The function to estimate lifetime cardiovascular risk –IBERLIFERISK– in Spanish working population, obtained a satisfactory discrimination; however, there was a slight overestimation of the risk in men and an underestimation of the risk in women.
ObjectiveTo recalibrate the current lifetime risk equation after extending the follow-up by 3 years.
MethodsRetrospective cohort study. 762.058 workers who underwent an occupational health examination between 2004 and 2007 were included. All episodes of temporary sickness and cardiovascular mortality up to December 2017 were evaluated. Regression models were combined to take into account the presence of competing risks in estimating cardiovascular risk in the derivation cohort. Calibration was performed by graphically representing the proportion of expected and observed events at 10 years of follow-up in the validation cohort, stratifying by risk deciles and calculating the Spiegelhalter Z statistic. Discrimination was evaluated graphically using the Receiver Operating Curve (ROC) and calculating Harrell’s C index.
ResultsThe mean age was 35.48 years (SD 10.56). 71.14% were men. Harrell’s C index was 0.78 (95% CI 0.76–0.79) in men and 0.73 (95% CI 0.69–0.77) in women. In general, there was a slight degree of underestimation in women and overestimation in men in the last decile of risk, although the Spiegelhalter Z statistic was not statistically significant in both sexes (p>0.05).
ConclusionsThe updated model continues to discriminate satisfactorily, although the model’s calibration has not substantially improved with the new update.
La función para estimar el riesgo cardiovascular de por vida en España –IBERLIFERISK– en población laboral obtuvo una discriminación satisfactoria; se registró una ligera sobreestimación del riesgo en hombres y una infraestimación del riesgo en mujeres.
ObjetivoRecalibrar la ecuación de riesgo de por vida actual tras alargar 3 años el seguimiento.
MétodosEstudio de cohortes retrospectivo. Se incluyeron 762.058 trabajadores que realizaron un examen de salud laboral entre los años 2004 y 2007. Se evaluaron todos los episodios de incapacidad temporal y mortalidad cardiovascular hasta diciembre de 2017. Se combinaron los modelos de regresión para tener en cuenta la presencia de riesgos competitivos en la estimación del riesgo cardiovascular en una cohorte de derivación. La calibración se realizó representando gráficamente la proporción de eventos esperados y observados a los 10 años de seguimiento en la cohorte de validación estratificando por deciles de riesgo y calculando el estadístico Z de Spiegelhalter. La discriminación se evaluó de forma gráfica mediante la curva Receiver Operating Curve (ROC) y calculando el índice C de Harrell.
ResultadosLa media de edad fue de 35,48 años (DE 10,56). El 71,14% eran hombres. El índice C de Harrell fue de 0,78 (IC 95% 0,76–0,79) en hombres y de 0,73 (IC 95% 0,69–0,77) en mujeres. En general, se registró un ligero grado de infraestimación en mujeres y de sobreestimación en hombres en el último decil de riesgo, aunque el estadístico Z de Spiegelhalter no resultó estadísticamente significativo en ambos sexos (p>0,05).
ConclusionesEl modelo actualizado continúa discriminando satisfactoriamente, aunque la calibración del modelo no ha mejorado sustancialmente con la nueva actualización.
The tables currently used to calculate cardiovascular risk (CVR) as the standard for decision-making, mainly about lifestyle and pharmacological treatment, estimate short-term risk, usually over 10 years. The majority of young adults with one or several risk factors are considered to be at low or intermediate risk over 10 years by current tables, although they would probably be considered high risk if the long-term risk over the remaining years of their life were taken into consideration. The lasts European Guides for Cardiovascular Prevention therefore recommend including and assessing lifetime or vascular age risk.1 Some algorithms for the primary prevention of cardiovascular diseases even recommend combining both tools to estimate lifetime CVR in those patients, above all young adults, with a low CVR over 10 years.2 Rossello et al.3 published a compilation and description of the different tools used to calculate CVR that are available to date.
