 levels and TXA2 production has also been demonstrated in diabetic (db/db) mice, accompanied by an increase in aortic and skeletal muscle arteriolar vascular tone.38,39 Increased COX-2 expression has also been observed in atherosclerotic plaque in diabetic patients. However, in coronary arterioles of diabetic patients, increased expression of vascular COX-2, associated with increased production of vasodilating PGs, PGE2 and PGI2, has been observed.40 This indicates a clear association between diabetes and altered COX-2 expression and activity, which may explain the alterations in prostanoid production in the mesenteric vascular bed observed in STZ-induced diabetic rats. Likewise, increased PG synthesis may not only be due to changes in COX activity but also to the amount of arachidonic acid available. Therefore, vanadium compounds may activate phospholipase A2, increasing arachidonic acid release and production of PGE2 or TXA2.41 However, another study has demonstrated that at concentrations of 10mM of sodium orthovanadate, no changes in COX-2 activity were observed in a macrophage cell culture.42 Although in vitro experiments have shown that vanadium compounds increase the expression of COX-2 protein or COX-2 activity, the concentrations at which this effect is observed are highly toxic in an in vivo model, making such effect almost impossible without resulting in hypoglycaemia, liver damage, severe acute renal failure and cellular respiration dysfunction.43,44 Finally, it has been shown that activity of the isoform COX-1 is not affected by vanadium compounds.45
In conclusion, the experiments outlined above show that, under our working conditions, sodium tungstate appears to be more effective than vanadyl sulfate for reversing metabolic and vascular conditions associated with STZ treatment.
Conflicts of interestNone.
This paper was financed by the following grants: Universidad de Buenos Aires (UBACyT 20020130200105BA and UBACYT 20020130100019BA) (2014–2017).
Please cite this article as: Lee HJ, Peredo HA, Cantú SM, Donoso AS, Puyó AM, Choi MR. Efectos del tungstato de sodio y sulfato de vanadilo sobre la liberación de prostanoides del lecho vascular mesentérico de ratas diabéticas. Clín Investig Arterioscler. 2018;30:249–257.