Cardiovascular diseases (CVDs) remain the leading cause of worldwide death, accounting for significant morbidity, mortality, disability, and reduced quality of life. The global prevalence of cardiovascular (CV) risk factors, such as type 2 diabetes mellitus, hypertension, dyslipidemia, and obesity, has grown exponentially in the last decades, particularly in low-medium income countries, and it's projected to increase rapidly in the coming years as the population progressively ages, leading to increased cardiovascular disease (CVD) and associated mortality. In fact, data from the global burden of disease study shows that CV mortality, associated disability-adjusted life years (DALYs), and years of life lost (YLL) have increased steadily, nearly doubling from 1990 to 2019.
Recent evidence proves the existence of an inverse association between hand grip strength (HGS), as a proxy for global muscle strength, with all-cause mortality, CV mortality, and the development of several chronic diseases. These associations have been demonstrated recurringly across the entire lifespan, beginning in childhood, and carrying on throughout adult life. Mounting evidence strongly indicates that HGS is an early predictor of chronic disease in premorbid populations and a therapeutic target for CVD prevention. Recent clinical trials have consistently shown that resistance exercise, which increases strength and potentially muscle mass, significantly improves the control of known CVD risk factors, reduces the risk of all-cause death and cardiovascular mortality.
In this review, we explore the latest evidence regarding the association between low muscle strength and diverse metabolic alterations, along with the interventions that could improve cardiometabolic risk factors, while simultaneously increasing muscle fitness.
Las enfermedades cardiovasculares (ECV) siguen siendo la causa principal de muerte a nivel mundial, con una gran significación en términos de morbilidad, mortalidad, incapacidad y reducción de la calidad de vida. La prevalencia global de factores de riesgo cardiovascular (CV), tales como diabetes mellitus tipo 2, hipertensión, dislipidemia y obesidad ha crecido exponencialmente en las últimas décadas, particularmente en los países con rentas bajas-medias, previéndose que se incremente rápidamente en los años venideros a medida que la población envejezca, originando un incremento de las ECV y la mortalidad asociada. De hecho, los datos procedentes del estudio sobre carga global de la enfermedad muestran que la mortalidad CV, los años de vida ajustados por discapacidad (DALY), y los años de vida perdidos (YLL) asociados se han incrementado de manera constante, y casi doblándose desde 1990 a 2019.
La evidencia reciente prueba la existencia de una asociación inversa entre la fuerza de agarre (HGS), como indicador de la fuerza muscular global, y la mortalidad por todas las causas, la mortalidad CV y el desarrollo de diversas enfermedades crónicas. Estas asociaciones han sido demostradas recurrentemente durante todo el periodo de vida, comenzando en la escuela, y prosiguiendo durante toda la vida adulta. La evidencia creciente indica enfáticamente que la HGS es un factor predictivo temprano de enfermedades crónicas en poblaciones premórbidas, y un objetivo terapéutico para la prevención de las ECV. Los ensayos clínicos recientes han reflejado de manera consistente que el ejercicio de resistencia, que incrementa la fuerza, y potencialmente la masa muscular, mejora considerablemente el control de los factores conocidos de ECV, reduce el riesgo de muerte por todas las causas, y la mortalidad cardiovascular.
En esta revisión exploramos la última evidencia relativa a la asociación entre la fuerza muscular baja y las diversas alteraciones metabólicas, junto con las intervenciones que podrían mejorar los factores de riesgo cardiometabólico, incrementando a la vez el estado muscular.
Cardiovascular diseases (CVDs) remain the leading cause of worldwide death, accounting for significant morbidity, mortality, disability, and reduced quality. The global trends for CVD prevalence, cardiovascular (CV) mortality, and associated disability-adjusted life years (DALYs) and years of life lost (YLL) have increased steadily in the past decades, nearly doubling from 1990 to 2019.1
The greatest clinical and economic burden attributable to CVDs is seen in low-income and middle-income countries (LIC and MIC), where CVDs are often diagnosed very late in the course of the disease, due to reduced access to health care services, coupled with the lack of population-level screening and treatment of CV risk factors, resulting in higher rates of both incident CVD and CV mortality.2
Recent evidence proves the existence of an inverse association between hand grip strength (HGS), a proxy for global muscle strength3 with all-cause mortality, cardiovascular (CV) mortality, and the development of several chronic diseases.4–8 This association is independent of demographic, anthropometric, or classic CV risk factors. Furthermore, exercise interventions aimed at increasing muscle strength are effective for the improvement of metabolic risk factors, potentially preventing incident CV cases and premature death. Therefore, it is crucial to design muscle-strengthening interventions on a population level, as well as creating awareness amongst the medical community and the public about the importance of muscle health. In this review, we explore the latest evidence regarding the association between low muscle strength and diverse metabolic alterations, along with the interventions that could improve cardiometabolic risk factors, while simultaneously increasing muscle fitness.