Several calculation tools have been developed which estimate lifetime CVR. They include the QRISK-LTR4 equation, which estimates lifetime CVR from 30 to 95 years old, and the equation developed by the American College of Cardiology/American Heart Association (ACC/AHA)5 which calculates CVR between the ages of 50 to 95 years old. The LIFE-CVD6 calculation tool was developed more recently, and this has been recommended by the new European guides for cardiovascular prevention. One study evaluated the change which would arise when lifetime CVR was calculated in comparison with CVR over 10 years using the QRISK-LTR and ACC/AHA7 tables. This showed that the percentage of patients who were reclassified as high risk by the long-term calculation amounted to 1.61% (95% CI 1.55–1.66) for QRISK-LTR, while for the ACC/AHA equation it was 27.1% (95% CI 27.11–27.70).
Furthermore, CVR factors have been shown to be significantly implicated in the prevalence and progression of subclinical arteriosclerosis in individuals younger than 50 years old.8 Within this line of research a function has been developed to estimate lifetime CVR in Spain –IBERLIFERISK– from 18 to 75 years old, in the Spanish working population. In this workers were subjected to a medical examination in the Ibermutua Insurance Company from 2004 to 2007,9 after which they were followed up until 2014. This model achieved a satisfactory degree of discrimination; nevertheless, calibration results indicated that the risk was over-estimated in men, specifically in the high risk deciles. More erratic behaviour was observed in the women, with a tendency to under-estimate risk except in the final decile, where the model over-estimated it. One possible reason for this calibration defect could be the low number of cardiovascular events, above all in the women. This study aims to recalibrate the current lifetime risk equation after prolonging the follow-up of the cohort by 3 years, with a higher number of cardiovascular events.
MethodsThe methodology of this study has already been described.9 A retrospective cohort study with a follow-up of up to 13 years was performed. The study cohort consisted of workers in companies which belonged to an Insurance company that worked with the Social Security, with broad national coverage (Ibermutua). They were aged from 18 to 65 years old and had no history of cardiovascular disease, and they were subjected to a work health examination in the Ibermutua Insurance Company from 2004 to 2007. The definition of the risk factors has already been published.10 All of the participants gave their informed consent prior to inclusion in the study, according to the principles laid down in the Helsinki Declaration.
All of the episodes of temporary disability of the subjects included in the study after the date they were included until 31 December 2017 were evaluated, based on the official register of Ibermutua. The following International Classification of Diseases codes, 9th revision, clinical modification (CIE-9-MC), 2002, were considered for the main result variable (the incidence of fatal or non-fatal cardiovascular events): coronary disease (codes 410–414), heart failure (code 428), cerebrovascular diseases (codes 431–438, except: 432.1, 437.2, 437.3, 437.7) and peripheral arterial disease (codes 440–444, except: 442, 443.0, 443.1). Additionally, deaths coded as due to hypertensive disease (codes 401–405) or arrhythmia (codes 426–427, except 427.5) were included as fatal cardiovascular events. Mapping to CIE-10 was carried out when this came into force, to include the same codes as those described.
Statistical analysisTo maximize the number of subjects included in the statistical analysis an iterative stochastic form of imputing lost values of the main variables was used, applying the Monte Carlo method in Markov chains. 20 imputations were replicated, after which they were combined following Rubin’s rules.
Using 70% of the sample, selected at random and following the classic models for estimating cause-specific risk, the regression models were combined to take into account the presence of competitive risks in the estimation of the risk. To this end 2 Cox proportional risk models were constructed, including the same variables in both equations and considering age to be a latent function of time. One of the models considered the appearance of (fatal and non-fatal) cardiovascular events to be a dependent variable and, in parallel, the appearance of any competitive event (death due to any other cause). The function of the accumulated incidence of cardiovascular disease was constructed by multiplying the contribution of the risk of cardiovascular disease at a certain age by the probability of being alive and free of the cardiovascular event at the same age, then adding these values through the range of the age of interest.
The formula obtained to calculate CVR in the derivation cohort was applied to all of the subjects of the validation cohort (the remaining 30% of the sample), and expected risk scores were calculated according to the model. Calibration was carried out by graphically representing the proportion of expected events and the proportion of observed events over the 10 year follow-up, stratifying the risk in deciles and calculating Spiegelhalter’s Z statistic. The proportion of observed events was calculated using the non-parametric Nelson–Aalen11 incidence of accumulated risk calculator, which takes into account the presence of competitive events. Discrimination was evaluated graphically using the Receiver Operating Curve (ROC) and calculating Harrell’s C index. Statistical analysis was carried out using the STATA/MP 14 programme.
