Metabolic syndrome (MetS) has been frequently observed in patients with acute myocardial infarction (AMI). However, there is limited research assessing the impact of metabolic syndrome on coronary artery severity in patients with acute myocardial infarction.
MethodsWe analyzed 199 patients with AMI who underwent invasive coronary angiography. This study aimed to determine the impact of MetS, MetS score and its components on coronary artery severity.
ResultsThe study comprised 199 eligible patients, with an average age of 64.5±11.3 years. Among the entire cohort, 136 patients (68.3%) were diagnosed with MetS. The MetS 3 subgroup with three components exhibited the highest percentage at 29.2%. The proportion of one-vessel, two-vessel, three-vessel, multi-vessel disease, or left main disease did not differ between the MetS and non-MetS groups (p>0.05). Our study revealed that the MetS group had a higher median Gensini score compared to the non-MetS group (p=0.002). Furthermore, the Gensini score was significantly correlated with the MetS score (Spearman correlation 0.2, p<0.05). Among metabolic syndrome components, elevated waist circumference and elevated blood glucose were associated with the Gensini score.
ConclusionsOur study revealed that MetS, MetS score and two components of MetS, elevated waist circumference and elevated blood glucose, were associated with the severity of angiographic coronary artery in patients with AMI.
El síndrome metabólico (MetS) se ha observado con frecuencia en pacientes con infarto agudo de miocardio (IAM). Sin embargo, hay una investigación limitada que evalúa el impacto del MetS en la gravedad de las arterias coronarias en pacientes con IAM.
MétodosSe analizaron 199 pacientes con IAM que se sometieron a angiografía coronaria invasiva. Este estudio tuvo como objetivo determinar el impacto del MetS, el puntaje de MetS y sus componentes en la gravedad de las arterias coronarias.
ResultadosEl estudio incluyó a 199 pacientes elegibles, con una edad promedio de 64,5±11,3 años. En toda la cohorte, 136 pacientes (68,3%) fueron diagnosticados con MetS. El subgrupo MetS 3 con tres componentes mostró el porcentaje más alto con un 29,2%. La proporción de enfermedad de una arteria, dos arterias, tres arterias, enfermedad multivaso o enfermedad de tronco de la coronaria izquierda no difirió entre los grupos MetS y no MetS (p>0,05). Nuestro estudio reveló que el grupo MetS tenía una puntuación media de Gensini más alta en comparación con el grupo no MetS (p=0,002). Además, la puntuación de Gensini se correlacionó significativamente con el puntaje de MetS (correlación de Spearman 0,2, p<0,05). Entre los componentes del MetS, la circunferencia de la cintura elevada y la glucosa en sangre elevada se asociaron con la puntuación de Gensini.
ConclusionesNuestro estudio reveló que el MetS, el puntaje de MetS y dos componentes del MetS, la circunferencia de la cintura elevada y la glucosa en sangre elevada, estaban asociados con la gravedad de las arterias coronarias angiográficas en pacientes con IAM.
Metabolic syndrome (MetS), known as Syndrome X or insulin resistance syndrome, is a collection of cardiovascular risk factors, including elevated blood pressure, elevated blood glucose, elevated waist circumference, and dyslipidemia.1 MetS increases the risk of type 2 diabetes mellitus and atherosclerotic cardiovascular diseases, especially AMI, by 2.2–2.7 times.2–4 The estimated prevalence of metabolic syndrome in the European population ranges from 12 to 26%, in the Asian population from 12 to 37%, and in the United States about 40%.5,6 In patients with AMI, the prevalence of metabolic syndrome is even higher, ranging from 46 to 62%.7–10
Metabolic syndrome, through its mechanisms involving arterial atherosclerosis, vascular calcification, and the impairment of the microvascular system – particularly the coronary microvascular system,11,12 contributes to increased in-hospital mortality and a higher rate of severe heart failure. It serves as a predictor for short-term mortality and increased major cardiovascular events in patients with AMI post-discharge.7,9,10
Each abnormal component independently contributes to arterial atherosclerosis. However, when these components are combined in metabolic syndrome, their impact on atherosclerosis and the extent of coronary vascular damage may exhibit variability. There were many researches with diverse results regarding the impact of metabolic syndrome components on the severity of coronary artery disease.13–15 However, in Asia, reseaches were primarily carried out in East and South Asia. Meanwhile, the Southeast Asian region, which experienced a high burden of cardiovascular diseases,16 especially coronary artery disease, had very limited research on this issue.
Therefore, we conducted this study to determine the impact of MetS on the severity of angiographic coronary arteries in patients with acute myocardial infarction who underwent invasive coronary angiography in a tertiary hospital in Vietnam, a developing country in Southest Asia.
Material and methodsSubjectsThis prospective cohort study was conducted at the Department of Interventional Cardiology, Cho Ray Hospital, from November 2022 to May 2023. The inclusion criteria were as follows: (1) age ≥18 years, (2) diagnosis of AMI type I according to the Fourth Universal Definition of Myocardial Infarction based on coronary angiography,17 and (3) willingness to participate in the study. The exclusion criteria included secondary hyperlipidemia, secondary hypertension, and the use of lipid-lowering drugs such as statins, fenofibrate, or ezetimibe within four weeks prior to hospital admission.
Ethical issues and informed consentThe study adhered to the principles of the Declaration of Helsinki and obtained approval from the ethics committee in biomedical research at the University of Medicine and Pharmacy in Ho Chi Minh City and Cho Ray Hospital (ID: 22592-DHYD) on 31st October 2022. All of the included patients were asked to sign informed consent and understood that research results can be used for publication but all personal identification will remain confidential.