Handgrip strengthIsometric HGS is a simple, non-invasive, inexpensive test used as a predictor of adverse health outcomes. HSG can be measured using a variety of instruments, such as digital, analog, hydraulic, and Smedley-type dynamometers. Amongst the available options, the JAMAR dynamometer has the most normative data and is used in most clinical studies evaluating HGS9 (Fig. 1).
However, HGS values may vary significantly depending on the patient's anthropometric characteristics, measuring instrument, and protocol employed. Factors such as the subject's position (standing or sitting), hand dominance, number of repeated measurements, and the placement of the upper extremity (alignment of the shoulder, elbow, arm, and hand) may condition HGS.10 This highlights the need for standardization of both the dynamometer and the protocol employed for measuring HGS.
Although HGS correlates with muscle mass and strength, it is also determined by weight, body mass index (BMI), height, bone mineral density, and other anthropometric parameters of the hand and arm (e.g., arm length and circumference, wrist circumference, breadth and width of the hand), reflecting the total body mass and size besides muscular fitness.11,12 Therefore, in non-athletic populations, obese or overweight individuals might be apparently stronger than subjects with a normal BMI, given that the former group has a greater body size and surface area.
BMI is proportional to an individual's weight regardless of body composition as it doesn’t discriminate between lean and fat mass. As the lean mass confers protection against metabolic diseases, and fat mass is a risk factor for a plethora of comorbidities, BMI might act as a confounding factor in the association between HGS and chronic diseases. To account for the effects of body mass and size on HGS, the raw value (absolute HGS) recorded from the dynamometer can be divided by weight or BMI, termed relative or normalized HGS. In comparison to absolute HGS, relative hand grip strength is a better surrogate of muscular strength and shows a stronger association with diverse chronic diseases.13,14
Normal HGS values also depend on the population being considered, as there is an important genetic component that determines muscular strength. In addition, normative values of HGS vary according to age and sex, being higher in males than females, and in younger versus older individuals. Reference HGS values for different populations have been published, to establish cut-points for low strength.15,16
Hand grip strength, obesity, and inflammationObesity has reached epidemic proportions amongst all age groups and is prevalence has tripled since 1975, accounting for approximately 4 million annual deaths.17 Data from the National Institutes of Health (NIH) shows that excessive weight is the second leading cause of preventable death in the United States and is one of the leading preventable risk factors for CVD, myocardial infarction (MI) and stroke.18 From an early age, obese and overweight individuals are less likely to perform sufficient physical activity, which is reflected in lower cardiorespiratory fitness (CRF) and lower muscle strength.19–21
Visceral obesity is linked to chronic low-grade inflammation, since it's enhancing the production of acute-phase reactants (e.g. C reactive protein [CRP]), and inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), leptin, and growth hormone (GH).22 In aging patients, appendicular skeletal muscle is progressively replaced by ectopic fatty tissue -a process termed myolipid infiltration, which is enhanced by concomitant obesity. The accumulation of adipose tissue contributes to chronic low-grade inflammation and directly causes the loss of muscle mass, strength, and fitness. Chronic low-grade inflammation impairs mitochondrial functioning, which in turn increases muscle catabolism, inhibits the anabolic actions of insulin-like growth factor type 1, and leads to IR, promoting myocyte apoptosis and the development of hypertension, diabetes, and metabolic syndrome.22
Both obesity and low HGS are associated with increased levels of pro-inflammatory signaling molecules in adolescents, adults, and the elderly.22 The imbalance between pro-inflammatory and anti-inflammatory chemokines is reflected in an increase of the leptin to adiponectin ratio, which constitutes a risk factor for CVD.23 Moreover, sarcopenic patients have higher levels of proinflammatory cytokines (e.g., serum IL-6, Interleukin-18, TNF-α, TNF-like weak inducer of apoptosis [TWEAK], and leptin) and lower levels of anti-inflammatory cytokines (e.g., adiponectin) than non-sarcopenic controls.24 Interestingly, an intensive lifestyle intervention (including a personalized routine of resistance training [RT] and a high-protein diet) that lasted for 12 weeks led to significant improvements in muscle mass, a trend for increased muscle strength, lower levels of pro-inflammatory signaling molecules, and higher concentrations of adiponectin, suggesting that muscle fitness helps to ameliorate chronic inflammation and improves the hormonal profile of sarcopenic patients.25,26
In fact, recent evidence supports that muscle fitness counteracts the inflammatory burden of adipose tissue regardless of BMI, highlighting its role in the prevention of metabolic diseases in obese patients. A cross-sectional study (n=90, males aged 18–30 years) that assessed the metabolic profile and inflammatory markers of obese untrained (OU), obese trained (OT), and normal-weight trained individuals (NT), reported that in comparison with OU, NT and OT exhibited lower levels of total cholesterol, low-density lipoprotein (LDL) and triglycerides (TG) and higher concentrations of high-density lipoprotein (HDL) and adiponectin. Additionally, inflammatory markers such as oxidated LDL, CRP, and leptin were lower in both strength-trained groups compared to OU (P<0.01).27
Other studies have demonstrated that muscle-strengthening activities can increase lean body mass, reduce adiposity and improve inflammation in the absence of weight loss, in individuals with diabetes and metabolic syndrome.26
All this evidence indicates that muscle fitness confers protection against chronic inflammation in both healthy and comorbid patients, regardless of sex, age and weight.
