A decrease in fibrinolytic activity and an increase in the thickness of the epicardial adipose tissue have been observed in patients with coronary artery disease. The aim of this study was to determine the association between epicardial adipose tissue and fibrinolytic activity by measuring the concentration of plasminogen activator inhibitor-1 (PAI-1).
MethodsA cross-sectional study was conducted on 56 apparently healthy women aged 45–60 years. Anthropometric measurements and biochemical determinations were performed on all participants. The fibrinolytic activity was determined by measuring PAI-1 by ELISA. Epicardial thickness was assessed by transthoracic echocardiography.
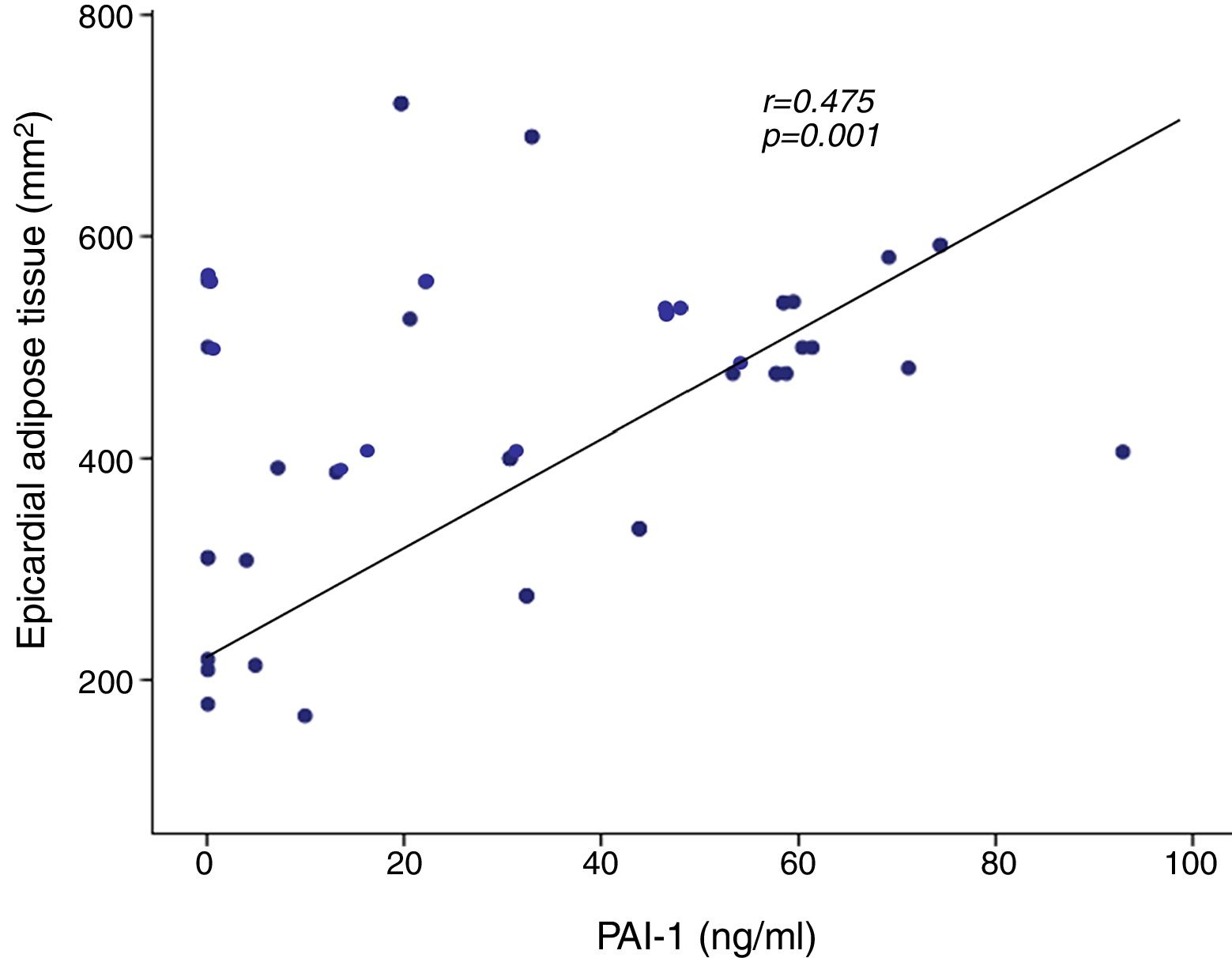
ResultsThe concentration of PAI-1 was directly associated with the thickness of the epicardial adipose tissue (r=0.475, p=0.001), glucose, triglycerides, insulin resistance, body mass index (BMI), visceral adipose tissue and total body fat. The multivariate regression analysis indicated that epicardial fat independently predicts the concentrations of PAI-1.