Study variablesAll patients who met the study criteria were given detailed medical history and demographics. Weight, height, and waist circumference were measured by the same researcher. Waist circumference was calculated at the midpoint between the lower rib margin and the top of the iliac crest in the horizontal plane when the patient stood upright and measurements were taken at the end of normal exhalation. Blood pressure measurements were obtained while the patient seated and the average of three measurements was recorded. Fasting blood glucose, total cholesterol, triglycerides, LDL-C, and HDL-C were obtained at 5:00 a.m. after the day of admission. LDL-C was calculated using the Friedewald's formula based on total cholesterol, HDL-C, and triglycerides.18
Metabolic syndrome is diagnosed based on consensus on the definition of MS for the Asian population published in 2009,19 with the endorsement of IDF, AHA, ACC, WHF, IAS, and IASO. The syndrome is considered to be present when at least three out of five criteria are met: (1) elevated waist circumference (≥80cm in females or ≥90cm in males), (2) elevated blood pressure (SBP ≥130mmHg and/or DBP ≥85mmHg, or currently undergoing treatment for hypertension), (3) elevated blood glucose (fasting blood glucose ≥100mg/dL or currently on diabetes treatment), (4) elevated triglycerides (≥150mg/dL or currently on lipid-lowering treatment for elevated triglycerides), (5) reduced HDL-C (<40mg/dL in males or <50mg/dL in females, or currently on lipid-lowering treatment for reduced HDL-C). According to the existence of these criteria, the metabolic syndrome score (MetS score) was calculated based on the number of metabolic syndrome components, ranging from 0 to 5, corresponding to MetS0 to MetS5.
All patients underwent invasive coronary angiography and the results were evaluated by a minimum of two interventional physicians. The severity of coronary artery disease was determined by the Gensini score assessment system.20 The degree of stenosis and the coronary artery lesion site were scored as follows: 1 point for less than 25% stenosis, 2 points for 26–50% stenosis, 4 points for 51–75% stenosis, 8 points for 76–90% stenosis, 16 points for 91–99% stenosis, and 32 points for total occlusion. Thereafter, each lesion score was multiplied by a factor that took into account the importance of the lesion's position in the coronary circulation (5 for the left main coronary artery, 2.5 for the proximal segment of the