Advanced glycation end products (AGEs) are pro-oxidant and cytotoxic compounds involved in the progression of chronic diseases as cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). The total body burden of AGEs also depend of those consume through the diet. Our aim was to analyze whether the reduction of AGE levels, after the consumption of two-healthy diets were associated with a greater decrease of intima-media thickness of both common carotid arteries (IMT-CC) in patients with T2DM and coronary heart disease (CHD).
Methods540 CHD patients with T2DM, at baseline, from the CORDIOPREV study, were divided into two groups: (1) Responders, patients whose IMT-CC was reduced or not changed after dietary intervention and (2) Non-responders, patients whose IMT-CC was increased after dietary intervention. A total of 423 completed baseline and the 5-year follow-up carotid ultrasounds were analyzed in this study.
ResultsOur data showed that Responders, despite had a higher baseline IMT-CC and serum methylglyoxal (MG) levels than Non-responders, showed a reduction of serum levels of this glycotoxin after dietary intervention. Conversely, in patients whose IMT-CC was increased after dietary intervention (Non-responders), serum MG levels were increased. Moreover, an increase of circulating level of AGEs (and in particular, MG), after dietary intervention, could be considered a risk factor for the progression of atherosclerosis in patients with T2DM and CHD.
ConclusionThese results support the importance of identifying underlying mechanisms in the context of secondary prevention of CVD that would provide therapeutic targets to reduce the high risk of cardiovascular events of these patients.
Clinical Trial registration-URL: https://clinicaltrials.gov/ct2/show/NCT00924937.
Unique Identifier: NCT00924937.
Los productos finales de glicación avanzada (AGE) son compuestos prooxidantes y citotóxicos involucrados en la progresión de enfermedades crónicas, como las enfermedades cardiovasculares (ECV) y la diabetes mellitus tipo 2 (DMT2). La carga corporal total de AGE también depende de aquellos que se consumen a través de la dieta. Nuestro objetivo fue analizar si la reducción de los niveles de AGE, tras el consumo de dos dietas cardiosaludables, se asociaba con una mayor disminución del grosor íntima-media de ambas arterias carótidas comunes (GIM-CC) en pacientes con DMT2 y enfermedad coronaria establecida (EC).
MétodosUn total de 540 pacientes con EC y DMT2, al inicio del estudio CORDIOPREV, se dividieron en dos grupos: 1) respondedores, pacientes cuyo GIM-CC disminuyó o no se modificó tras la intervención dietética, y 2) no respondedores, pacientes cuyo GIM-CC aumentó tras la intervención dietética. En este estudio se incluyeron, finalmente, un total de 423 pacientes, aquellos que completaron el estudio de ecografía carotídea tanto en el basal como a los 5 años de intervención dietética.
ResultadosNuestros resultados mostraron que los respondedores, a pesar de tener un GIM-CC y niveles séricos de metilglioxal (MG) más elevados que los no respondedores, mostraron una reducción de los niveles séricos de esta glicotoxina tras la intervención dietética. Por el contrario, en los pacientes cuyo IMT-CC aumentó tras la intervención dietética (no respondedores), los niveles séricos de MG aumentaron. Un aumento de los niveles circulantes de AGE (y en particular de MG) tras la intervención dietética podría considerarse un factor de riesgo para la progresión de la aterosclerosis en pacientes con DMT2 y CE.
ConclusiónEstos resultados apoyan la importancia de identificar los mecanismos subyacentes en el contexto de la prevención secundaria de la ECV que proporcionarían dianas y estrategias terapéuticas para reducir el alto riesgo de eventos cardiovasculares de estos pacientes.
Coronary heart disease (CHD) is the most prevalent form of cardiovascular disease (CVD) and is a major cause of mortality worldwide.1 Despite advances in the treatment and understanding of the etiological and pathophysiological factors underlying the disease, one in three patients with CHD has a new cardiovascular event.2,3 Therefore, there is a need to establish preventive and effective strategies to prevent or delay the onset of CVD and its comorbidities.
Advanced glycation end products (AGEs) constitute a group of pro-oxidant and cytotoxic compounds generated by non-enzymatically reactions by condensation between the carbonyl groups of reducing sugars and the free amino groups of nucleic acids, proteins or lipids leading to stable and irreversible end products.4 Small amounts of AGEs are generated as a consequence of a normal metabolism, but in the context of chronic diseases (type 2 diabetes mellitus-T2DM, metabolic syndrome, obesity or CVD), AGE production increases being involved in their pathology and progression.5 In T2DM patients, AGEs tend to accumulate because their production is boosted by chronic hyperglycemia, thus worsening AGE-induced deleterious effects.4 Recent findings showed that, in patients with T2DM, the incidence of cardiovascular events correlated with baseline N-carboxymethyllysine (CML), the most well AGEs studied serving as a marker of AGE accumulation in several tissues.6,7 Moreover, elevated levels of circulating AGEs have been associated with CVD and correlated with increased arterial wall stiffness in diabetic patients.8
While endogenous AGE formation represents a minor component of the total body load of AGEs, the diet is the most important exogenous sources of AGEs, which depend on the nutrient composition and the food processing methods applied.9,10 We have recently published that the consumption of a Mediterranean diet model, in comparison with a Western-style diet, which is rich in saturated fats (SFAs), provides a low content in dietary AGEs.11,12 Moreover, the Mediterranean diet is able to reduce circulating levels of AGEs and modulate their metabolism, with the consequent decrease in oxidative stress and inflammation grade in both elderly adults and metabolic syndrome patients.11,12
Evaluation of the intima-media thickness of both common carotid arteries (IMT-CC), using B mode ultrasound is a noninvasive, is a well-validated clinical method considered a surrogate marker of subclinical atherosclerosis and a strong predictor of new myocardial infarction and stroke.13,14
Taking all the above into consideration, the main aim of this study was to analyze whether the reduction of AGE levels and the modulation of gene expression related to AGE metabolism, after the consumption of two-healthy dietary approaches (a Mediterranean diet and a low-fat diet) were associated with a greater decrease of IMT-CC in CHD patients with T2DM.
