Gran Canaria is a region of genetic isolation of familial hypercholesterolemia due to a founder mutation, p.[Tyr400_Phe402del], in the LDL receptor (LDLR) gene. Initial data suggest that its carriers could have a high prevalence of diabetes.
Material and methodsPatients over 30 years of age with familial hypercholesterolemia and a confirmed mutation in LDLR were recruited from a tertiary hospital in Gran Canaria. The prevalence of diabetes and other clinical data were compared among carriers of p.[Tyr400_Phe402del] and those with other LDLR mutations.
Results76.4% of the 89 participants were carriers of p.[Tyr400_Phe402del]. The prevalence of diabetes in this group was significantly higher (25 vs. 4%, p = .045). These cases also had a higher prevalence of cardiovascular disease and higher levels of LDL cholesterol and triglycerides. There were no differences in age, weight, body mass index, waist, age of onset, and time of statin treatment. However, they required PCSK9 inhibitors more often (51.5 vs. 24%, p = .027).
ConclusionsThe mutation p.[Tyr400_Phe402del] is associated with a high prevalence of diabetes, not explained by classic risk factors, such as age, obesity, or long-term use of statins.
Gran Canaria es una región de aislamiento genético para hipercolesterolemia familiar, debido a una mutación fundadora, p.[Tyr400_Phe402del], en el gen del receptor de LDL (LDLR). Datos iniciales indican que sus portadores podrían tener una alta prevalencia de diabetes.
Material y métodosSe reclutó a los pacientes mayores de 30 años con hipercolesterolemia familiar y mutación confirmada en LDLR en un hospital de tercer nivel de Gran Canaria y se comparó la prevalencia de diabetes y otros datos clínicos entre los portadores de p.[Tyr400_Phe402del] y los de otras mutaciones en LDLR.
ResultadosEl 76,4% de los 89 participantes era portador de p.[Tyr400_Phe402del]. En ese grupo la prevalencia de diabetes fue significativamente más alta (25 vs. 4%, p = 0,045). Dichos casos también tenían mayor prevalencia de enfermedad cardiovascular y niveles más altos de colesterol LDL y triglicéridos. No hubo diferencias en edad, peso, índice de masa corporal, cintura, edad de inicio y tiempo de tratamiento con estatinas. Sin embargo, sí precisaban más a menudo inhibidores de PCSK9 (51,5 vs. 24%, p = 0,027).
ConclusionesLa mutación p.[Tyr400_Phe402del] se asocia a una elevada prevalencia de diabetes, no explicada por factores de riesgo clásicos, como la edad, la obesidad o el uso prolongado de estatinas.
Familial hypercholesterolaemia (FH) is characterised by very high concentrations of LDL cholesterol (LDL-c) and a high risk of premature cardiovascular disease (CVD).1 It has an autosomal dominant inheritance and is caused by mutations of different genes involved in the metabolism of LDL-c: LDL receptor (LDLR), apolipoprotein B (APOB), apolipoprotein E (APOE), proprotein convertase subtilisin/kexin type 9 (PCSK9) and LDL receptor-adaptive protein type 1 (LDLRAP1), with the latter being responsible for a recessive form of the disease.2
In Spain over 400 mutations in LDLR have been identified, none of which explain more than 7% of cases.3 However, one study recently conducted in the island of Gran Canaria has outlined the existence of a founder mutation effect, p.[Tyr400_Phe402del] in the LDLR gene, which represents almost 70% of patients with a positive genetic diagnosis in this region, and for which it is considered a population of genetic isolation for FH.4
One peculiar characteristic, initially observed in the carriers of p.[Tyr400_Phe402del] was the high prevalence of diabetes (DM) type 2, present in 17.8% of the subjects studied.4 This is a higher figure than expected in the general population and much higher than that described in patients with FH carriers of mutations in LDLR in other populations.5 Patients with FH generally receive prolonged treatment with statins and this has been associated with a higher risk of DM of between 9% and 25%.6 However, studies published up until now indicate that, unlike that observed in the general population, patients with FH appear to be protected against this diabetogenic effect of statins.7 In fact, several studies have shown that the prevalence of DM is lower among patients with FH than among their family members not affected by the disease.5 This finding was also corroborated in Spain, in patients with heterozygous FH from the National Register of Dyslipidaemias of the Spanish Arteriosclerosis Society where prevalence of DM was somewhat lower than that observed in the general Spanish society (5.94% vs. 9.44%),8 which could be at least partly related to a healthier lifestyle among people with FH.9
The high prevalence of DM in individuals who present with the founder mutation effect in Gran Canaria has no clear explanation. The aim of this study was to compare the frequency and characteristics of diabetes in the carriers of p.[Tyr400_Phe402del] compared with the carriers of other mutations of LDLR on the island.