left anterior descending coronary artery, 2.5 for the proximal segment of the circumflex artery, 1.5 for the mid-segment of the left anterior descending coronary artery, 1.0 for the right coronary artery, the distal segment of the left anterior descending coronary artery, the posterolateral artery, and the obtuse marginal artery, and 0.5 for other segments). The Gensini score was determined by adding up the scores of each individual coronary segment.
StatisticsData entry and processing were performed utilizing Stata 14.2 software on a Windows operating system (StataCorp, 2015. Stata Statistical Software: Release 14. College Station, TX, StataCorp LP). The Shapiro–Wilk test was employed to assess the normality of numerical variables. Continuous variables exhibiting a normal distribution were presented as mean±standard deviation. In cases where the distributions deviated from normality, the description utilized the median (25th–75th quartiles). Categorical and ordinal variables were defined in terms of frequencies and percentages. Differences in means between groups were compared using the t-test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. Differences in the frequency distributions of categorical variables were assessed using the chi-square test (χ2) or Fisher's exact test. We compared Gensini score in metabolic syndrome score subgroups using the Kruskal–Wallis test. Find the correlation between the metabolic syndrome components with the Gensini score using Sperman correlation. A p-value of<0.05 was considered statistically significant.
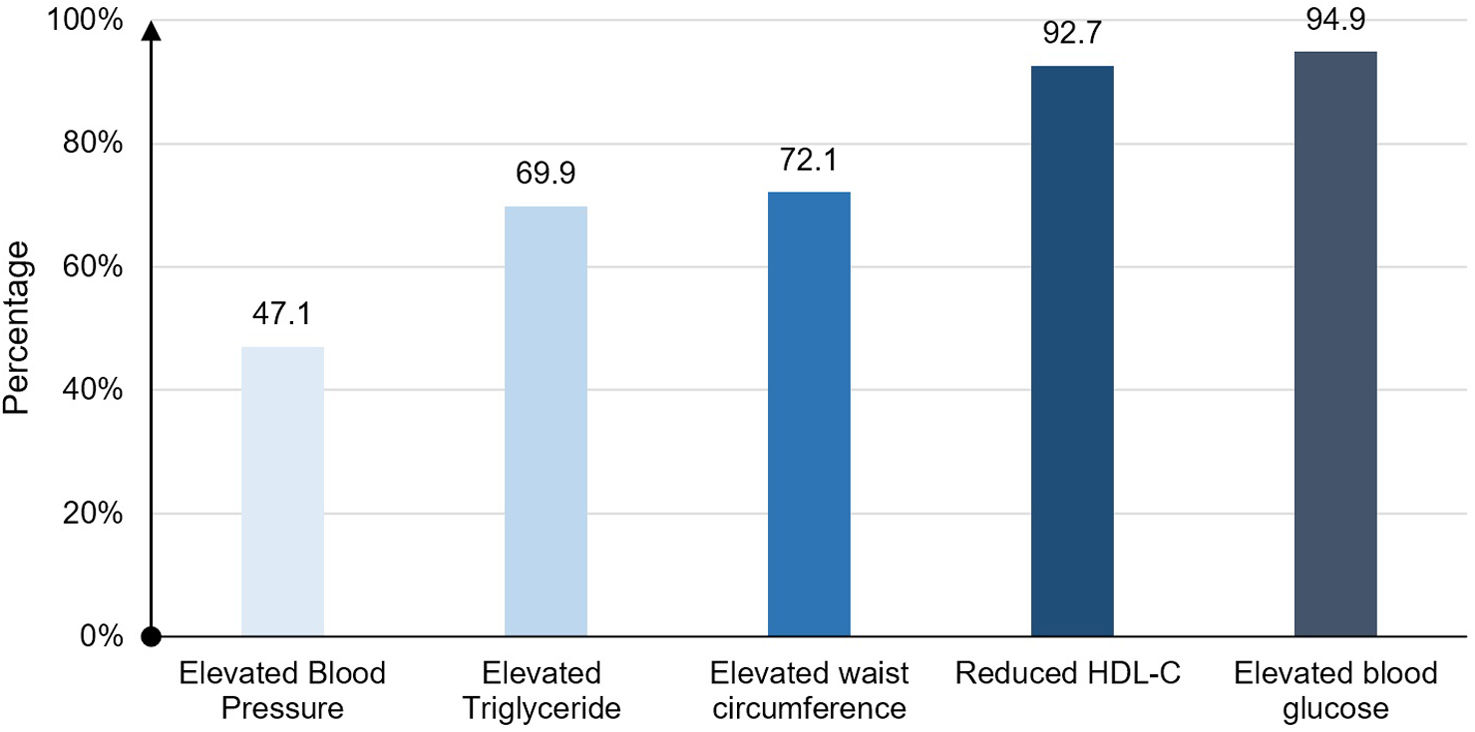
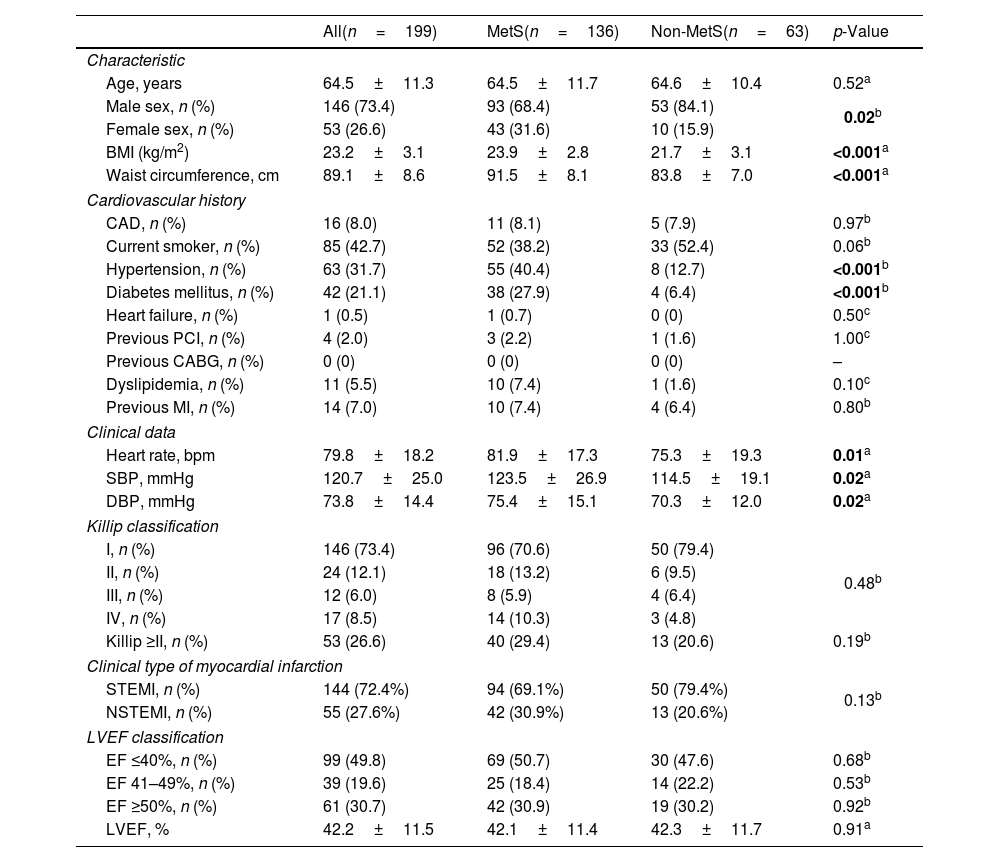
ResultsBaseline characteristicsFrom November 2022 to May 2023, we enrolled 199 patients with acute myocardial infarction who met the inclusion criteria. In our study, the mean age was 64.5±11.3, of which 146 (73.4%) were male. A total of 136 (68.3%) patients had metabolic syndrome. The most common component in the MetS group was elevated blood glucose (94.9%) (Fig. 1).
Baseline characteristics of the study population of the two groups with and without MetS were presented in Table 1. Patients with MetS had a higher proportion of women, a higher BMI, larger waist circumference, and higher proportions of hypertension and diabetes mellitus than the non-MetS group (p<0.05). There was no significant difference between the two groups regarding the Killip class at admission, Killip class ≥II, proportion of STEMI and LVEF classification.