MethodsDesign and study populationThe current work was conducted within the framework of the CORDIOPREV (CORonary Diet Intervention with Olive oil and cardiovascular PREVention) study. Details of the rationale, study methods, inclusion and exclusion criteria, cardiovascular risk factors and baseline characteristics of the patients have been recently described.15 Briefly, the CORDIOPREV study was a single-center, randomized, single-blind, parallel-group and secondary prevention trial including 1002 CHD patients, conducted in Spain, to compare 2 controlled dietary interventions: (a) Mediterranean-type diet, vs. (b) Low-fat diet, on the risk of suffering new cardiovascular events. Eligible participants were men and women (aged over 20, but under 76 years) who had established CHD and without any clinical events in the previous 6 months, no severe illnesses or an expected life expectancy lower than the length of the study.
For the specific aims of this work, we selected those participants with T2DM at baseline, according to the American Diabetes Association (n=540),16 which were divided into two groups depending on the change in IMT-CC produced by the dietary intervention: (1) Responders, patients whose IMT-CC was reduced or not changed after dietary intervention and (2) Non-responders, patients whose IMT-CC was increased after dietary intervention.
Ethics approvalThe protocol was written in accordance with the principles of the Declaration of Helsinki. The respective Institutional Review Board by the Human Investigation Review Committee of the Reina Sofa University Hospital (Córdoba, Spain) approved the study protocol. The trial was registered in 2009 at ClinicalTrials.gov (number NCT00924937). Recruitment took place from July 2009 to February 2012. All subjects provided written informed consent.
Randomization and dietary interventionRandomization was performed by the Andalusian School of Public Health, as previously described.15 The study dietitians were the only members of the intervention team to know about each participant's dietary group. Briefly, the randomization was based on the following variables: sex (male, female), age (<60 and ≥60 years old) and previous myocardial infarction (yes, no). Each patient was randomly stratified, in addition to the conventional treatment for CHD, to one of two potentially healthy diets: (a) the Mediterranean diet, with a minimum of 35% of total calories from fat [22% MUFA, 6% polyunsaturated (PUFA), and <10% SFA], 15% proteins, and a maximum of 50% carbohydrates and (b) a low-fat, high complex carbohydrate diet, as recommended by the National Cholesterol Education Program, with <30% of total calories from fat (12–14% MUFAs, 6–8% PUFAs, <10% SFAs), ≥55% from carbohydrates and 15% from protein. In both diets, the cholesterol content was adjusted to <300mg/day. Both study diets included foods from all major food groups, but no total calorie restriction was set. Full details on dietary assessment, adherence and recommendations, as well as follow-up visits, have been published elsewhere.15,17 No intervention to increase physical activity or lose weight was included. Participants in both intervention groups received the same intensive dietary counseling. The present study was conducted over a follow-up period of 5 years. Details of the specific recommended diets, mean baseline values and changes in energy and nutrient intake after 5 years of intervention with both dietary patterns have been previously described.17
Laboratory testsAt 8.00am, following a 12-h fast, the patients were admitted to the laboratory for anthropometric and biochemical tests [weight, BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL-cholesterol, LDL-cholesterol, triglycerides, total cholesterol, high sensitive C-reactive protein (hsCRP), fasting glucose and insulin and hemoglobin A1c (HbA1c) as described previously.15 Glomerular filtration rate (eGFR) was estimated with the Chronic Kidney Disease and Epidemiology equation (CKD-EPI).18
Carotid ultrasonographyThe carotid study was ultrasonically assessed bilaterally by quantification of IMT-CC as well as carotid plaque number and height, at the beginning of the study and after 5 years of dietary intervention. Briefly, carotid arteries were examined using a Doppler ultrasound high-resolution B-mode (Envisor C Ultrasound System, Philips, Eindhoven, The Netherlands), following the recommendations of the American Society of Echocardiography Carotid Intima-Media Thickness Task Force.19 All images were analyzed off-line using dedicated analysis tools (QLAB Advance Ultrasound Quantification Software, v5.0, Phillips, USA). Analysis was performed by technicians blinded to clinical information and previous imaging. A full description of the methodology has been recently described.20
Out of the 540 CHD patients with T2DM from the CORDIOPREV study, a total of 519 completed the carotid ultrasound study at baseline. Of these patients, data from 117 patients were missing because they did not complete the ultrasonography study (at baseline or during follow-up) due to problems related to disapproval of the technique, refusal to participate, death, or withdrawal for other reasons. In this sense, a total of 423 CHD patients with T2DM completed the 5-year follow-up carotid ultrasound study and were analyzed in this study. Baseline characteristics of those patients with complete the ultrasonography study (during follow-up, n=423) did not differ with patients who did not complete it (n=117) (Supplementary Table 1).