Material and methodsAll patients over 30 years who attended the Lipid Unit of the Complejo Hospitalario Universitario Insular Materno-Infantil with heterozygous FH and mutations confirmed in the LDLR gene were recruited and the carriers of p.[Tyr400_Phe402del] were compared with the others, extending the sample of the previous study,4 from which individuals under 30 years of age were also excluded so as to better estimate the prevalence of DM in the adult population.
Patients in treatment with glucocorticoids with a history of pancreatectomy, DM type one of other known causes of secondary DM were excluded.
Study protocolThe following were recorded for each participant: age, sex, known history of diabetes, age at diagnosis of the disease when known, time of evolution, type of hypoglycaemic treatment, chronic complications, and history of diabetes in first-degree relatives. Other data were also analysed, including the outcomes of the genetic LDLR study, personal background of CVD, type and age at onset, background tobacco habit and high blood pressure and type of hypolipidemic treatment (age at start and duration of treatment). The baseline serum levels were recorded and those most recently available with hypolipidemic treatment of total cholesterol, HDL cholesterol (HDL-c), LDL-c, triglycerides, lipoprotein (a) [lp(a)], and baseline glycaemia and glycated haemoglobin (HbA1c). The physical examination included weight, height, waist circumference, blood pressure, existence of corneal arc and tendon xanthomas. Body mass index (BMI) was also calculated.
The diagnosis of DM was established in those individuals who had a previous documented diagnosis in the clinical record or when the baseline glycaemia was >125 mg/dl and/or HbA1c era ≥6,5%.
All patients were informed about the procedure of the study and signed an informed consent form in compliance with the ethical standards of the 1975 Declaration of Helsinki. The study was approved by the ethics committee of the centre.
Genetic analysisThe variant p.[Tyr400_Phe402del] in LDLR was genotyped in all the subjects by a previously described procedure.4 In those whose analysis of this variant was negative, we proceeded with gene sequencing LDLR, APOB, PCSK9, APOE, LDLRAP1 y STAP1 (Progénika Biopharma, Gendiag Laboratories). The mutations were classified as non-pathogenic or benign, possibly non-pathogenic, of uncertain clinical significance, possibly pathogenic or pathogenic according to the Scientific Association of Clinical Genetics.10 For this analysis we only included the carriers of mutations in LDLR and we compared the carriers of the prevalent mutation p.[Tyr400_Phe402del] with the others.
Statistical analysisData was summarised as means ± SD or medians (interquartile range) for continuous variables and frequencies (%) for categorical variables. For the different comparisons between groups, the percentages were compared using the Chi-squared test (χ2), the means by the Student’s T-test, and the medians through the Wilcoxon test for independent data. Statistical significance was established as p < .05. Data was analysed using the statistical package SPSS, version 20.0 (IBM Corporation, Armonk, NY).