ResultsA total of 762,058 individuals fulfilled the inclusion criteria. Their average age was 35.48 years, with a standard deviation of 10.56 years. 71.14% were men. The derivation cohort was composed of a randomized selection of 70% of the total number of participants (n=533,441). It recorded a total of 3493 and 364 cardiovascular events in the men and women, respectively, with a total follow-up of 4,398,638.58 individuals-year, and 6343 and 903 competitive events (deaths with a non-cardiovascular cause) in the men and women, respectively. 1501 cardiovascular events were recorded in the men and 149 in the women of the validation cohort, together with 2645 and 375 competitive events, respectively.
Both study cohorts had similar basal characteristics, in the men (Table 1) as well as in the women (Table 2). In general, the men had more manual jobs than the women (74.80% vs. 41.04%), more of them smoked (48.47% vs. 40.32%) and the men were also more likely to be risk consumers of alcohol (20.82% vs. 4.38%). A higher proportion of men than women were diagnosed with diabetes mellitus (1.44% vs. 0.56%), hypertension (5.18% vs. 2.77%) and dyslipidaemia (6.52% vs. 3.95%).
Description of the basal characteristics of the sample (men).
| Variable name | Definition/units | Derivation | Validation | ||
|---|---|---|---|---|---|
| N=379,448 | N=162,689 | ||||
| Professional category, n (%) | Non-manual (CNO94 1–499) | 93,485 | (24.64%) | 40,121 | (24.66%) |
| Manual (CNO94 500–999) | 283,837 | (74.80%) | 121,670 | (74.79%) | |
| DK/DA | 2126 | (0.56%) | 898 | (0.55%) | |
| Smoking, n (%) | Non-smoker | 136,159 | (35.88%) | 58,192 | (35.77%) |
| Ex-smoker | 59,389 | (15.65%) | 25,297 | (15.55%) | |
| 1–10 cigarettes/occasional | 70,195 | (18.50%) | 30,163 | (18.54%) | |
| 11–20 cigarettes | 81,975 | (21.60%) | 35,328 | (21.72%) | |
| >20 cigarettes | 29,002 | (7.64%) | 12,557 | (7.72%) | |
| Pipe and cigars | 2728 | (0.72%) | 1152 | (0.71%) | |
| Alcohol consumption, n (%) | No | 300,458 | (79.18%) | 128,742 | (79.13%) |
| Yes | 78,990 | (20.82%) | 33,947 | (20.87%) | |
| Family history of cardiovascular disease, n (%) | No | 358,847 | (94.57%) | 153,896 | (94.60%) |
| Yes | 20,601 | (5.43%) | 8793 | (5.40%) | |
| DM, n (%) | No | 373,999 | (98.56%) | 160,294 | (98.53%) |
| Yes | 5449 | (1.44%) | 2395 | (1.47%) | |
| Kidney disease, n (%) | No | 365,649 | (96.36%) | 156,625 | (96.27%) |
| Yes | 6695 | (1.76%) | 2994 | (1.84%) | |
| Antihypertensive treatment, n (%) | No | 366,872 | (96.69%) | 157,224 | (96.64%) |
| Yes | 12,576 | (3.31%) | 5465 | (3.36%) | |
| Body mass index, average (SD) [n] | 26.48 (4.13) | 26,46 (4.13) | |||
| [n=376,299] | [n=161,354] | ||||
| SAP (mmHg), average (SD) [n] | 129,10 (15.77) | 129,08 (15.84) | |||
| [n=377,363] | [n=161,829] | ||||
| DAP (mmHg), average (SD) [n] | 76.96 (10.92) | 76.97 (10.94) | |||
| [n=377,379] | [n=161,808] | ||||
| Total cholesterol (mg/dl), average (SD) [n] | 196,05 (42.13) | 196,08 (42.16) | |||
| [n=368,919] | [n=158,133] | ||||
| HDL cholesterol (mg/dl), average (SD) [n] | 50.82 (12.44) | 50.89 (12.46) | |||
| [n=354,595] | [n=152,189] | ||||
CNO-94: 1994 National Classification of Occupations; SD: standard deviation; DM: diabetes mellitus; DK/DA: does not know/does not answer; DAP: diastolic arterial pressure; SAP: systolic arterial pressure.