Determination of serum levels of AGEsSerum and plasma samples were collected at baseline and after 5 years of follow-up of dietary intervention and separated from whole blood by centrifugation at 1500×g for 20min at 20°C and 1500×g for 15min at 4°C, respectively within 1h of extraction.
Methylglyoxal (MG) is a highly reactive dicarbonyl compound and precursor of AGEs 21 and N-carboxymethyllysine (CML), one of the most well AGEs studied and serve as markers of AGE accumulation in several tissues.6 Serum MG and CML were determined by well validated competitive ELISAs based on non-cross-reactive monoclonal antibodies (mabs) for protein-bound CML (4G9 mab) and protein-bound MG derivatives [lysine-MG-H1 (3D11 mab)], characterized by high-performance liquid chromatography (HPLC), and used as immunogens.22,23 The resulting values reflect relatively stable protein- or peptide-associated CML and MG and not the free compounds. AGEs (inter-assay coefficients of variation, 2.8% and 5.2% for CML and MG, respectively; intra-assay coefficients of variation, 2.6% and 4.1% for CML and MG, respectively).24
Quantification of the gene expression related to AGEs metabolism by Real-Time PCRBlood was processed and peripheral blood mononuclear cells (PBMCs) were isolated as previously described.25 PBMCs were isolated at baseline and after 5 years of follow-up of dietary intervention. Total PBMC RNA was extracted using the Trizol method and quantified using a Nanodrop ND-1000 v3.5.2 spectrophotometer (Nanodrop Technology®, Cambridge, UK). RNA integrity was verified on agarose gel electrophoresis and stored at −80°C. Samples were digested with DNAse I (AMPD-1 KT, Sigma) before Real Time-PCR. Real-time PCR reactions were carried out using the Bio-Rad PCR platform following the manufacturer's instructions. Each reaction was performed with 5μl of a 1:5 (v/v) dilution of the first cDNA synthesized from 1μg of total RNA using the commercial kit High Capacity cDNA Reverse Transcription Kit with RNAse inhibitor (Applied Biosystems), following the manufacturer's instructions. Primer pairs were selected from the database TaqMan Gene Expression assays (Applied Biosystems, Carlsbad, CA, USA; https://products.appliedbiosystems.com/ab/en/US/adirect/ab?cmd=catNavigate2&catID=601267), for the following genes: AGE receptor-1 (AGER1; DDOST, Hs00193263_m1), receptor for AGEs (RAGE; AGER, Hs00153957_m1), glyoxalase I (GloxI; GLO, Hs00198702). The relative expression for each analyzed gene was calculated with glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GAPDH, Hs99999905_m1) as a housekeeping gene. The dataset were analyzed by OpenArray® Real-Time qPCR Analysis Software (Applied Biosystems, Carlsbad, CA, USA).
Statistical analysisThe statistical analyses were carried out using SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov normality test was performed to evaluate the distribution of the quantitative variables, and continuous variables that deviated significantly from the assumption of normality were transformed. Categorical variables were compared using Chi-Square tests. Continuous data were compared using unpaired t-tests when comparing two groups or analysis of variance (ANOVA), adjusted for potential cofounders or potential effect modifiers (age, sex, smoking habit, and medications – lipid-lowering therapy, and anti-hypertensive drugs). To evaluate the changes in time Δchanges (changes produced between post and pre-intervention: as the value after five years of dietary intervention, minus value at baseline) was calculated.
Multiple logistic analysis was carried out to estimate the independent contribution of the modulation of AGE metabolism (changes in serum levels of MG and gene expression of GloxI and RAGE, after dietary intervention) as well as baseline IMT-CC, age and sex to changes in IMT-CC (Responders/Non-responders).
The differences were considered significant when p<0.05. All the data presented in figures and tables are expressed as means±standard error (SE).
ResultsCharacteristics of the study populationBaseline clinical and metabolic characteristics, lipid profiles and treatment regimens of patients who completed baseline and the follow-up carotid ultrasound study (n=423) are presented in Table 1. No significant differences were observed between Responders and Non-responders.