ConclusionsWomen with thicker epicardial adipose tissue have reduced fibrinolytic activity due to presenting increased PAI-1 levels, and consequently greater thrombotic risk.
En pacientes con enfermedad coronaria se ha observado una disminución de la actividad fibrinolítica y aumento del grosor del tejido adiposo epicárdico. El objetivo del estudio fue determinar la relación entre la grasa epicárdica y la actividad fibrinolítica, midiendo la concentración del inhibidor del activador del plasminógeno tipo-1 (PAI-1).
MétodosEstudio transversal que incluyó a 56 mujeres aparentemente sanas, con edad de 45-60 años. A las participantes se les realizaron mediciones antropométricas y bioquímicas, la actividad fibrinolítica se determinó midiendo PAI-1 por la técnica de ELISA. El grosor epicárdico se evaluó por ecocardiografía transtorácica.
ResultadosLa concentración de PAI-1 se asoció directamente con el grosor del tejido adiposo epicárdico (r=0,475, p=0,001), glucosa, triglicéridos, resistencia a la insulina, IMC, tejido adiposo visceral y grasa corporal total. El análisis de regresión multivariado indicó que la grasa epicárdica predice en forma independiente el valor de PAI-1.
ConclusionesLas mujeres con incremento de tejido adiposo epicárdico muestran menor actividad fibrinolítica por presentar niveles aumentados de PAI-1 y, en consecuencia, un posible mayor riesgo trombótico.
In Mexico, according to the Encuesta Nacional de Salud y Nutrición de Medio Camino 2016 (ENSANUT MC) [2016 Medio Camino National Survey of Health and Nutrition], the prevalence of overweight and obesity in females is 75.6% and higher in the 40-to-79 age group.1 Obesity represents a major risk factor for coronary artery disease; a body mass index (BMI)>29 increases the likelihood of suffering a cardiovascular event three-fold.2,3 Coronary artery disease is associated more with abdominal obesity than with BMI, suggesting that cardiovascular risk does not only depend on the amount of adipose tissue, but also how it is distributed.4 Abdominal visceral adipose tissue produces a large number of pro-inflammatory adipokines with local and systemic effects which favour the development of cardiovascular disease.5
Epicardial adipose tissue is another reservoir of visceral fat. It is located in the atrioventricular and interventricular sulci and extends through the apex, surrounding the coronary arteries. One of the characteristics of this type of epicardial adipose tissue is that it shares circulation with the myocardium.6 In healthy individuals, epicardial adipose tissue provides the myocardium with the lipids necessary for oxidation and obtaining energy and protects it from lipotoxicity.7,8 In conditions such as obesity, epicardial fat dysfunction occurs and more pro-inflammatory adipokines are produced, potentially participating in the process of coronary atheromatosis.9,10
Cardiovascular risk increases considerably in women over the age of 50.11 Among other factors, this is caused by the decrease in oestrogen production leading to a redistribution of body fat which results in a pattern of abdominal obesity, and changes in haemostasis and serum lipid profile, which all lead to the creation of a pro-thrombotic state.12,13
One thrombotic risk marker is plasminogen activator inhibitor-1 (PAI-1), which is the main protein involved in fibrinolysis.14 In patients with obesity and metabolic syndrome, the concentration of PAI-1 is increased.15 That increase leads to a state of hypofibrinolysis as tissue plasminogen activator (t-PA) is inhibited and fibrin deposits increase in the atherosclerotic plaque, eventually forming an occlusive thrombus.14,16,17
The aim of this study was to determine the relationship between epicardial fat and fibrinolytic activity by measuring PAI-1 concentration in women aged 45 to 60.