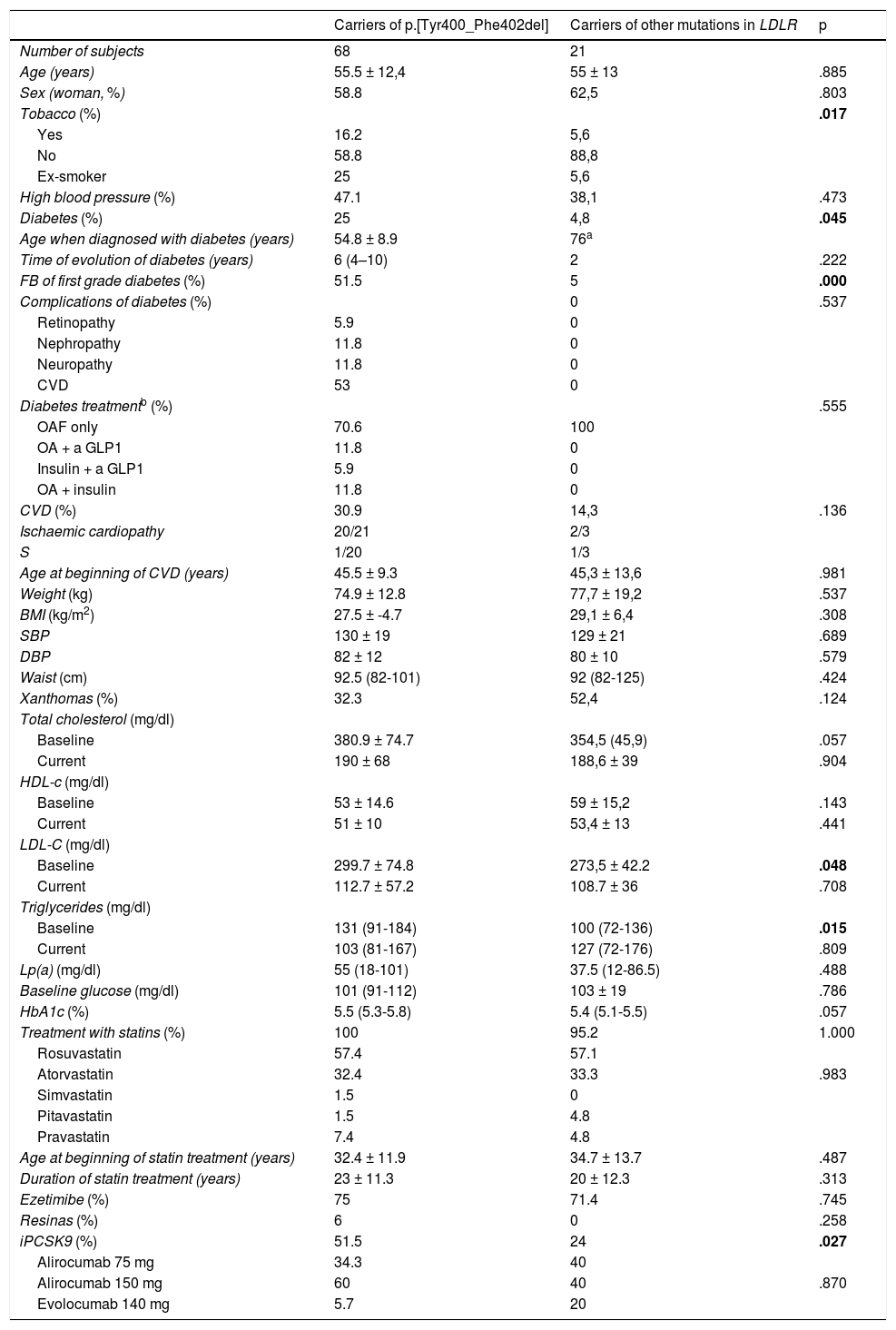
ResultsEight nine patients belonging to 46 families with FH with positive genetic diagnosis were included, of whom 68 (76.4%) were carriers of the mutation p.[Tyr400_Phe402del]. The clinical characteristics of participants were collected in Table 1, in accordance with the presence or absence of the founder mutation. The prevalence of diabetes was higher among those with the p.[Tyr400_Phe402del] (25 vs. 4.8%; p = .045) variant. The mean age of diabetes presentation was 55 years. 51.5% of patients with the founder mutation had a history of diabetes among first-degree relatives, compared with just 5% of the carriers of other mutations (p < .0001) and the percentage of smokers and ex-smokers was higher (16.5 and 25 vs. 5.6 and 5.6%; p = .017). Among the patients with diabetes, 12% of the carriers of the variant p.[Tyr400_Phe402del] had developed nephropathy and others had developed diabetic polyneuropathy. There were no differences between groups among variables characteristically associated with diabetes, like age, BMI, waist circumference or blood pressure figures. The prevalence of CVD established was almost double among the carriers of the mutation than among the others (31% vs.14%), although these differences were not statistically significant. The baseline levels of LDL-c and triglycerides were higher in the carriers of p.[Tyr400_Phe402del], but there were no differences in the post-treatment lipids between groups. Regarding treatment, a high percentage of participants were in treatment with high intensity statins and ezetimiba, with no differences between groups, but the use of iPCSK9 was higher among the carriers of the founder mutation (51.5% vs. 24%, p = .027). There were no differences in either age at the beginning of statin treatment nor in the total treatment time.