Baseline clinical and metabolic characteristics, lipid profiles and treatment regimens of the CHD patient with T2DM who completed baseline and the follow-up carotid ultrasound study.
| Total population(n=423) | Respondersa(n=225) | Non-respondersa(n=198) | p value | |
|---|---|---|---|---|
| Age (years) | 61.0±0.4 | 61.7±0.5 | 60.1±0.6 | 0.055 |
| Men/women (n) | 350/73 | 192/33 | 158/40 | 0.133 |
| Weight (kg) | 86.9±0.7 | 86.9±0.9 | 86.7±1.0 | 0.889 |
| BMI (kg/m2)b | 31.8±0.2 | 31.6±0.3 | 32.0±0.3 | 0.376 |
| Waist circumference (mm) | 107.0±0.5 | 107.4±0.7 | 106.6±0.8 | 0.424 |
| Diastolic blood pressure (mmHg) | 76.7±0.5 | 76.9±0.8 | 76.8±0.7 | 0.883 |
| Systolic blood pressure (mmHg) | 140.2±1.4 | 141.7±1.3 | 138.5±1.0 | 0.105 |
| Hypertension (%)c | 90.5 | 90.2 | 90.7 | 0.890 |
| LDL-cholesterol (mg/dL) | 85.6±1.7 | 86.4±1.6 | 84.6±1.2 | 0.438 |
| HDL-cholesterol (mg/dL) | 40.5±0.6 | 40.3±0.7 | 40.7±0.5 | 0.723 |
| Total cholesterol (mg/dL) | 155.5±2.0 | 157.0±2.0 | 153.7±1.5 | 0.261 |
| Triglycerides (mg/dL) | 139.6±3.2 | 142.6±4.4 | 136.3±4.6 | 0.325 |
| hsCRP (mg/mL) | 2.70±0.10 | 2.68±0.14 | 2.71±0.15 | 0.895 |
| Fasting glucose (mg/dL) | 130.2±2.1 | 131.5±3.0 | 128.7±2.9 | 0.500 |
| Fasting insulin (mU/L) | 12.5±0.7 | 13.1±1.0 | 11.9±1.0 | 0.426 |
| HbA1c (%) | 7.25±0.06 | 7.19±0.08 | 7.32±0.09 | 0.293 |
| eGFR (mL/min/1.73m2)d | 87.1±0.8 | 86.0±1.1 | 88.5±1.2 | 0.136 |
| Smoking (%) | 11.4 | 11.9 | 10.8 | 0.194 |
| Use of antihypertensive drugs (%) | ||||
| ACEIs or ARB | 83.5 | 86.2 | 83.4 | 0.102 |
| Calcium channel blockers | 20.0 | 21.4 | 19.3 | 0.295 |
| Beta-blockers | 82.7 | 83.6 | 79.2 | 0.632 |
| Nitrates | 10.0 | 9.8 | 10.1 | 0.845 |
| Diuretics | 39.5 | 40.0 | 39.1 | 0.509 |
| Use of lipid lowering drugs (%) | ||||
| Statins | 88.7 | 89.7 | 87.0 | 0.423 |
| Fibrates | 3.6 | 3.6 | 3.7 | 0.901 |
Values are represented as the mean±standard error or percentage of participants, unless otherwise stated. We used unpaired t tests for quantitative variables and Chi-squared tests for categorical variables (p<0.05, Responders vs Non-responders).
Responders, patients whose intima-media thickness of both common carotid arteries (IMT-CC) was reduced or not changed after dietary intervention and Non-responders, patients whose IMT-CC was increased after dietary intervention.
Hypertension was defined as a systolic blood pressure≥140mm Hg, a diastolic blood pressure≥90mm Hg, or the use of antihypertensive therapy.
Serum creatinine (sCr)-based estimated glomerular filtration rate (eGFR) was evaluated using the CKD-Epi (CKD Epidemiology Collaboration) equation (18).
CHD, coronary heart disease; T2DM, type 2 diabetes mellitus; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high sensitive C-reactive protein; HbA1c, glycated hemoglobin; ACEIs, angiotensin converting enzyme inhibitors; ARB, angiotensin-receptor blockers.
Responders showed a higher baseline IMT-CC compared to Non-responders (p<0.001). No significant differences were found in other parameters related to carotid ultrasonography (carotid plaque presence, number or height) between Responders and Non-responders (Table 2).
Baseline characteristics related to carotid ultrasonography and AGE metabolism of the CHD patients with T2DM who completed baseline and the follow-up carotid ultrasound study.
| Total population(n=423) | Respondersa(n=225) | Non-respondersa(n=198) | p value | |
|---|---|---|---|---|
| Carotid ultrasonography | ||||
| IMT-CC (mm) | 0.76±0.01 | 0.82±0.01 | 0.68±0.01 | <0.001 |
| Carotid plaque presence (%) | 82.3 | 82.3 | 82.4 | 0.733 |
| Carotid plaque number (n) | 1.61±0.10 | 1.62±0.10 | 1.60±0.07 | 0.912 |
| Carotid plaque height (mm) | 1.88±0.06 | 1.83±0.08 | 1.94±0.08 | 0.352 |
| AGE metabolism | ||||
| Serum Methylglyoxal (mg/mL) | 3.79±0.09 | 3.94±0.13 | 3.61±0.11 | 0.012 |
| Serum N-carboxymethyllysine (mg/mL) | 0.51±0.04 | 0.50±0.04 | 0.52±0.07 | 0.864 |
| AGER1 gene expression (AU) | 1.05±0.05 | 1.03±0.07 | 1.08±0.08 | 0.688 |
| RAGE gene expression (AU) | 1.14±0.09 | 1.02±0.07 | 1.28±0.08 | 0.035 |
| GloxI gene expression (AU) | 39.5 | 40.0 | 39.1 | 0.333 |
Values are represented as the mean±standard error or percentage of participants, unless otherwise stated. We used unpaired t tests for quantitative variables and Chi-squared tests for categorical variables (p<0.05).