The mechanism that links poor muscle fitness and inflammation extends beyond body composition changes related to aging, beginning as early as the gestational period. Available evidence indicates that individuals with low birth weight (LBW) are predisposed to have low muscular strength and chronic low-grade inflammation, possibly due to a phenomenon known as fetal programming.28,29 This concept proposes that in-utero exposure to a nutrient-deprived environment leads to permanent organ changes (e.g., less hepatic, pancreatic, and muscular tissue), resulting in altered energy expenditure pathways, decreased capacity to achieve metabolic homeostasis, and decreased muscle mass/strength. Remarkably, both maternal obesity and undernutrition can elicit a fetal programming, which induces metabolic changes that carry on into adulthood, being responsible for a higher prevalence of obesity and chronic diseases later in life. Some epigenetic mutations acquired during the gestational period can also confer protection against the development of chronic diseases during adult life. For instance, by increasing the expression of adiponectin-coding genes, while simultaneously decreasing the expression of angiotensin II (Ang II), resulting in a lower probability of becoming obese or having metabolic derangements in the future.
Muscular strength and hypertensionHypertension (HTN) is the main contributor to the global burden of disease and the leading risk factor for CVD.4 Recent observational and interventional studies have demonstrated that HGS is inversely associated with blood pressure (BP) and constitutes an early, independent risk factor for HTN across all ages and ethnicities, suggesting that muscle strength could be a possible therapeutic target.30,31
To the present, the largest prospective study evaluating the association between HGS and HTN (n=712,442 adults aged 20–69 years) was based on data from 4 successive Chinese National Surveys on Adults’ Fitness (2000–2014), showing that each interquartile decrease in relative HGS was associated with an increased risk of hypertension in the range of 43–58%, independently of BMI. In addition, this study demonstrated that a relatively small improvement of absolute HGS, which can be easily achieved by exercising, can potentially prevent a significant number of incident hypertension cases; having profound implications for public health care (e.g. Increasing absolute HGS by 2.02kg in a 60–69-year-old woman weighing 50kg reduces the risk of HTN by 43%).32
However, other studies in Caucasian and Chinese cohorts that have used absolute HGS reported a positive association between HGS and BP in both adolescents and adults.33,34 For example, a study (n=1200: 650 middle-aged and 550 older adults), showed that in men and women over 85 years old, absolute HGS was associated with higher systolic BP (SBP), mean arterial pressure (MAP), and pulse pressure (PP) after adjusting for comorbidities and medication use.33 Another study based on data from the National Health and Nutrition Survey (n=4597, age ≥18 years) reported a positive correlation between absolute HGS and DBP, representing a risk factor for hypertension in obese and overweight men (OR=1.31, P<0.05).34 These conflicting results can potentially be explained by the employment of absolute HGS values instead of relative HGS measurements. However, longitudinal studies using relative HGS have also failed to find a significant association between HGS and BP. For instance, a prospective study in Finish adults (n=463, average follow-up of 16 years) reported that per each 1 SD increase in relative HGS (HGS/body weight), the incident hypertension decreased by about 25%. However, this association lost significance after adjustment for multiple risk factors.35 To explain these conflicting results, it has been proposed that chronic inflammation that mediates the development of hypertension and it is also associated with low muscle strength, an over-adjusting model for hs-CPR might have blunted the association between normalized HGS and HTN.36 This proposal is endorsed by the results of another longitudinal study (n=9083, age 20–80 years) that analyzed data from the Korea National Health and Nutrition Examination Survey (KHANES) 2015–2016, reporting that relative HGS was positively correlated with systolic blood pressure. However, prior to adjustment for hs-CRP, relative HGS was negatively correlated to both systolic and diastolic blood as expected.7