Comparison of the clinical and biochemical characteristics of the mutation carriers p.[Tyr400_Phe402del] with the carriers of other mutations in LDLR.
| Carriers of p.[Tyr400_Phe402del] | Carriers of other mutations in LDLR | p | |
|---|---|---|---|
| Number of subjects | 68 | 21 | |
| Age (years) | 55.5 ± 12,4 | 55 ± 13 | .885 |
| Sex (woman, %) | 58.8 | 62,5 | .803 |
| Tobacco (%) | .017 | ||
| Yes | 16.2 | 5,6 | |
| No | 58.8 | 88,8 | |
| Ex-smoker | 25 | 5,6 | |
| High blood pressure (%) | 47.1 | 38,1 | .473 |
| Diabetes (%) | 25 | 4,8 | .045 |
| Age when diagnosed with diabetes (years) | 54.8 ± 8.9 | 76a | |
| Time of evolution of diabetes (years) | 6 (4–10) | 2 | .222 |
| FB of first grade diabetes (%) | 51.5 | 5 | .000 |
| Complications of diabetes (%) | 0 | .537 | |
| Retinopathy | 5.9 | 0 | |
| Nephropathy | 11.8 | 0 | |
| Neuropathy | 11.8 | 0 | |
| CVD | 53 | 0 | |
| Diabetes treatmentb (%) | .555 | ||
| OAF only | 70.6 | 100 | |
| OA + a GLP1 | 11.8 | 0 | |
| Insulin + a GLP1 | 5.9 | 0 | |
| OA + insulin | 11.8 | 0 | |
| CVD (%) | 30.9 | 14,3 | .136 |
| Ischaemic cardiopathy | 20/21 | 2/3 | |
| S | 1/20 | 1/3 | |
| Age at beginning of CVD (years) | 45.5 ± 9.3 | 45,3 ± 13,6 | .981 |
| Weight (kg) | 74.9 ± 12.8 | 77,7 ± 19,2 | .537 |
| BMI (kg/m2) | 27.5 ± -4.7 | 29,1 ± 6,4 | .308 |
| SBP | 130 ± 19 | 129 ± 21 | .689 |
| DBP | 82 ± 12 | 80 ± 10 | .579 |
| Waist (cm) | 92.5 (82-101) | 92 (82-125) | .424 |
| Xanthomas (%) | 32.3 | 52,4 | .124 |
| Total cholesterol (mg/dl) | |||
| Baseline | 380.9 ± 74.7 | 354,5 (45,9) | .057 |
| Current | 190 ± 68 | 188,6 ± 39 | .904 |
| HDL-c (mg/dl) | |||
| Baseline | 53 ± 14.6 | 59 ± 15,2 | .143 |
| Current | 51 ± 10 | 53,4 ± 13 | .441 |
| LDL-C (mg/dl) | |||
| Baseline | 299.7 ± 74.8 | 273,5 ± 42.2 | .048 |
| Current | 112.7 ± 57.2 | 108.7 ± 36 | .708 |
| Triglycerides (mg/dl) | |||
| Baseline | 131 (91-184) | 100 (72-136) | .015 |
| Current | 103 (81-167) | 127 (72-176) | .809 |
| Lp(a) (mg/dl) | 55 (18-101) | 37.5 (12-86.5) | .488 |
| Baseline glucose (mg/dl) | 101 (91-112) | 103 ± 19 | .786 |
| HbA1c (%) | 5.5 (5.3-5.8) | 5.4 (5.1-5.5) | .057 |
| Treatment with statins (%) | 100 | 95.2 | 1.000 |
| Rosuvastatin | 57.4 | 57.1 | |
| Atorvastatin | 32.4 | 33.3 | .983 |
| Simvastatin | 1.5 | 0 | |
| Pitavastatin | 1.5 | 4.8 | |
| Pravastatin | 7.4 | 4.8 | |
| Age at beginning of statin treatment (years) | 32.4 ± 11.9 | 34.7 ± 13.7 | .487 |
| Duration of statin treatment (years) | 23 ± 11.3 | 20 ± 12.3 | .313 |
| Ezetimibe (%) | 75 | 71.4 | .745 |
| Resinas (%) | 6 | 0 | .258 |
| iPCSK9 (%) | 51.5 | 24 | .027 |
| Alirocumab 75 mg | 34.3 | 40 | |
| Alirocumab 150 mg | 60 | 40 | .870 |
| Evolocumab 140 mg | 5.7 | 20 | |
BMI: body mass index; CVD: cardiovascular disease; FB: family background; HbA1c: glycated haemoglobin; HDL: high density lipoproteins; LDL: low density lipoproteins; Lp(a): lipoprotein (a); OA: oral agents; PAD: diastolic blood pressure; PCSK9i: PCSK9 inhibitors; S: stroke; SBP: systolic blood pressure.