Responders, patients whose intima-media thickness of both common carotid arteries (IMT-CC) was reduced or not changed after dietary intervention and Non-responders, patients whose IMT-CC was increased after dietary intervention.
CHD, coronary heart disease; T2DM, type 2 diabetes mellitus; AGE, advanced glycation end products; AGER1, AGE receptor-1; RAGE, receptor for AGEs; GloxI, glyoxalase I.
In relation to baseline characteristics related to AGE metabolism, Responders exhibited higher serum levels of MG and lower RAGE expression compared to Non-responders (p=0.012 and 0.035, respectively). We did not find any differences in serum levels of CML and AGER1 and GloxI expression between both groups of patients (Table 2).
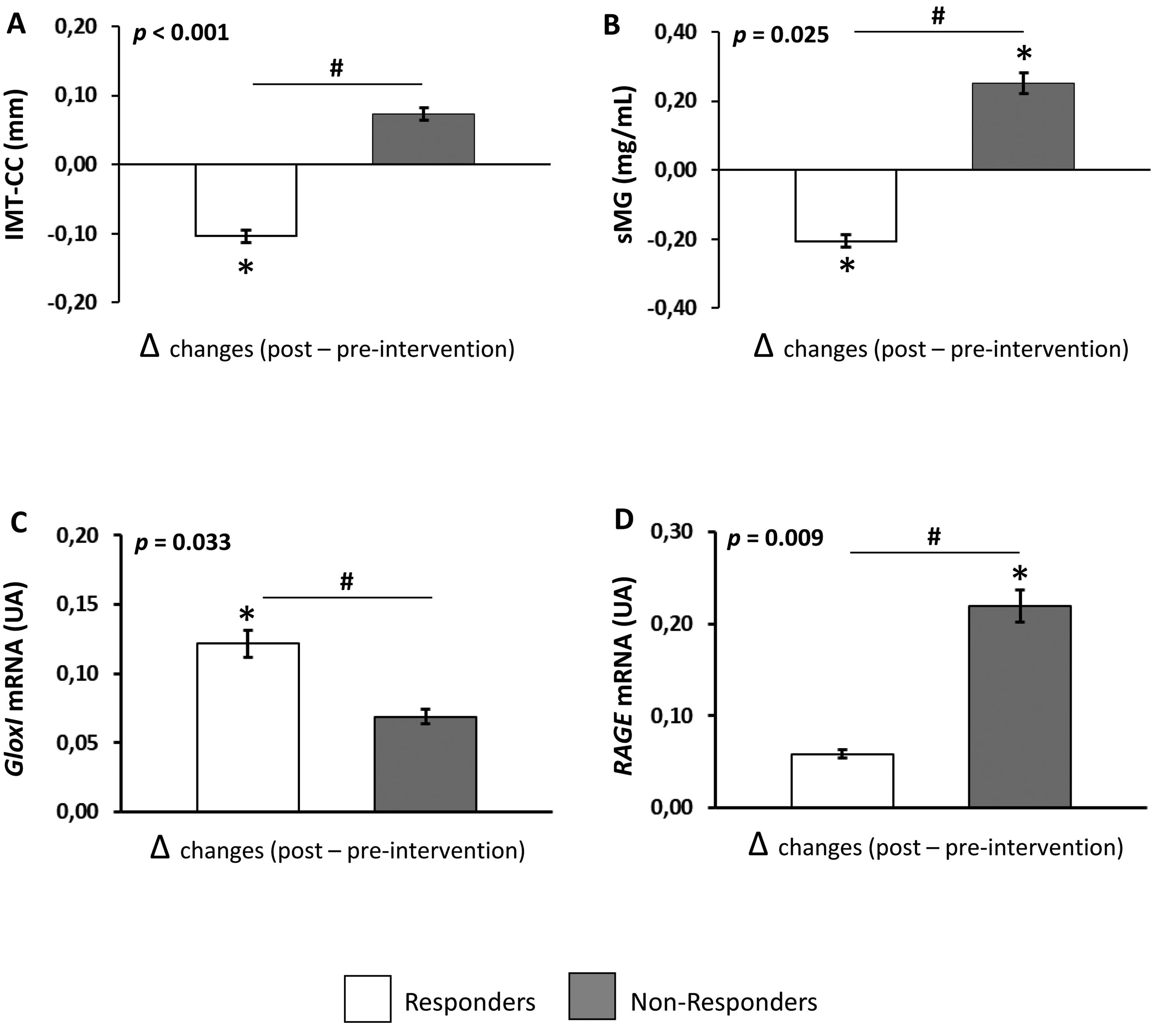
Dietary intervention modulates MG levels and gene expression related to AGE metabolismThe effect of dietary intervention (Δchanges produced between post and pre-intervention) on AGE levels and gene expression related to AGE metabolism are shown in Fig. 1. Dietary intervention produced a decrease in serum levels of MG in Responders and an increase in the levels of this glycotoxin in Non-responders compared to pre-intervention (p=0.011 and 0.009, respectively). In addition, Responders showed lower serum levels of MG after dietary intervention compared to Non-responders (p=0.025) (Fig. 1B). We did not find any differences in serum levels of CML comparing pre- and post-intervention or between Responders and Non-responders (data not shown).