Material and methodsWe carried out a cross-sectional study in 56 apparently healthy women aged 45–60 who attended the Medical Research Unit in Endocrine Diseases of the Hospital de Especialidades del Centro Médico Nacional del Instituto Mexicano del Seguro Social (IMSS) [Mexican Institute for Social Security National Medical Centre Specialist Hospital], from February 2013 to August 2016. None of the participants were taking hormone therapy. Women with established diagnosis of diabetes, renal and hepatic failure, endocrine disorders, haematological diseases, previous history of cardiovascular disease and thrombosis were excluded from the study. This study protocol was approved by the IMSS Specialist Hospital Research Committee. The participants were informed and signed the corresponding consent form.
Medical assessmentMedical history and anthropometric measurements were taken from all patients. Weight and height were measured without shoes and wearing light clothes using scales and a Bame stadiometer, respectively. Blood pressure was measured with an Aneroid Baumanometer. BMI was calculated dividing height (m2) by weight (kg); patients were considered to be obese with a BMI>30 and overweight with a BMI of 25 to 29.9.
Biochemistry analysisAntecubital venous blood samples were obtained between 07:00 and 08:00h after fasting for at least 12h. Samples were collected in citrate tubes without anticoagulant. Glucose, HDL-cholesterol and triglycerides were measured in serum by enzymatic methods using the semi-automated chemical analyser Ekem Kontrol Lab. Oestradiol was measured in serum by means of chemiluminescence with the IMMULITE 1000 device. Plasma concentrations of PAI-1 were measured by enzyme-linked immunosorbent assay (ELISA) (BioVendor, USA). Insulin resistance was measured using the Matthews formula: HOMA-IR=insulin (μU/ml)×fasting glucose (mmol/l)/22.5.18
Body analysisBody composition was assessed by bioelectrical impedance analysis (Body Composition Analyzer 353ioi JAWON). The analysis was carried out in the morning after fasting for 12h. Values for abdominal visceral adipose tissue and the percentage of total body fat were obtained.
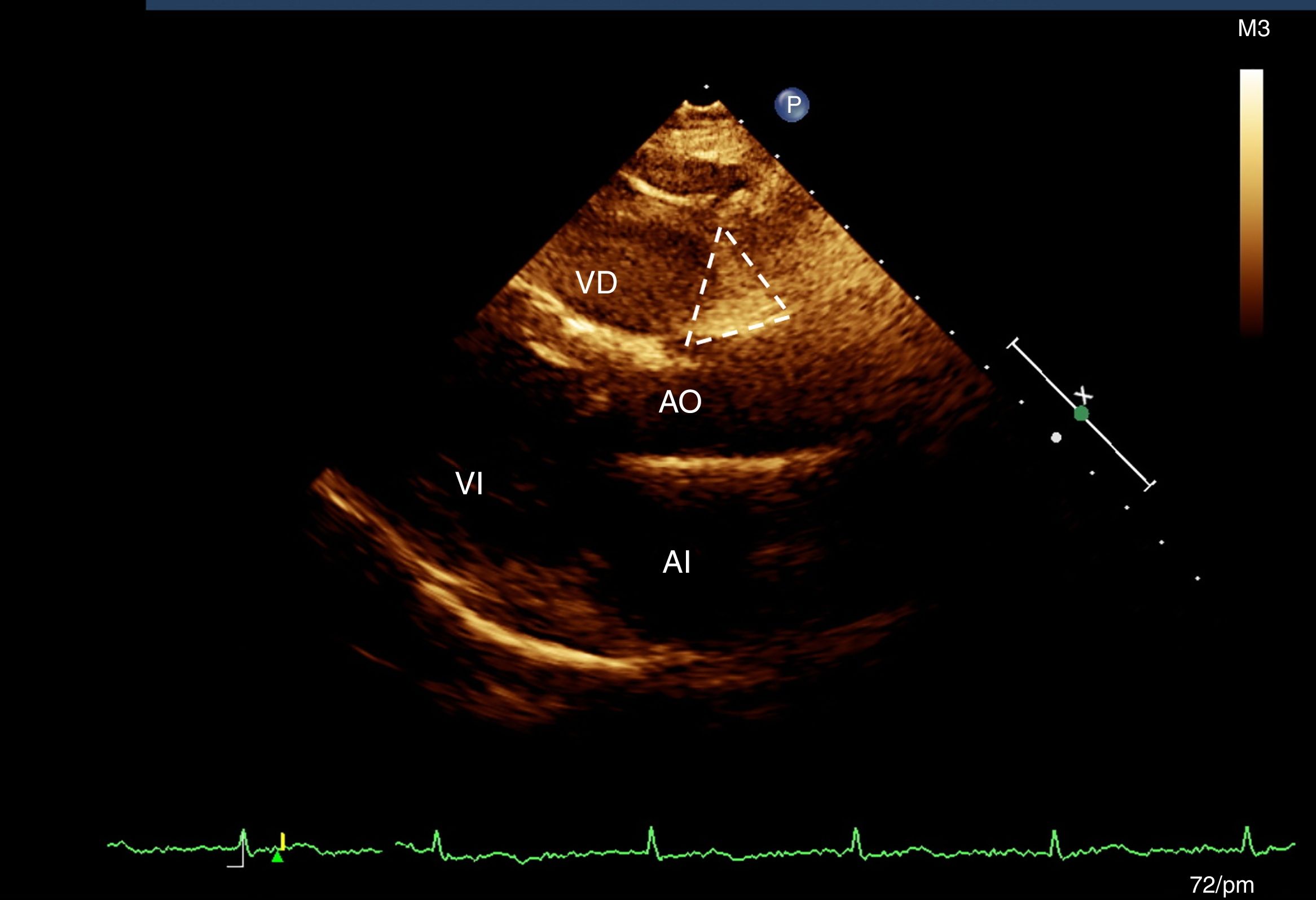