Baseline characteristics of study population.
| All(n=199) | MetS(n=136) | Non-MetS(n=63) | p-Value | |
|---|---|---|---|---|
| Characteristic | ||||
| Age, years | 64.5±11.3 | 64.5±11.7 | 64.6±10.4 | 0.52a |
| Male sex, n (%) | 146 (73.4) | 93 (68.4) | 53 (84.1) | 0.02b |
| Female sex, n (%) | 53 (26.6) | 43 (31.6) | 10 (15.9) | |
| BMI (kg/m2) | 23.2±3.1 | 23.9±2.8 | 21.7±3.1 | <0.001a |
| Waist circumference, cm | 89.1±8.6 | 91.5±8.1 | 83.8±7.0 | <0.001a |
| Cardiovascular history | ||||
| CAD, n (%) | 16 (8.0) | 11 (8.1) | 5 (7.9) | 0.97b |
| Current smoker, n (%) | 85 (42.7) | 52 (38.2) | 33 (52.4) | 0.06b |
| Hypertension, n (%) | 63 (31.7) | 55 (40.4) | 8 (12.7) | <0.001b |
| Diabetes mellitus, n (%) | 42 (21.1) | 38 (27.9) | 4 (6.4) | <0.001b |
| Heart failure, n (%) | 1 (0.5) | 1 (0.7) | 0 (0) | 0.50c |
| Previous PCI, n (%) | 4 (2.0) | 3 (2.2) | 1 (1.6) | 1.00c |
| Previous CABG, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Dyslipidemia, n (%) | 11 (5.5) | 10 (7.4) | 1 (1.6) | 0.10c |
| Previous MI, n (%) | 14 (7.0) | 10 (7.4) | 4 (6.4) | 0.80b |
| Clinical data | ||||
| Heart rate, bpm | 79.8±18.2 | 81.9±17.3 | 75.3±19.3 | 0.01a |
| SBP, mmHg | 120.7±25.0 | 123.5±26.9 | 114.5±19.1 | 0.02a |
| DBP, mmHg | 73.8±14.4 | 75.4±15.1 | 70.3±12.0 | 0.02a |
| Killip classification | ||||
| I, n (%) | 146 (73.4) | 96 (70.6) | 50 (79.4) | 0.48b |
| II, n (%) | 24 (12.1) | 18 (13.2) | 6 (9.5) | |
| III, n (%) | 12 (6.0) | 8 (5.9) | 4 (6.4) | |
| IV, n (%) | 17 (8.5) | 14 (10.3) | 3 (4.8) | |
| Killip ≥II, n (%) | 53 (26.6) | 40 (29.4) | 13 (20.6) | 0.19b |
| Clinical type of myocardial infarction | ||||
| STEMI, n (%) | 144 (72.4%) | 94 (69.1%) | 50 (79.4%) | 0.13b |
| NSTEMI, n (%) | 55 (27.6%) | 42 (30.9%) | 13 (20.6%) | |
| LVEF classification | ||||
| EF ≤40%, n (%) | 99 (49.8) | 69 (50.7) | 30 (47.6) | 0.68b |
| EF 41–49%, n (%) | 39 (19.6) | 25 (18.4) | 14 (22.2) | 0.53b |
| EF ≥50%, n (%) | 61 (30.7) | 42 (30.9) | 19 (30.2) | 0.92b |
| LVEF, % | 42.2±11.5 | 42.1±11.4 | 42.3±11.7 | 0.91a |
Values were presented as mean±standard deviation or frequency (percentage). In bold, statistical significance. SD: standard deviation; BMI: body mass index; CAD: coronary artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft surgery; MI: myocardial infarction; SBP: systolic blood pressure; DBP: diastolic blood pressure; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; LVEF: left ventricular ejection fraction.
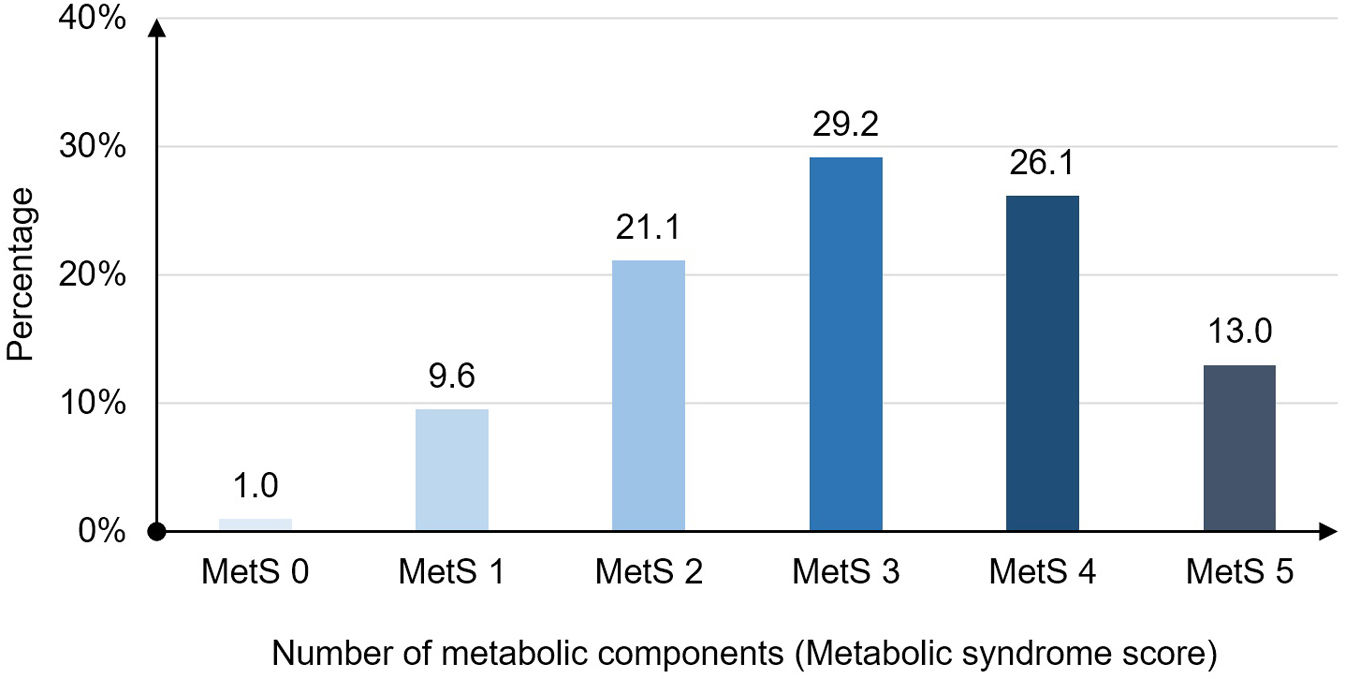
The distribution of patients according MetS score 0–5 (number of metabolic components) was presented in Fig. 2. MetS 3 subgroup with three components had the highest rate at 29.2%.