Effect of dietary intervention on IMT-CC and parameters related to AGE metabolism in CHD patients with T2DM. (A) IMT-CC, (B) serum levels of MG, (C) gene expression of GloxI, (D) gene expression of RAGE. Values are represented as the mean (Δchanges produced between post and preintervention)±standard error. Responders, patients whose intima-media thickness of both common carotid arteries (IMT-CC) was reduced or not changed after dietary intervention and non-responders, patients whose IMT-CC was increased after dietary intervention. *p<0.05, differences between 5-years compared to pre-intervention, #p<0.05, differences in changes at 5-years between Responders and Non-responders by univariate ANOVA.
Regarding the gene expression of AGE metabolism, dietary intervention produced an increase in GloxI expression in Responders (p=0.005), but not in Non-responders, compared to baseline. However, dietary intervention increased RAGE expression in Non-responders (p=0.001), but not in Responders, compared to baseline (Fig. 1C and D, respectively). Moreover, after dietary intervention, Responders showed a higher GloxI expression and a lower RAGE expression compared to Non-responders (p=0.033 and 0.009, respectively). No significant differences were found in AGER1 expression comparing pre- and post-intervention or between Responders and Non-responders (data not shown).
Changes in clinical, metabolic characteristics and lipid profiles after dietary interventionDietary intervention reduced LDL-cholesterol and total cholesterol levels compared to pre-intervention in Responders (p=0.021 and 0.019, respectively) and reduced triglycerides levels compared to pre-intervention in both groups of patients. We did not find differences between Responders and Non-responders among the different variables analyzed (Table 3).
Changes in clinical, metabolic characteristics and lipid profiles of the CHD patients with T2DM who completed baseline and the follow-up carotid ultrasound study.
| Change between baseline and after 5 years | |||
|---|---|---|---|
| Responders(n=225) | Non-responders(n=198) | p value* | |
| BMI (kg/m2) | −0.64±0.07 | −0.54±0.05 | 0.121 |
| LDL-cholesterol (mg/dL) | −4.42±0.40# | −1.52±0.37 | 0.028 |
| HDL-cholesterol (mg/dL) | −1.39±0.51 | −2.32±0.43 | 0.081 |
| Total cholesterol (mg/dL) | −8.61±0.19# | −5.49±0.23 | 0.046 |
| Triglycerides (mg/dL) | −14.5±0.27# | −12.8±1.99# | 0.373 |
| hsCRP (mg/mL) | 0.31±0.10 | 0.56±0.09 | 0.105 |
| Fasting glucose (mg/dL) | 6.26±1.07 | 9.86±0.67 | 0.135 |
| Fasting insulin (mU/L) | 4.65±0.60 | 3.84±0.25 | 0.131 |
| HbA1c (%) | −0.24±0.05 | −0.29±0.08 | 0.309 |
| eGFR (mL/min/1.73m2) | −5.13±0.68 | −5.52±0.41 | 0.723 |
Values are represented as the mean (Δchanges produced between post and preintervention)±standard error.
Responders, patients whose intima-media thickness of both common carotid arteries (IMT-CC) was reduced or not changed after dietary intervention and non-responders, patients whose IMT-CC was increased after dietary intervention.
p<0.05, differences in changes at 5-years between Responders and Non-responders by univariate ANOVA.
p<0.05, differences between 5-years compared to pre-intervention.
CHD, cardiovascular heart disease; T2DM, type 2 diabetes mellitus; BMI, Body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high sensitive C-reactive protein; HbA1c, glycated hemoglobin; eGFR, Serum creatinine (sCr)-based estimated glomerular filtration rate.