The mechanism that links HTN with low muscle strength is not completely understood. Aging and obesity lead to progressive muscle mass loss, declining strength and microinflammation. Chronic inflammation favors an upregulation of the renin-angiotensin system, producing vasoconstriction, increased oxidative stress, endothelial dysfunction, impaired blood vessel relaxation by decreased nitric oxide (NO) activity and increase of BP. Also, lower levels of adiponectin enhance vasoconstriction in response to angiotensin II (Ang II) and blunt the vasodilatory response to acetylcholine, further contributing to the rise of BP. Moreover, low HGS could be associated with HTN due to the impaired autonomic regulation of BP and HR seen in patients with low muscle strength.37,38
Recent evidence demonstrates the existence of an inverse association between arterial stiffness and HGS, which could partially explain the correlation between HGS and HTN and the high incident CVD rates seen in dynapenic patients.
A longitudinal study (n=1508 adults, 39.1% hypertensive) evaluated the association between HGS, different surrogates of arterial stiffness and atherosclerosis (represented by carotid intima-media thickness [IMT]). To determine peripheric arterial stiffness, participants were assessed for the augmentation index (AIx) and brachial-ankle pulse wave velocity (baPWV). Results indicated that AIx was inversely associated with HGS (r=0.437, P<0.001).37 Other cross-sectional and longitudinal studies have reported a negative association between HGS and both preclinical atherosclerosis and arterial stiffness, in non-hypertensive participants.48 This suggests that low muscle fitness is an early marker of vascular dysfunction, and its involvement in the development of HTN, atherosclerosis, and eventually CVD, from the early stages of the process.
Muscular strength and diabetesMounting evidence indicates that there is an inverse association between HGS and incidence, prevalence, and risk of type 2 diabetes mellitus (T2DM), allowing low muscle strength to serve as a possible early marker for disease, and a therapeutic target from an early age.39,40 The inverse association between low muscular strength and higher levels of glycemia has been demonstrated in both cross-sectional and longitudinal studies, persisting after adjustment by anthropometric, demographic, and social factors, as well as cardiorespiratory fitness.41–46 Moreover, low HGS constitutes a predictor of mortality and CVD amongst patients with T2DM or hyperglycemia.5,6
As previously discussed, relative HGS has been found to be a better predictor of metabolic alterations than raw HGS, including T2DM. In a longitudinal study comprising 66,100 European participants with age>50 years, non-diabetic at baseline data was used to calculate the predictive capacity of an office-based risk score for T2DM that included age, gender, body mass index, smoking, and hypertension, the addition of HGS demonstrate that it is an independent predictor of new-onset diabetes and that relative HGS is a better predictor of T2DM than absolute HGS.44
A large meta-analysis (n=1,713,468 US adults aged 45-64yr) showed that each SD increase in HGS decreased the risk of T2DM by 13% in both men and women, after adjusting for anthropometric markers of adiposity.45 A prospective cohort study including 166,894 participants from the UK Biobank (follow-up for 5.3 years on average, age 37–73 years) showed that, in comparison to the highest quintile of absolute grip strength, men in the lowest quintile had a 50% higher risk of diabetes, while women in the lowest quintile had a 25% higher risk of T2DM. For relative grip strength, risk of diabetes in the lowest quintile was more than double for men (HR: 2.22 [95% CI: 1.84–2.67]) and 96% higher for women (HR: 1.96, 95% CI: 1.52–2.53) in comparison to the highest quintile. The association between HGS and T2DM persisted after adjusting for multiple confounding factors, including adiposity. Remarkably, associations were stronger when grip strength was expressed relative to body weight, which could reflect the importance of muscle quality and confirms that relative HGS is the best parameter to evaluate cardiometabolic health.8
Available evidence suggests that low muscle strength is directly implicated in the progression from normoglycemia to dysglycemia, as HGS is not only associated with T2DM, but also with all the sequential metabolic alterations that precede it, such as IR, beta-cell dysfunction, hyperglycemia, and hyperinsulinemia in premorbid populations since childhood and youth.46–48