Description of the basal characteristics of the sample (women).
| Variable name | Definition/units | Derivation | N=153,993 | Validation | N=65,928 |
|---|---|---|---|---|---|
| Professional category, n (%) | Non-manual (1–499) | 89,894 | (58.38%) | 38,543 | (58.47%) |
| Manual (500–999) | 63,191 | (41.04%) | 27,017 | (40.98%) | |
| DK/DA | 891 | (0.58%) | 362 | (0.55%) | |
| Smoking, n (%) | Non-smoker | 70,335 | (45.68%) | 30,351 | (46.04%) |
| Ex-smoker | 21,553 | (14.00%) | 9066 | (13.75%) | |
| 1–10 cigarettes/occasional | 36,876 | (23.95%) | 15,705 | (23.82%) | |
| 11–20 cigarettes | 22,357 | (14.52%) | 9528 | (14.45%) | |
| >20 cigarettes | 2855 | (1.85%) | 1272 | (1.93%) | |
| Alcohol consumption, n (%) | No | 147,227 | (95.62%) | 63,026 | (95.61%) |
| Yes | 6749 | (4.38%) | 2896 | (4.39%) | |
| Family history of cardiovascular disease, n (%) | No | 143,156 | (92.97%) | 61,427 | (93.18%) |
| Yes | 10,820 | (7.03%) | 4495 | (6.82%) | |
| DM, n (%) | No | 153,108 | (99.44%) | 65,536 | (99.41%) |
| Yes | 868 | (0.56%) | 386 | (0.59%) | |
| Kidney disease, n (%) | No | 153,695 | (99.82%) | 65,780 | (99.78%) |
| Yes | 4324 | (2.81%) | 1915 | (2.90%) | |
| Antihypertensive treatment, n (%) | No | 151,049 | (98.10%) | 64,669 | (98.10%) |
| Yes | 2927 | (1.90%) | 1253 | (1.90%) | |
| Body mass index, average (SD) [n] | 23.85 (4.32) [n=152,125] | 23.87 (4.30) [n=65,101] | |||
| SAP (mmHg), average (SD) [n] | 116.51 (14.72) [n=153,139] | 116.55 (14.69) [n=65,537] | |||
| DAP (mmHg), average (SD) [n] | 72.60 (9.96) [n=153,199] | 72.64 (10.01) [n=65,554] | |||
| Total cholesterol (mg/dl), average (SD) [n] | 190.06 (36.14) [n=148,859] | 190.33 (36.47) [n=63,744] | |||
| HDL cholesterol (mg/dl), average (SD) [n] | 62.74 (14.61) [n=144,121] | 62.77 (14.59) [n=61,711] |
SD: standard deviation; DM: diabetes mellitus; DK/DA: does not know/does not answer; DAP: diastolic arterial pressure; SAP: systolic arterial pressure.
Predictors included in the recalibrated CVR models (Tables 3 and 4) included occupation, smoking, alcohol consumption, body mass index, a history of cardiovascular disease in first degree family members, kidney disease, a history of diabetes mellitus, antihypertensive and lipid reducing treatment, systolic and diastolic blood pressure values and total cholesterol.
Equation coefficients for men.