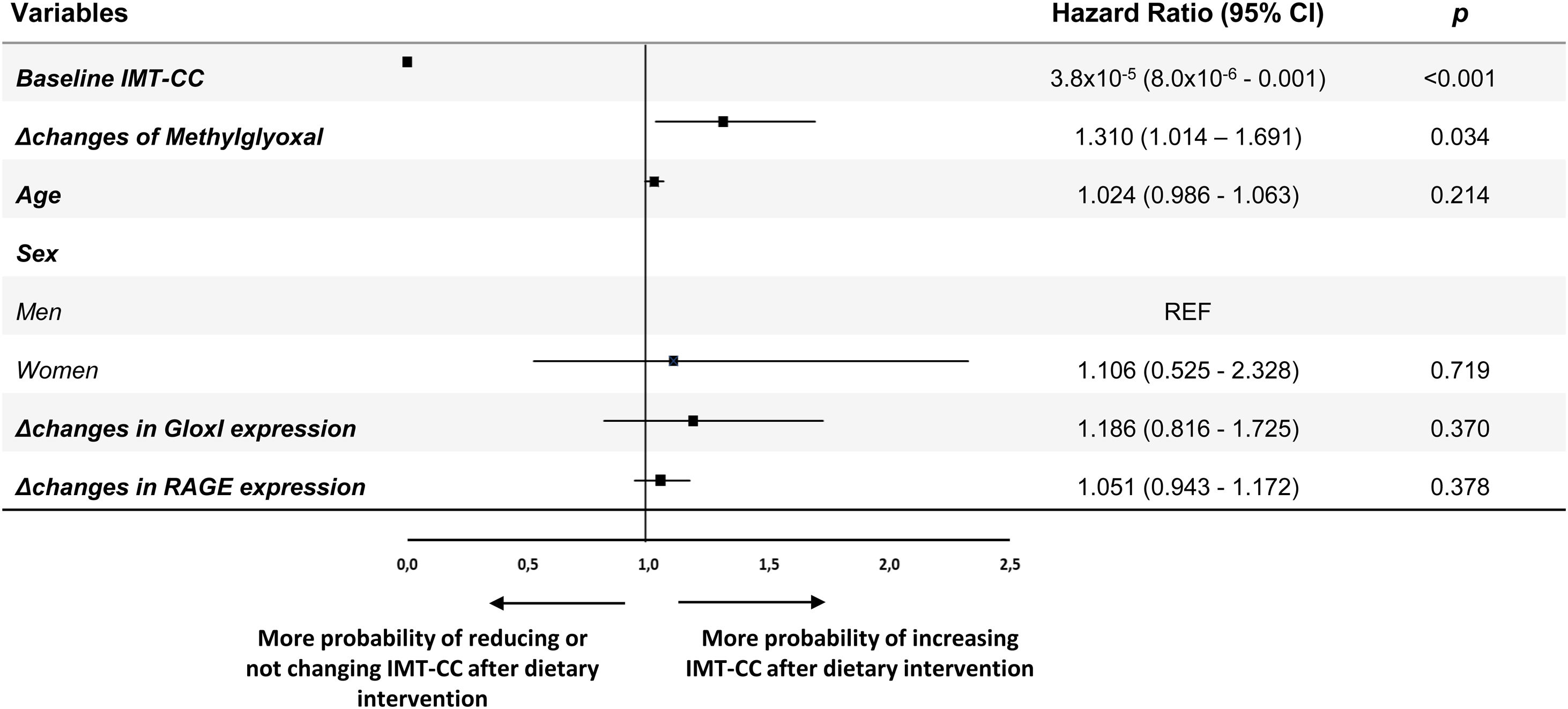
We performed a multiple logistic regression analysis to evaluate the contribution of the modulation of AGE metabolism to the changes in IMT-CC after dietary intervention (Fig. 2). Changes in serum levels of MG and gene expression of GloxI and RAGE as well as baseline IMT-CC, age and sex were included in the analysis. In our model, an increase of a SD of Δchanges in serum levels of MG determined an increase of 1.310-fold (95% CI, 1.014–1.691) the probability of increasing IMT-CC after dietary intervention. Moreover, baseline IMT-CC (odds ratio [OR], 25×104; 95% CI, 1.0×104–1.25×104) increased the probability of reducing IMT-CC after dietary intervention.
Multiple logistic regression analysis to determine the contribution of AGEs metabolism to the changes of IMT-CC, after dietary intervention, in CHD patients with T2DM. Squares denote hazard ratios; horizontal lines represent 95% confidence intervals. R2=0.345, constant=5.094 (p=0.000). Predictive variables tested by Enter method: Age (years), Sex (men and women), Baseline IMT-CC (mm), Changes in Methylglyoxal (mg/mL, Δ post-intervention minus pre-intervention), Changes in GloxI expression (AU, Δ post-intervention minus pre-intervention), Changes in RAGE expression (AU, Δ post-intervention minus pre-intervention). AGEs, Advanced glycation end products; IMT-CC, intima-media thickness of both common carotid arteries; GloxI, Glyoxalase 1; RAGE, Receptor for AGEs; CHD, cardiovascular heart disease.
This study presents new findings about the involvement of AGEs, and their management through consumption of two healthy dietary approaches, in the molecular mechanisms underlying the progression of atherosclerosis in patients with T2DM and CHD. Our data showed that those patients who reduced or maintained their IMT-CC, after 5 years of intervention with both dietary patterns (Responders), despite had a higher baseline IMT-CC and serum levels of MG than Non-responders, showed a reduction of serum levels of this glycotoxin after dietary intervention. Conversely, in patients whose IMT-CC was increased after dietary intervention (Non-responders), serum levels of MG were increased.
Structural and morphological alterations in common carotid arteries have an important role in the etiology of CVD. Indeed, evaluation of IMT-CC is one of the most commonly studied parameter when evaluating subclinical atherosclerosis and is associated with prevalent atherosclerotic disease.14 Different clinical studies support the beneficial effect of certain nutrients and specific dietary strategies in modulating and decreasing IMT-CC. In this sense, we recently found that consumption of a Mediterranean diet, which is rich in minimally-processed plant-based foods and MUFA from olive oil, but lower in SFA, meat and dairy products, reduced IMT-CC in patients with CHD.20 The intake of fiber, especially pectin, and whole-grains appear to protect against IMT-CC progression.26,27 By contrast, the intake of a Western diet-style, characterized by highly processed and refined foods and high contents of sugars, salt, SFA and protein from red meat are associated with increased IMT-CC in midlife women.28 However, the mechanisms by which diet exerts its cardioprotective role are not well elucidated.