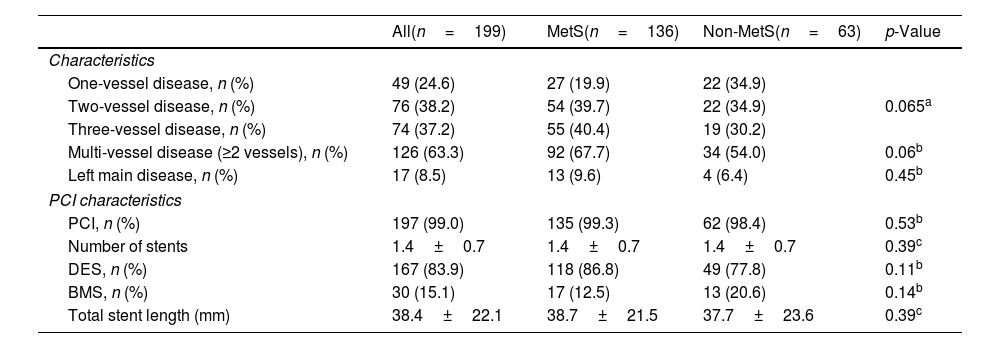
Association between metabolic syndrome, metabolic syndrome score, its components and Gensini scoreThere was no significant difference between the two groups regarding the proportion of one-vessel, two-vessel, three-vessel, multi-vessel disease or left main disease (p>0.05) (Table 2). A total of 197 (99.0%) patients were received percutaneous coronary intervention.
Coronary angiography characteristics.
| All(n=199) | MetS(n=136) | Non-MetS(n=63) | p-Value | |
|---|---|---|---|---|
| Characteristics | ||||
| One-vessel disease, n (%) | 49 (24.6) | 27 (19.9) | 22 (34.9) | 0.065a |
| Two-vessel disease, n (%) | 76 (38.2) | 54 (39.7) | 22 (34.9) | |
| Three-vessel disease, n (%) | 74 (37.2) | 55 (40.4) | 19 (30.2) | |
| Multi-vessel disease (≥2 vessels), n (%) | 126 (63.3) | 92 (67.7) | 34 (54.0) | 0.06b |
| Left main disease, n (%) | 17 (8.5) | 13 (9.6) | 4 (6.4) | 0.45b |
| PCI characteristics | ||||
| PCI, n (%) | 197 (99.0) | 135 (99.3) | 62 (98.4) | 0.53b |
| Number of stents | 1.4±0.7 | 1.4±0.7 | 1.4±0.7 | 0.39c |
| DES, n (%) | 167 (83.9) | 118 (86.8) | 49 (77.8) | 0.11b |
| BMS, n (%) | 30 (15.1) | 17 (12.5) | 13 (20.6) | 0.14b |
| Total stent length (mm) | 38.4±22.1 | 38.7±21.5 | 37.7±23.6 | 0.39c |
Values were presented as mean±standard deviation or median (IQR) or frequency (percentage). IQR: interquartile range; DES: drug eluting stent; BMS: bare metal stent; SD: standard deviation; PCI: percutaneous coronary intervention; MetS: metabolic syndrome.