The value of p < .05 is in bold, in each case.
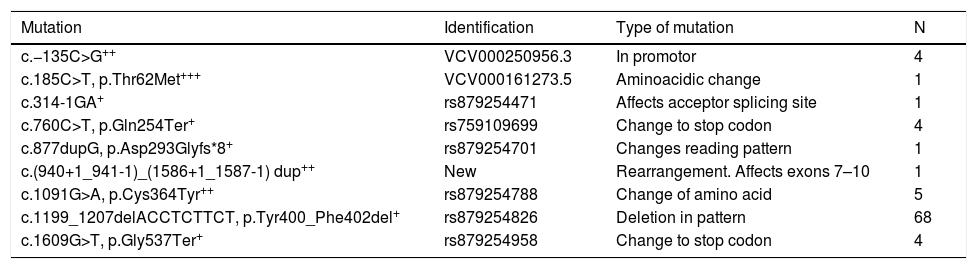
Apart from p.[Tyr400_Phe402del], another 8 pathogenic or possibly pathogenic mutations were found in the LDLR (Table 2). The founder mutation is a deletion of 3 amino acids in the YWTD domain of the protein, which could affect the interaction with PCSK9 and/or their processing.4 The [c.877dupG, p.Asp293Glyfs*8] was described for the first time in this population,4 and is a duplication which breaks the reading framework generating a truncated protein, identified in a male aged 45 years with ischaemic cardiopathy at 33 years of age and levels without treatment of total cholesterol of 346 mg/dl and LDL-c of 258 mg/dl. The variant c.(940+1_941-1)_(1586+1_1587-1) dup was not previously described and is a rearrangement with changes of the connection affecting exons 7–10, generating a null allele, and was identified in a male patient of 42 with total cholesterol levels and LDL-c of 340 mg/dl and 274 mg/dl respectively, with 2 siblings with hypercholesterolaemia and ischaemic cardiopathy at 40 years of age.
Mutations found in the population.
| Mutation | Identification | Type of mutation | N |
|---|---|---|---|
| c.−135C>G++ | VCV000250956.3 | In promotor | 4 |
| c.185C>T, p.Thr62Met+++ | VCV000161273.5 | Aminoacidic change | 1 |
| c.314-1GA+ | rs879254471 | Affects acceptor splicing site | 1 |
| c.760C>T, p.Gln254Ter+ | rs759109699 | Change to stop codon | 4 |
| c.877dupG, p.Asp293Glyfs*8+ | rs879254701 | Changes reading pattern | 1 |
| c.(940+1_941-1)_(1586+1_1587-1) dup++ | New | Rearrangement. Affects exons 7–10 | 1 |
| c.1091G>A, p.Cys364Tyr++ | rs879254788 | Change of amino acid | 5 |
| c.1199_1207delACCTCTTCT, p.Tyr400_Phe402del+ | rs879254826 | Deletion in pattern | 68 |
| c.1609G>T, p.Gly537Ter+ | rs879254958 | Change to stop codon | 4 |
The numbers indicated in the table refer to the transcription of reference NM_000527.5, which results in the peptide of reference NP_000518.1.
Identification of the variants refers to ClinVar (VCV; https://www.ncbi.nlm.nih.gov/clinvar) o a dbSNP (rs; https://www.ncbi.nlm.nih.gov/snp).