| CVD model | Model for other exitus | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Professional category (ref. non-manual) | ||
| Manual | 1.123 (1.036–1.218) | 1.484 (1.389–1.585) |
| DA | 0.530 (0.275–1.023) | 0.681 (0.427–1.085) |
| Smoking (ref. non-smoker) | ||
| Ex-smoker | 1.842 (1.651–2.055) | 1.862 (1.714–2.023) |
| Occasional/1–10 | 1.654 (1.464–1.869) | 1.568 (1.434–1.715) |
| 11–20 | 2.634 (2.376–2.920) | 2.645 (2.452–2.853) |
| >20 | 4.106 (3.671–4.592) | 4.378 (4.030–4.756) |
| Pipe/cigars | 2.038 (1.512–2.747) | 2.471 (1.991–3.069) |
| Alcohol (ref. non-risk consumption) | 1.150 (1.069–1.238) | 1.614 (1.530–1.703) |
| Body mass index (kg/m2) | 1.016 (1.007–1.024) | 0.990 (0.984–0.997) |
| Family history of cardiovascular disease | 1.332 (1.186–1.497) | 0.935 (0.841–1.038) |
| Diabetes mellitus | 2.869 (2.506–3.285) | 2.593 (2.314–2.906) |
| Kidney disease | 1.633 (1.395–1.912) | 2.021 (1.788–2.285) |
| Antihypertensive treatment | 1.972 (1.767–2.201) | 1.818 (1.655–1.996) |
| Centred SAP (mmHg) | 1.014 (1.012–1.017) | 1.015 (1.013–1.017) |
| Centred DAP (mmHg) | 1.019 (1.015–1.023) | 1.008 (1.004–1.011) |
| Lipid reducing treatment | 1.404 (1.015–1.023) | 1.271 (1.119–1.444) |
| Centred total cholesterol (mg/dl) | 1.008 (1.007–1.008) | 1.002 (1.001–1.003) |
| Centred HDL (mg/dl) | 0.989 (0.986–0.992) | 1.007 (1.005–1.009) |
DAP: diastolic arterial pressure; SAP: systolic arterial pressure.
Equation coefficients for women.
| CVD model | Model for other exitus | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Professional category (ref. non-manual) | ||
| Manual | 1.277 (1.034–1.578) | 1.321 (1.155–1.510) |
| DA | 1.042 (0.258– 4.203) | 1.257 (0.561–2.814) |
| Smoking (ref. non-smoker) | ||
| Ex-smoker | 1.472 (1.066–2.033) | 1.053 (0.851–1.302) |
| Occasional/1–10 | 1.649 (1.245–2.185) | 1.145 (0.954–1.374) |
| 11–20 | 2.222 (1.674–2.948) | 1.868 (1.565–2.229) |
| >20 | 2.960 (1.811–4.840) | 3.633 (2.752–4.796) |
| Alcohol (ref. non-risk consumption) | 1.099 (0.723–1.671) | 1.691 (1.347–2.122) |
| Body mass index (kg/m2) | 0.998 (0.975–1.022) | 1.021 (1.006–1.037) |
| Family history of cardiovascular disease | 1.841 (1.379–2.456) | 0.952 (0.748–1.213) |
| Diabetes mellitus | 3.407 (2.009–5.776) | 1.916 (1.198–3.065) |
| Kidney disease | 1.171 (0.733–1.871) | 1.976 (1.528–2.556) |
| Antihypertensive treatment | 2.275 (1.564–3.310) | 1.828 (1.372–2.435) |
| Centred SAP (mmHg) | 1.026 (1.018–1.035) | 1.016 (1.010–1.022) |
| Centred DAP (mmHg) | 1.001 (0.987–1.015) | 0.995 (0.986–1.004) |
| Lipid reducing treatment | 1.347 (0.762–2.381) | 1.886 (1.298–2.740) |
| Centred total cholesterol (mg/dl) | 1.008 (1.005–1.011) | 1.006 (1.004–1.008) |
| Centred HDL (mg/dl) | 0.980 (0.972–0.988) | 0.995 (0.990–0.999) |
DAP: diastolic arterial pressure; SAP: systolic arterial pressure.