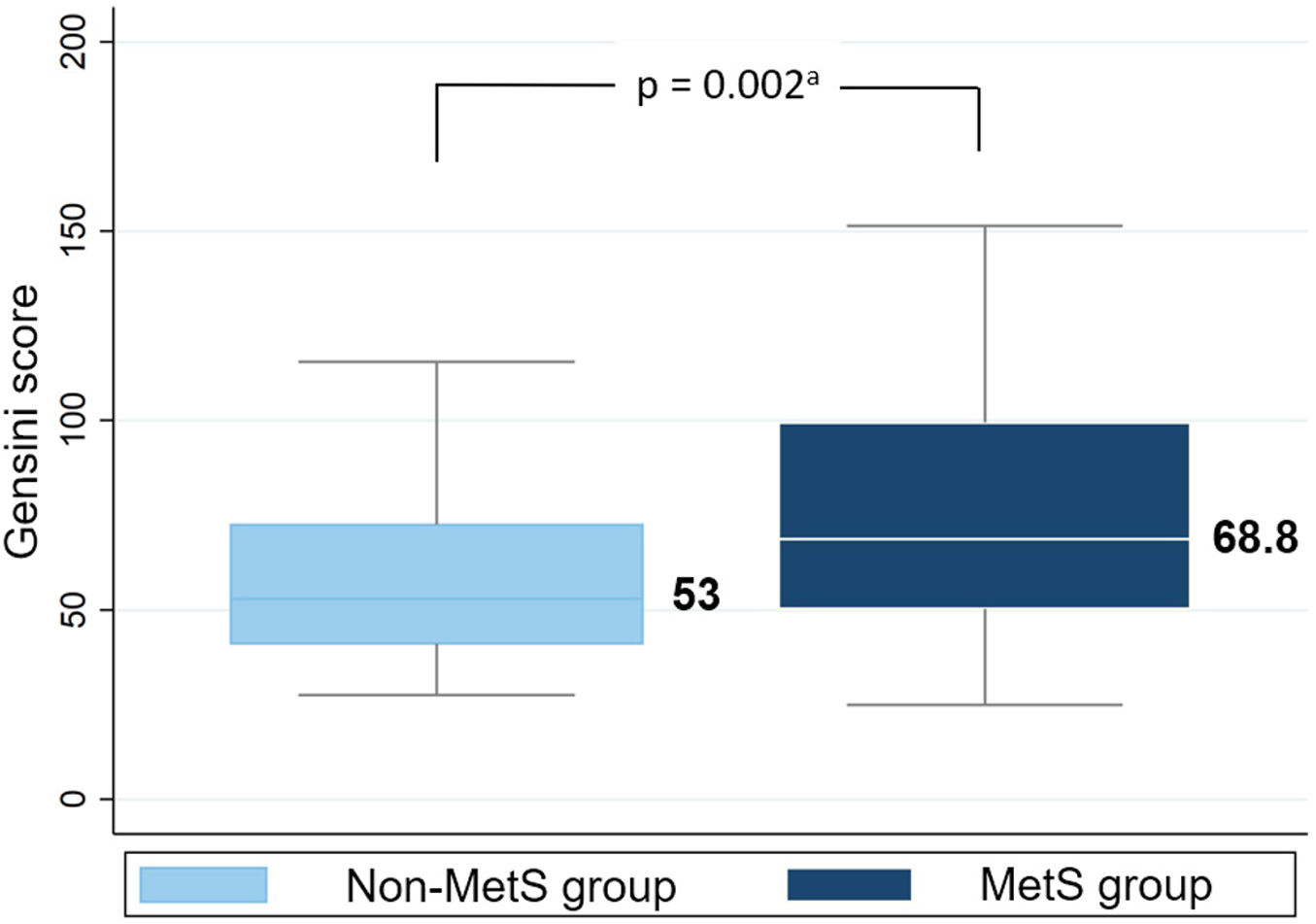
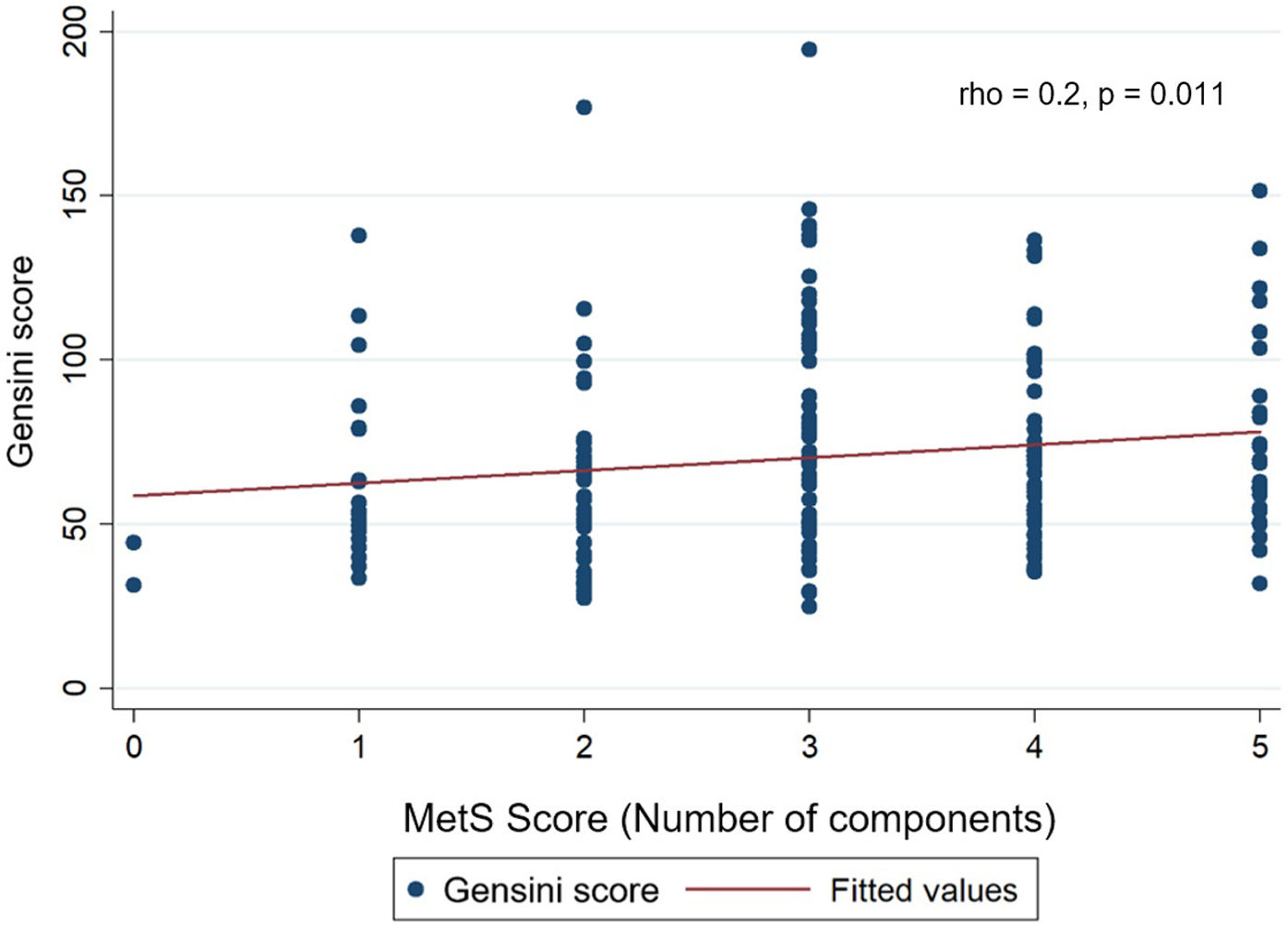
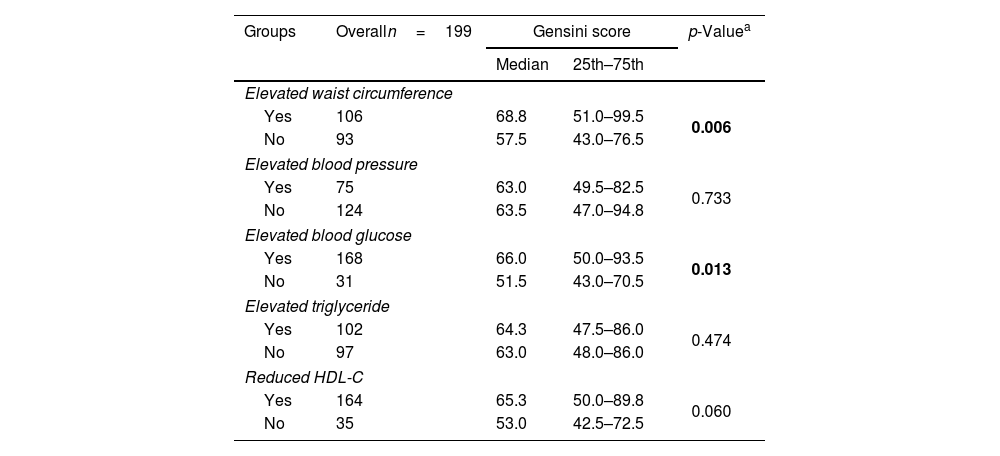
The median Gensini score in MetS group (68.8) was higher than non-MetS group (53) with p=0.002 (Mann–Whitney U test) (Fig. 3). Gensini score was significantly weak, positive correlated with MetS score (Spearman correlation rho=0.2, p=0.011) (Fig. 4). Among MetS components, elevated waist circumference and elevated blood glucose were significantly associated with the Gensini score (Table 3).
Association between MetS components and Gensini score.
| Groups | Overalln=199 | Gensini score | p-Valuea | |
|---|---|---|---|---|
| Median | 25th–75th | |||
| Elevated waist circumference | ||||
| Yes | 106 | 68.8 | 51.0–99.5 | 0.006 |
| No | 93 | 57.5 | 43.0–76.5 | |
| Elevated blood pressure | ||||
| Yes | 75 | 63.0 | 49.5–82.5 | 0.733 |
| No | 124 | 63.5 | 47.0–94.8 | |
| Elevated blood glucose | ||||
| Yes | 168 | 66.0 | 50.0–93.5 | 0.013 |
| No | 31 | 51.5 | 43.0–70.5 | |
| Elevated triglyceride | ||||
| Yes | 102 | 64.3 | 47.5–86.0 | 0.474 |
| No | 97 | 63.0 | 48.0–86.0 | |
| Reduced HDL-C | ||||
| Yes | 164 | 65.3 | 50.0–89.8 | 0.060 |
| No | 35 | 53.0 | 42.5–72.5 | |
In bold, statistical significance. HDL-C: high-density lipoprotein cholesterol; MetS: metabolic syndrome.
Our study observed 136 patients with metabolic syndrome (68.3%). Elevated blood glucose was the most common component at 94.9%, and the MetS 3 subgroup with three components had the highest rate at 29.2%. There was no difference in the proportion of one-vessel, two-vessel, three-vessel, multi-vessel disease, or left main disease between MetS group and non-MetS group. Gensini score was significantly weak correlated with MetS score (Spearman correlation rho=0.2, p=0.011). Among the components of MetS, only elevated waist circumference and elevated blood glucose were found to be related to the Gensini score (p<0.05).