This study confirms the initial observation4 that the population with the variant p.[Tyr400_Phe402del] in LDLR, a founder mutation of FH characteristic of Gran Canaria, has a high prevalence of DM (25%), much higher than the existing one between carriers of other mutations of this gene coming from the island (4%). Several studies have examined the relationship between FH and DM and up until now it has been observed that the percentage of patients with DM in the population with FH is lower than that of the general population, being lower in those with more aggressive mutations in LDLR.5,11 The data found in the group of FH with other mutations in LDLR in our population confirm this low prevalence, despite being in prolonged treatment with high intensity statins (20 ± 12 years) and have a mean BMI in the overweight range, which is associated with a higher risk of diabetes.6 In the Spanish Register of Dyslipedaemias of the SEA a prevalence of diabetes of 5.94% was obtained among the heterozygous FH population, 40% less than in the general Spanish population. The risk factors for diabetes in this study were age, male sex, high BMI, presence of high blood pressure, high levels of triglycerides and duration of treatment with statins. However, the diabetes was not linked to baseline concentrations of LDL-c or the presence of any specific mutation.8
The frequency of DM in the carriers of the mutation p.[Tyr400_Phe402del] is located at higher levels than those of the general Spanish population12 and those previously observed in the same geographic area of Gran Canaria.13 In the age group of patients in this study, whose mean age was 55.5 ± 12 years, the prevalence of diabetes in the general population is that of 10%–15% in Spain12 and of 16.5% in Gran Canaria.13 No age differences were found, or differences in BMI, waist circumference, time of treatment and age at beginning with statins between the 2 groups analysed. These factors could explain a higher prevalence of DM. The carriers of p.[Tyr400_Phe402del] also exhibited a more adverse lipid profile, with higher levels of LDL-c and triglycerides, a finding which could precisely be explained by the greater frequency of DM this finding coincides with a recent study of patients with type 2 DM and FH of the national register of the SEA.14 Furthermore, the clarification of particulars rich in triglycerides is also realized from the LDL receptor. The higher concentration of triglycerides of this group of patients could be alternatively associated with the singular effects of the actual mutation. This hypertriglyceridaemia could contribute to the development of resistance to insulin and the dysfunction of beta cells, increasing the susceptibility to diabetes.15
Regarding complications, patients with DM did not present with many microvascular complications, but with a high percentage of established CVD (53%). DM is a separate risk factor in the development of CVD in patients with FH14 and this may explain the higher frequency of CVD in the carriers of p.[Tyr400_Phe402del] (31% vs. 14%).
Lastly, the group of individuals with the founder mutation required treatment with iPCSK9 (51,5 vs. 24%, p = 0,027) more frequently. During the last few years studies have appeared that relate the use of iPCSK9 with significant increases in glucose and HbA1c,16 and therefore a possible relationship between the activity of PCSK9 with hydrocarbonate metabolism has been suggested.17 The p.[Tyr400_Phe402del] mutation affects the highly preserved YWTD domain, which could give rise to greater affinity by PCSK9.4 This last point could indicate that certain metabolic routes, possibly directly or indirectly affecting the activity of PCSK9, could simultaneously include hypercholesterolemia, resistance to insulin and changes to the metabolism of glucose. Also, it is not to be ruled out that in this population of genetic isolation, as of yet unknown, anomalies may exist, that could jointly segregate with the mutation p.[Tyr400_Phe402del], either because they are found in genetic linkage or because they are found more frequently in this group, as all their members have a common ancestor. These new genetic variants, yet unknown, would then be the ones which could modify the homeostasis of glucose in the carriers of this mutation in LDLR, with contradictory effects to those that seem to provoke other already known pathogenic mutations.
ConclusionsThe island of Gran Canaria is a region of genetic isolation for the mutation p.[Tyr400_Phe402del] of LDLR. This mutation is associated with a very high prevalence of diabetes, which could explain the more adverse phenotype of these patients. This high prevalence cannot be explained by risk factors for diabetes, such as age, being overweight, obesity or prolonged treatment with statins. More extensive familial studies are required to assess the aggregation between this mutation and diabetes, to better explain this finding.
LimitationsThe main limitation of this study is the sample size, particularly regarding the subjects with FH carriers of non-prevalent mutations. This hinders the estimation of diabetes prevalence within this group. However, the prevalence in the group of carriers of p.(Tyr400_Phe402del), which is a larger group, is superior to the Spanish and Canary Island population and this offers robustness to the observation.
Another limitation is that the levels of plasmatic insulin were not measured, and it was impossible to confirm whether differences existed between groups regarding insulin-resistance (HOMA-IR) or parameters of dysfunction of the beta cell (HOMA-beta).
FinancingThis study received financing from the Spanish Society of Arteriosclerosis, Spain (Clinical epidemiological grant 2015) and from the Official College of Physicians of Las Palmas, Spain (González Jaraba 2015 grant).
Conflict of interestsThe authors have no conflicts of interest to declare regarding this study.
Please cite this article as: Sánchez-Hernández RM, González-Lleó AM, Tugores A, Brito-Casillas Y, Civeira F, Boronat M, et al. Hipercolesterolemia familiar en Gran Canaria: mutación con efecto fundador y alta frecuencia de diabetes. Clin Investig Arterioscler. 2021;33:247–253.