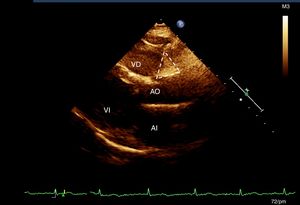
Assessment of epicardial adipose tissueAll the participants had an M-mode, two-dimensional, transthoracic echocardiogram and Doppler (Phillips IE33 echocardiograph, version 5.2.0.289). According to the previously described technique,19 two sites of epicardial fat deposition were selected using depth-of-field to improve two-dimensional visualisation.19 With the long longitudinal axis view, the groove was located between the root of the aorta and the right ventricle, which forms a triangle just at the junction of the aorta with the right ventricle (AoRV).19 In the four-chamber view, epicardial fat was measured at the apex, which is also seen to be triangular in shape, at the site of the junction of the apical regions of both ventricles (Fig. 1). All the measurements were made by the same cardiologist sonographer.19
Statistical analysisVariables are expressed as mean±standard deviation. The Spearman test was used to identify the correlation between the variables and the Mann–Whitney U-test to identify the differences between the groups. A multivariate analysis was carried out to identify the effect of the different variables on PAI-1 concentration. The statistical computing program SPSS v.21 was used and a value was considered significant when p≤0.05.
Based on the results of this study and the number of patients included, the statistical power of the association between epicardial fat and PAI-1 was calculated as 0.98.
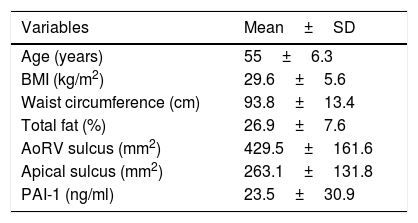
ResultsA total of 56 women were studied, with an average age of 55.0±6.3 years, and BMI of 29.6±5.6. Out of the total, 72.3% were obese or overweight and 27.7% had normal weight; the general characteristics of the participants are shown in Table 1.
General characteristics of the patients.
| Variables | Mean±SD |
|---|---|
| Age (years) | 55±6.3 |
| BMI (kg/m2) | 29.6±5.6 |
| Waist circumference (cm) | 93.8±13.4 |
| Total fat (%) | 26.9±7.6 |
| AoRV sulcus (mm2) | 429.5±161.6 |
| Apical sulcus (mm2) | 263.1±131.8 |
| PAI-1 (ng/ml) | 23.5±30.9 |
AoRV: right aortoventricular adipose tissue; BMI: body mass index; PAI-1: plasminogen activator inhibitor 1.