Metabolic syndrome had a high prevalence in the population with AMI, ranging from 50% to 60%, according to some reports in Asia.8,21 Metabolic syndrome involves various important pathophysiological mechanisms such as atherosclerosis, arterial calcification,11,12 thereby increasing in damage to the coronary vascular system. This was reflected in a higher Gensini score in the MetS group compared to the non-MetS group. This finding was consistent with Kim et al.’s study, which also reported that the MetS group had significantly higher Gensini scores (p=0.002).14 Additionally, we observed a weak positive Spearman correlation (rho=0.2, p=0.011) between the MetS score and the Gensini score. Gui et al., 22 also reported a significant association between the Gensini score and the MetS score with a p-value of 0.001. Yavuz et al.15 found a positive correlation between the MetS score and the Gensini score with a correlation coefficient (r) of 0.402 and p<0.001. Yavuz et al. showed a stronger correlation than our study; however, it is worth noting that their research excluded all patients with acute coronary syndrome within one month and those who underwent PCI and CABG previously.
In patients with MetS, the frequency and progression of coronary artery calcification occur more frequently. When obesity is present, there is a greater accumulation of fat tissue around the coronary arteries, and atherosclerotic plaques mainly form in arteries with perivascular adipose tissue (PVAT) surrounding them. This process is often explained through a Outside-In Theory, in which adipokines from the fat tissue surrounding blood vessels on the outside have the potential to impair blood vessel function and contribute to the development of arterial atherosclerosis in nearby blood vessels.11 Quininir Salvatici et al.23 also observed that elevated waist circumference was associated with the Gensini score (higher quartile 27.02±37.3 vs. lower quartile 17.23±28.6, p=0.03). Abdominal obesity or elevated waist circumference was believed to be related to a mild inflammatory state.24 This result was supported by Rana et al.,25 who demonstrated that patient with elevated waist circumference in their study was associated with elevated levels of CRP, sPLA2 (only in females), fibrinogen, and adiponectin. These findings provided a basis for the idea that patients with AMI who had metabolic syndrome, or elevated waist circumference may experience more complex coronary artery disease.
Elevated blood glucose promotes atherosclerosis through three important factors, including advanced glycation end products (AGEs), increased oxidative stress production and activation of protein kinase C (PKC).26–28 Additionally, hyperglycemic-induced PKC signaling leads to increased endothelial inflammation, endothelial dysfunction, foam cell formation, increased expression of vascular endothelial growth factor (VEGF) and reduced nitric oxide (NO) production. All these mechanisms demonstrate that hyperglycemic levels contribute to the development of atherosclerosis. Muhammed et al.29 found that patients with impaired fasting glucose had higher Gensini scores compared to those with normal blood glucose levels (p<0.01), with no significant difference from the diabetes group (p=0.9). Zhao et al.30 demonstrated that impaired fasting glucose was an independent predictor of Gensini scores in patients without diabetes. These studies collectively highlighted the detrimental impact of elevated blood glucose on the severity of coronary artery disease and emphasized the importance of glycemic control in managing cardiovascular health.
The lack of association between elevated blood triglyceride level and Gensini score, as reported in our study and some other Asian studies (such as Zhang et al.31 and Gui et al.22). Elevated blood triglyceride level was considered a risk factor for atherosclerotic heart disease.32 However, the relationship between triglyceride level and the risk of atherosclerotic heart disease remains controversial. Some studies suggested that there might be no direct association between elevated blood triglyceride level and the risk of atherosclerotic heart disease when adjusting for other factors, including total cholesterol, LDL-C, and HDL-C levels.33 Additionally, some basic research had suggested that elevated blood triglyceride level might have a potentially protective role against the toxicity of lipid-induced fatty acids.34 Moreover, low blood lipid levels could be detrimental to maintaining the stability of cell membranes.35 These findings might help explain why elevated blood triglyceride level was not associated with Gensini scores in our study.
The relationship between reduced HDL-C level and Gensini score appears to differ between out study and Gui et al.’s study. In our study, there was no significant association (p=0.06), while Gui et al. found a correlation between reduced HDL-C and Gensini score (p=0.003). Reduced HDL-C is associated with the development of arterial atherosclerosis. However, Harrison et al.’s research had shown that the function of HDL-C (determined by its composition and post-translational modifications) was a more critical determinants for protection against cardiovascular disease compared than the levels of HDL-C. The difference between these studies can be explained by the fact that the cardioprotective benefit of HDL-C primarily depends on its function rather than its level. Since many studies did not assess the function of HDL-C, evaluating HDL-C concentration alone in relation to coronary artery lesions can yield conflicting results.
This study has several limitations. Firstly, this study was conducted at single-center study with a small sample size. A multicenter study with a larger sample size is needed to further evaluate the correlations between metabolic syndrome, MetS score, Its components and Gensini score and to determine the predictive value for short-term and long-term adverse prognosis. Secondly, this study did not clarify whether interventions for metabolic syndrome can reverse its adverse effects.
ConclusionIn our study, MetS, MetS score and two components of MetS, elevated waist circumference and elevated blood glucose, were associated with the severity of coronary artery disease in Vietnamese patients with acute myocardial infarction.
Informed consentAll of the included patients were asked to sign informed consent and understood that research results can be used for publication but all personal identification will remain confidential.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsHai PNT, Sy VH has designed and performed the study. Tai NN, Kha MN, and Sang QL have drafted the manuscript and did critical editing. Tai NN, Kha MN, Sy VH have carefully supervised this manuscript preparation and writing.
All authors had complete access to the data, made substantial contributions to the study, gave approval for the last version to be published, and assumed responsibility for its accuracy and integrity.
Conflicts of interestEach author has stated that they have no conflicts of interest
Data availabilityThe authors declare that data supporting the findings of this study are available within the article.
This study was conducted in the Interventional Cardiology Department, Cho Ray Hospital. We would like to thank the medical staff who helped with the data collection.