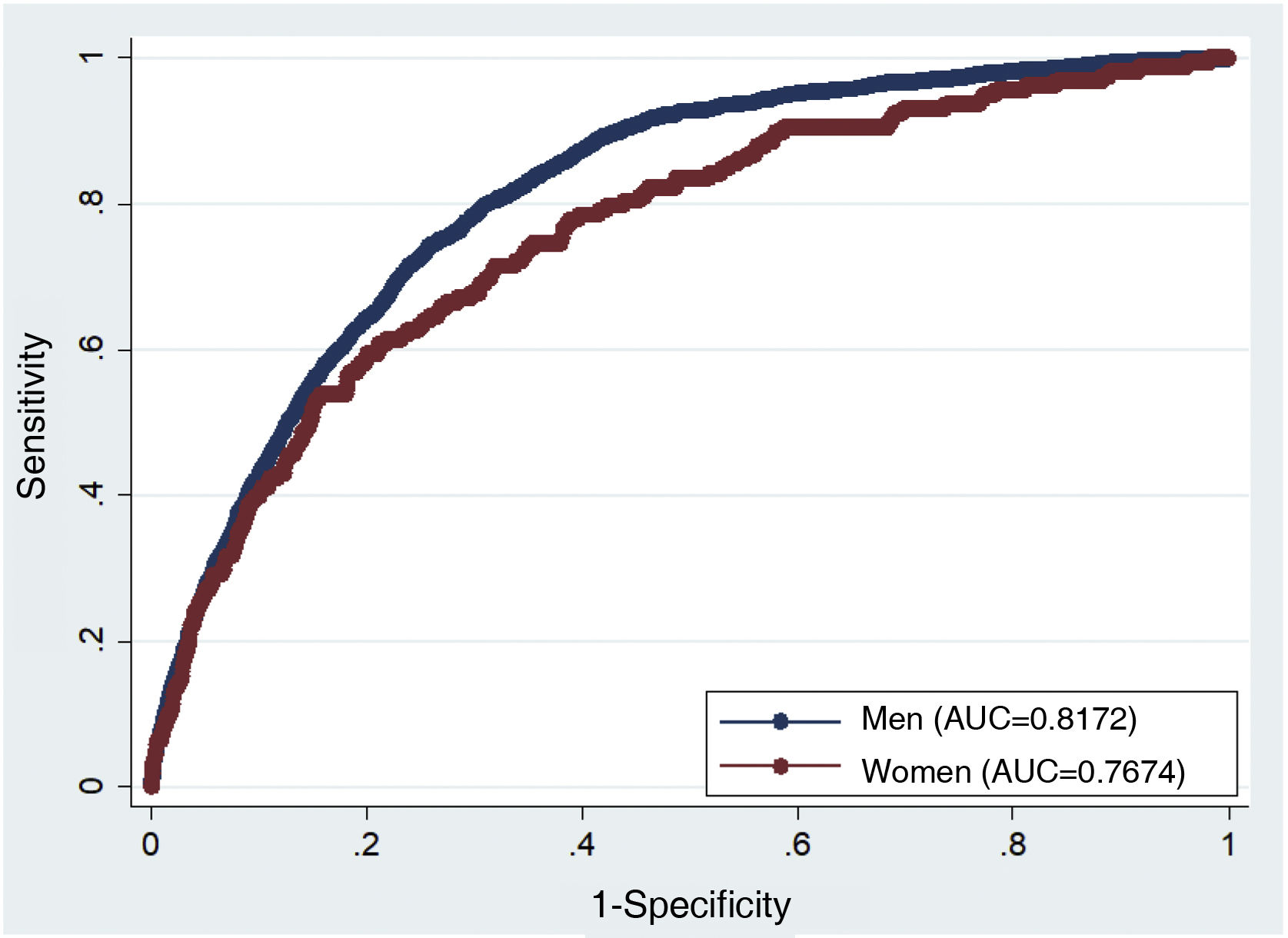
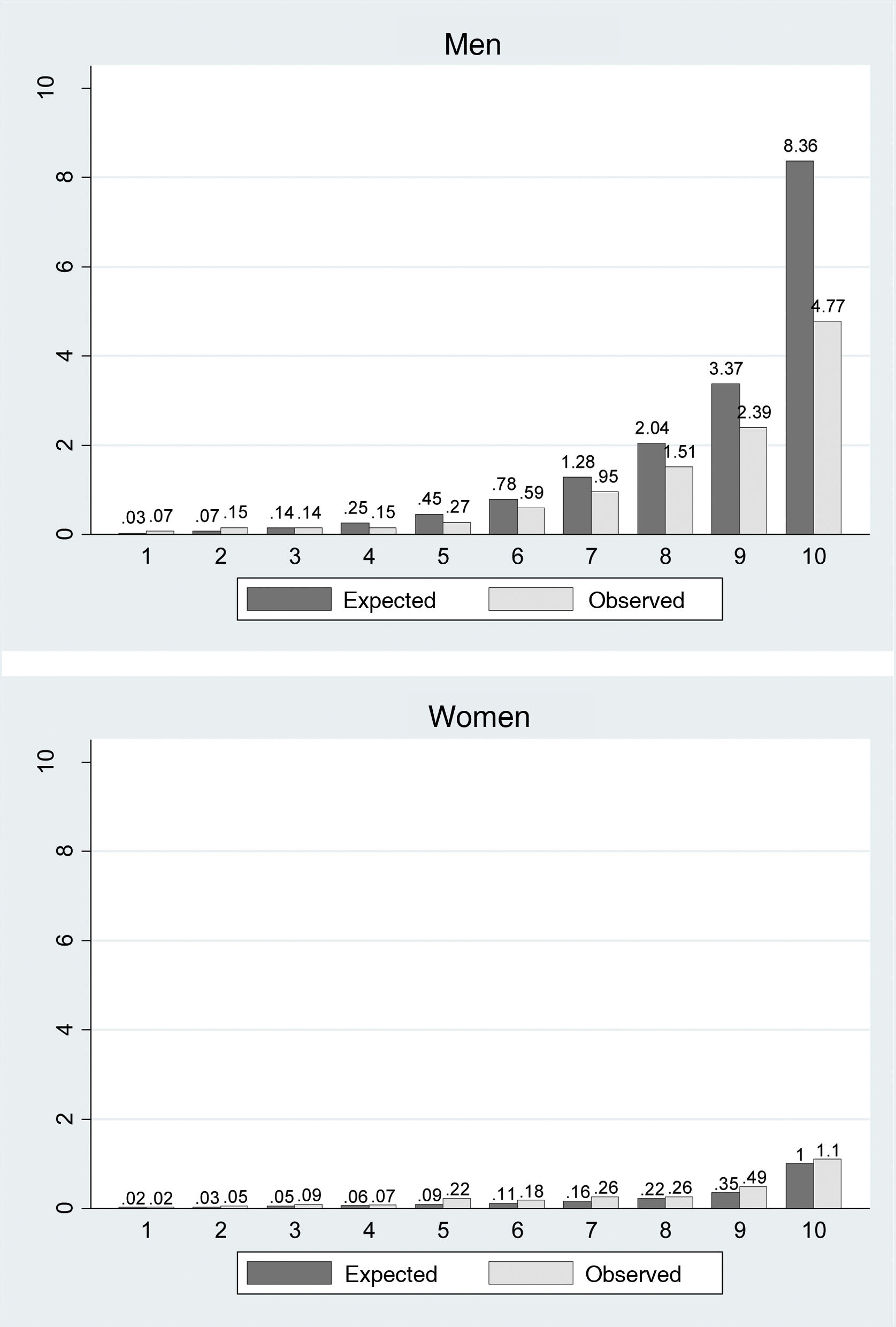
The area under the curve was 0.82 for the men and 0.73 for the women (Fig. 1). Harrell’s C index was 0.78 (95% CI 0.76–0.79) in the men and 0.73 (95% CI 0.69–0.77) in the women. Fig. 2 makes it possible to evaluate the calibration of the model, using the graphic representation of the observed risks and the expected risks, while taking into consideration the presence of competitive events over a 10 year follow-up per risk deciles. More than 75% of the men were classified in the first 3 risk deciles. In general a slight degree of under-estimation was found for the women, together with a slight degree of over-estimation in the men. Spiegelhalter’s Z statistic was not found to be statistically significant in both sexes (p>0.05).
DiscussionThis study obtained an updated model for calculating lifetime CVR (IBERLIFERISK2) from 18 to 75 years old in the Spanish working population. The risk predictors included in the new model are the same as the ones used in the original IBERLIFERISK equation. The psychometric properties of the model are still regular. The updated model still discriminates in a satisfactory way, and the area under the curve is very similar in both models (in the original model, men 0.84 and women 0.7332; in the updated model, men 0.88 and women 0.77). In terms of the calibration of the model, the recalibrated one systematically over-estimates in men in deciles 4–8 somewhat more than the original model, although it does so less than the original model in the final deciles 9 and 10, which are high risk deciles, while similar results to the original ones are recorded for women.