AGEs generated from endogenous or exogenous sources exhibit a significant cardiometabolic impact in both diabetic and non-diabetic populations being considered important mediators of the pathogenesis of atherosclerosis and CVD despite the control of hyperglycemia.29 AGE accumulation can induce complex signaling pathways leading to increased inflammation, oxidative stress, enhanced calcium deposition, and increased vascular smooth muscle apoptosis that lead the development of atherosclerosis.29,30 This is in line with our data in which we found that an increase in circulating MG levels, after dietary intervention, determined a high probability of increasing IMT-CC in patients with T2DM and CHD.
In response to high AGEs content, the host defense system employs different mechanisms to restrict their toxicity and pathogenicity. Glyoxalase system (mainly GloxI) is a part of the innate and adaptive defense to regulate the deleterious effects of AGEs, catalyzing the metabolism of α-dicarbonyls (as MG) and prevents their binding with proteins to generate AGEs.31 According to that, we observed a reduction in MG content in Responders which could be explained by the fact that these patients exhibit an activation of GloxI, which does not occur in Non-responder patients. Cell-bound receptors for AGEs (AGER1 and RAGE) are antagonistic in terms of their function in relation to AGEs 24,32: while AGER1 suppresses AGEs (as CML)-related oxidative stress/inflammation response,33 RAGE increases reactive oxygen species levels and promotes the release of pro-inflammatory cytokines, pro-thrombotic and cell adhesion molecules, all involved in the pathophysiology of CVD.34 In our results, the gene expression of RAGE was increased in patients who increased their IMT-CC (Non-responders), after dietary intervention, whilst there were no changes in the gene expression of this receptor in Responders. The non-changes observed in serum levels of CML in both groups of patients could be explained by the fact that the gene expression of AGER1 is not altered. It is well described that an activation of AGER1 is necessary to uptake and degrade AGEs (as CML is) which contributes to the clearance circulating levels of CML.35
Endogenous AGE formation represents a minor component of the total body AGE content. Exogenous AGEs, in particular those consumed from the diet, are the most important source of AGEs.9,10 Different factors affect the formation of AGEs in foods, as cooking method (high cooking temperature for extended periods of time boosts their formation) and the nutrient composition (foods rich in protein and fat generate more AGE during cooking), which could be used as a strategy to reduce them.10 Evaluation of AGEs and their metabolism would allow the implementation of dietary strategies to modulate and decrease them, providing the basis for future approaches, in longitudinal studies, to prevent or delay the onset of CVD and its comorbidities.
Our study has several limitations. This research is based on a long-term, well-controlled dietary intervention, which ensures the quality of the study but may not reflect the level of compliance in a free-living population. Secondly, the results are limited to patients with T2DM and CHD and may not be suitable for extrapolation to other populations. Finally, further molecular studies are needed to offer a complete explanation which join all the results showed in our study.
ConclusionsOur findings suggest that an increase of circulating level of AGEs (and in particular, MG), after dietary intervention, could be considered a risk factor for the progression of atherosclerosis in patients with T2DM and CHD. These results support the importance of identifying underlying mechanisms CVD, in the context of secondary prevention, that provide therapeutic targets to reduce the high risk of cardiovascular events of these patients.
Author's contributionJLM and EMYS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JLM, EMYS and PPM. Acquisition, analysis, or interpretation of data: EMYS, JFAD, FMGM, PGL. Drafting of the manuscript: EMYS and FMGM. Critical revision of the manuscript for important intellectual content: JLM, PMM and JFAD. Statistical analysis: EMYS, FMGM, JFAD and PGM. Obtained funding: JLM and EMYS. Administrative, technical, or material support: PGL and FMGM.
FundingThe CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero (Cordioprev-CEAS, 1/2016 to Jose Lopez-Miranda). This study also received research grants from Ministerio de Ciencia e Innovación (AGL2012-39615, AGL2015-67896-P and PID2019-104362RB-I00 to Jose Lopez-Miranda), from Consejería de Salud-Junta de Andalucía (PC-0283-2017 to Elena M. Yubero-Serrano and FIS PI18/01822 to Elena M. Yubero-Serrano), integrated into the framework of the National Plan for Scientific Research, Technological Development and Innovation 2013-2016, co-financed by the Instituto de Salud Carlos III (ISCIII) of Spain and also by the Directorate General for Assessment and Promotion of Research and the EU's European Regional Development Fund (FEDER). Elena M Yubero-Serrano was awarded with Beca 2018 FEA – Nutrición Manuel de Oya.
Funding for open access charge: Universidad de Cordoba/CBUA.
Elena M. Yubero-Serrano is also the recipient of the Nicolas Monardes Programme from the “Servicio Andaluz de Salud, Junta de Andalucia”, Spain (C1-0005-2019). The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestJuan F. Alcala-Diaz reports personal fees from Bayer, Grunenthal Pharma, Esteve, Ferrer, and Boehringer Ingelheim outside the submitted work. Jose Lopez-Miranda reports personal fees from AMGEN, SANOFI, FERRER, Esteve, and Boehringer Ingelheim-Lilly outside the submitted work.
We would like to thank the EASP (Escuela Andaluza de Salud Publica), Granada (Spain), for carrying out the randomization process in this study.
The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.