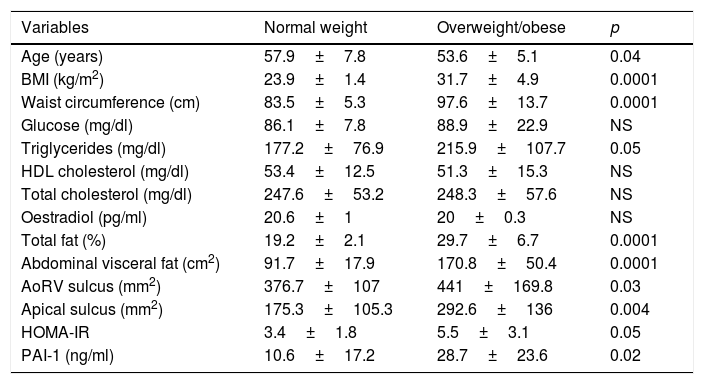
The group of participants who were obese or overweight were found to have an increased amount of epicardial adipose tissue in the AoRV and apical sulci; increased concentrations of triglycerides, total fat, abdominal visceral fat and PAI-1 were also found in this group (Table 2).
Classification of participants according to BMI.
| Variables | Normal weight | Overweight/obese | p |
|---|---|---|---|
| Age (years) | 57.9±7.8 | 53.6±5.1 | 0.04 |
| BMI (kg/m2) | 23.9±1.4 | 31.7±4.9 | 0.0001 |
| Waist circumference (cm) | 83.5±5.3 | 97.6±13.7 | 0.0001 |
| Glucose (mg/dl) | 86.1±7.8 | 88.9±22.9 | NS |
| Triglycerides (mg/dl) | 177.2±76.9 | 215.9±107.7 | 0.05 |
| HDL cholesterol (mg/dl) | 53.4±12.5 | 51.3±15.3 | NS |
| Total cholesterol (mg/dl) | 247.6±53.2 | 248.3±57.6 | NS |
| Oestradiol (pg/ml) | 20.6±1 | 20±0.3 | NS |
| Total fat (%) | 19.2±2.1 | 29.7±6.7 | 0.0001 |
| Abdominal visceral fat (cm2) | 91.7±17.9 | 170.8±50.4 | 0.0001 |
| AoRV sulcus (mm2) | 376.7±107 | 441±169.8 | 0.03 |
| Apical sulcus (mm2) | 175.3±105.3 | 292.6±136 | 0.004 |
| HOMA-IR | 3.4±1.8 | 5.5±3.1 | 0.05 |
| PAI-1 (ng/ml) | 10.6±17.2 | 28.7±23.6 | 0.02 |
AoRV: right aortoventricular; HOMA: homeostatic model assessment; BMI: body mass index; PAI-1: plasminogen activator inhibitor 1.
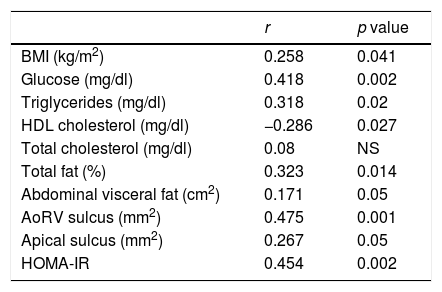
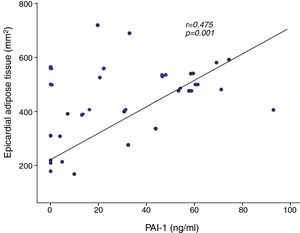
A positive correlation was found between PAI-1 and epicardial adipose tissue in the AoRV sulcus (r=0.475, p=0.001) (Fig. 2), which persisted after adjusting for BMI (r=0.304, p<0.05). PAI-1 concentration also had a positive correlation with apical sulcus fat, glucose, triglycerides, HOMA-IR, total body fat and abdominal fat and was inversely correlated with HDL-cholesterol (Table 3).