The IBERLIFERISK and IBERLIFERISK2 equations were developed using the same methodology that was described for the QRISK-LTR equation. They were developed taking into account the fact that the data were drawn from subjects with different follow-up times, and who entered the observation period at different ages. Age was considered to be a latent function of time, and this made it possible to make the model more flexible, as subjects were considered event-free until the moment, so that risk could be calculated for different age bands. The discrimination obtained in the new model IBERLIFERISK2 is satisfactory and similar to that of the original model (IBERLIFERISK). It is comparable to the discrimination in the QRISK-LTR model, discriminating in a similar way for men and somewhat worse for women.4
Nevertheless, the calibration of the model has not substantially improved with the new update. One reason for these differences in calibration may be the small number of cardiovascular events registered, in spite of the prolongation of the follow-up period, above all in the women. As the mortality data were obtained from the Nacional Institute of Statistics it is improbable that deaths have been lost. On the other hand, it cannot be ruled out that non-mortal cardiovascular events may have been lost. Although events of this type are systematically recorded in insurance companies that cover workers when they give rise to disability at work, information may have been lost for those subjects who changed their work insurance company during follow-up, became unemployed or had even changed category without stating that they had ceased working in the most recent years. In spite of the prolongation of the follow-up period, our study registered only 0.72% of cardiovascular events and 1.36% of competitive events, as opposed to 5.19% and 6.34% of cardiovascular and competitive events, respectively, as registered in the QRISK-LTR calculator. These differences can be explained, at least in part, because our population is much younger, with an average age of 35 years, while in QRISK-LTR the average age was 48 years. The QRISK-LTR equation makes it possible to calculate CVR from 30 to 95 years, while the IBERLIFERISK2 equation does so from the age of 18 years up to 75 years. The fact that age is decisive when constructing the equation, as the IBERLIFERISK2 population is much younger and vascular disease usually appears at advanced ages represented more heavily in the QRISK-LTR equation, affects both the incidence of events as well as the calculated CVR value.
Another aspect that should be taken into consideration is the positive impact of several preventive programmes in primary care, and that these and screening programmes may reduce the number of events. A study that analysed the change over time in residual lifetime CVR tendencies in adults during 3 periods in 6 decades (1960–1979, 1980–1999, and 2000–2018).12 It concluded that the increase in life expectancy during this time had also involved a reduction in residual lifetime CVR in the community.
Lifetime CVR models have their limitations, and questions still arise that should be resolved before they can be applied in clinical practice. For example, when should a subject be considered to be high risk based on their lifetime CVR? The QRISK-LTR4 models defines a shut-off point to classifying a subject as high risk. This is set at percentile 90, which is when lifetime CVR rises above 50% in the derivation cohort, although it is unclear whether this threshold is used in the decision-making of normal clinical practice. Nor is there any evidence to support the benefits of treating young patients pharmacologically during such a long time with antihypertensive and/or lipid reducing drugs, although studies such as the Heart Protection Study13 seem to indicate that there are long-term benefits.
Lifetime CVR, together with vascular age,1 may be useful in improving and complementing the information patients receive, making it more comprehensible and increasing their awareness of its impact on their health. A clinical trial evaluated the impact of improving the information patients receive about their CVR, showing CVR in the form of figures and in terms of absolute and relative risk as well as vascular age in high risk patients. When this was compared with habitual clinical practice, a significant reduction in CVR was detected, and healthier habits had been adopted after one year of follow-up.14 It can be hypothesized that subjects with a low short-term risk may consider making their lifestyle healthier when they are informed that their lifetime CVR is high.
The model was validated using a parallel cohort to the one used for its derivation. A study is underway to validate the equation in 2 external cohorts, one composed of a similar working population to the original one, and another composed of patients who visited a primary care centre. A platform exists to calculate CVR (http://www.iberliferisk.com/), and it has been updated with the IBERLIFERISK2 equation.
ConclusionsAn updated model has been obtained to calculate lifetime CVR (IBERLIFERISK2), from 18 to 75 years old in the Spanish working population. Compared with the original IBERLIFERISK, discrimination remains stable and the calibration improves in men in the highest risk deciles, although it increases over-estimation in the medium risk deciles. The risk predictors included in the new model are occupation, smoking, alcohol consumption, body mass index, a history of cardiovascular disease in first degree family members, kidney disease, a history of diabetes mellitus, antihypertensive or lipid-reducing treatments and the values of systolic and diastolic blood pressure and total cholesterol.
FinancingThis work was financed by the Fundación Española de Arteriosclerosis and the Sociedad Española de Arteriosclerosis [Grant for Epidemiological Clinical Research in Arteriosclerosis, 2019].
Conflict of interestsThe authors have no conflict of interests to declare.