Association of PAI-1 concentration with other variables.
| r | p value | |
|---|---|---|
| BMI (kg/m2) | 0.258 | 0.041 |
| Glucose (mg/dl) | 0.418 | 0.002 |
| Triglycerides (mg/dl) | 0.318 | 0.02 |
| HDL cholesterol (mg/dl) | −0.286 | 0.027 |
| Total cholesterol (mg/dl) | 0.08 | NS |
| Total fat (%) | 0.323 | 0.014 |
| Abdominal visceral fat (cm2) | 0.171 | 0.05 |
| AoRV sulcus (mm2) | 0.475 | 0.001 |
| Apical sulcus (mm2) | 0.267 | 0.05 |
| HOMA-IR | 0.454 | 0.002 |
AoRV: right aortoventricular; HOMA: homeostatic model assessment; BMI: body mass index.
We carried out a multiple regression analysis to identify the contribution of the different variables to the concentration of PAI-1. The prediction variables were: BMI, visceral fat, total fat, epicardial fat in the AoRV sulcus, oestradiol and HOMA-IR. We identified that epicardial fat independently predicts PAI-1 concentration (r=0.558, p=0.03, beta coefficient=0.387).
DiscussionThis study demonstrated a significant relationship between increased epicardial fat thickness and thrombotic risk assessed by PAI-1 concentrations. The group studied consisted of women aged 45–60; this age group corresponding to a stage of life when cardiovascular risk increases. There is a limited amount of information published on this subject and, to our knowledge, there have been no previous studies in Mexico. However, a similar correlation between the level of PAI-1 and the thickness of epicardial fat was found in a study on 42 Italian women.20
Increased epicardial fat thickness has been related to the development of coronary events, and this association is independent of traditional cardiovascular risk factors.21 An increase in epicardial fat has been found in patients with atheromatous plaques in their coronary arteries.22 This same association was also found in a study in the Mexican population.23 The results of these studies suggest that epicardial adipose tissue is directly involved in the development of coronary atherosclerosis.21 The mechanisms that promote the development of atherosclerosis in patients who have increased epicardial fat are not yet fully understood. Adipokines produced by epicardial adipose tissue probably affect the coronary arteries.24–26 One of these molecules could be PAI-1.
In our study, the association of epicardial adipose tissue was with the circulating concentration of PAI-1, which was probably synthesised in the endothelium, adipocytes and other tissues. It has been shown in biopsies from patients with coronary artery disease that PAI-1 is also expressed in epicardial adipose tissue.27 It is therefore feasible that the coronary arteries are affected by both circulating PAI-1 and PAI-1 produced in epicardial fat. The molecules synthesised in the epicardial fat may reach the coronary arteries by diffusion from the periadventitial fat in the epicardium, and/or by the release to the vasa vasorum, passing through the arterial layers to the intimal layer.28,29
Although PAI-1 and epicardial adipose tissue may be associated through intermediate variables such as BMI and insulin resistance, multivariate analysis indicated that epicardial fat independently predicts PAI-1 concentration.
The results also showed that our population is at high risk of cardiovascular disease, to the extent that the measurement of epicardial fat and PAI-1 would be useful in the assessment and prediction of cardiovascular risk.
Epicardial fat was measured by echocardiography and this may be considered as a limitation of the study. However, the echocardiography technique has a very high correlation with the gold standard, magnetic resonance imaging, and also has as additional advantages its safety, reproducibility and low cost.5,19,30
The number of participants in our study was low. However, the correlation between epicardial fat and PAI-1 achieved adequate statistical power. Nevertheless, future studies will be necessary with a larger number of patients and in other population groups. Moreover, despite the fact that PAI-1 is the main inhibitor of fibrinolysis and is associated with the development and progression of coronary artery disease,31,32 in future projects it would be useful to complement the study of fibrinolysis by measuring other proteins involved in haemostasis, such as tissue plasminogen activator or t-PA.
ConclusionsThis study demonstrated a direct association between epicardial fat and a decrease in fibrinolysis. These findings suggest that the increase in the thickness of epicardial fat and the high concentration of PAI-1 may lead to the development of cardiovascular disease.
Conflict of interestsThe authors declare no conflicts of interest.
Please cite this article as: Basurto Acevedo L, Barrera Hernández S, Fernández Muñoz MJ, Saucedo García RP, Rodríguez Luna AK, Martínez Murillo C. El incremento de la grasa epicárdica en mujeres se asocia a riesgo trombótico. Clin Investig Arterioscler. 2018;30:112–117.