A longitudinal study between 1985 and 2019, comprising 263 male and female participants, measured grip strength in childhood (9–15 years), young adulthood (28–36 years), and mid-adulthood (38–49 years). A Bayesian relevant life course exposure model concluded that grip strength at each time point was equally associated with prediabetes or type 2 diabetes. For each 1 SD increase in cumulative grip strength, the odds of having prediabetes or T2DM in mid-adulthood decreased by 34% (OR 0.66, 95% CI 0.40, 0.98). These findings suggest that muscle fitness is a determinant of metabolic health from a very early age, many years before the onset of clinical disease.45 HGS is negatively associated with impaired glucose tolerance, HOMA IR and prediabetes even in normal weight adults.43
Currently, diabetes is recognized as a risk factor for dynapenia and sarcopenia, the latter being considered one of its many complications. The relationship between HGS and hyperglycemia seems to be bidirectional, which means high glucose levels could exacerbate the loss of muscle mass/strength and vice versa.47 The Guangzhou Biobank Cohort study reported that lowering blood glucose across the whole range of hyperglycemic contribute to the preservation of muscle strength, especially in aging women.48 Improving muscle strength can potentially help diabetic patients to achieve optimal glycemic control, which in turn contributes to the preservation of skeletal muscle tissue.
The mechanisms that link T2DM and low muscular strength are not completely understood. Adipocytes infiltrating muscle tissue produce free radicals that exert a direct toxic effect over myocytes, inhibiting the synthesis of structural muscle proteins such as myosin heavy chain, eventually leading to apoptosis and muscle atrophy. Both mitochondrial dysfunction and IR enhance apoptosis, which leads to a decreased expression of glucose cotransporters (GLUT4) at the myocyte's plasma membrane, as its proportional to fiber volume, resulting in a decreased muscular uptake of glucose and consequent hyperglycemia.39,44 High intracellular glucose concentrations alter muscle function due to molecular changes in structural proteins, negatively affecting strength and power.39
Furthermore, diabetic hand syndrome characterized by limited joint mobility or diabetic cheiroarthropaty and Dupuytren's disease, which are common among patients with T2DM, might also contribute to low HG measured with a dynamometer.40 Peripheral neuropathy affecting the upper or lower limbs can also affect different muscle strength measurements as handgrip and knee extensor strength).
Muscular strength, dyslipidemia and metabolic syndromeAccording to the National Health and Nutrition Examination Survey (NHANES 2003–2006), an estimated 53% of U.S. adults have lipid abnormalities49 and in Colombia 75% of adults older that 35 years have elevated levels of non-HDL-cholesterol.50 This high prevalence of dyslipidemia has been also reported in Latin America medical doctors.51
Emergence evidence from observational studies and clinical trials demonstrates that relative hand grip strength is inversely associated with the prevalence of dyslipidemia in both children and adults, after adjustment for multiple confounding factors, including alcohol consumption, smoking status, exercise, income, baseline comorbidities, and education level.52,53 However, other studies that didn’t find an association between grip strength and dyslipidemia used the raw values of HGS, rather than adjusting them by BMI or weight.54 As previously discussed, BMI might act as a confounding factor regarding the association between HGS and cardiovascular health.13,14
Dyslipidemia is a key component of the metabolic syndrome (MetS) entity that have a prevalence that reach until 40% in developing countries.55 There is an inverse, significant association between Mets and normalized HGS.56 A longitudinal study that included 3350 adults reported an inverse association between relative HGS and MetS in both sexes. In comparison to the highest quartile of strength (Q4), the respective HR for dyslipidemia in quartiles Q1–3 was: 1.76 (1.12, 2.78), 1.67 (1.08, 2.59), and 1.49 (0.95, 2.34) in men, and 1.30 (1.02, 1.57), 1.28 (1.03, 1.55) and 1.14 (0.82, 1.58) in women.57 Another large longitudinal study based on data from the NHANES (combining surveys from 2011–2012 and 2013–2014) and the China Health and Retirement Longitudinal Study (2011), which included 10,574 middle-aged and older adults, reported that relative HGS (adjusted by body mass) is inversely associated with the prevalence of MetS, its individual components (diabetes, hyperglycemia, hypertriglyceridemia, low HDL cholesterol, hypertension) and physical disabilities in both U.S. and Chinese adults.58 The odds ratios (ORs) for MetS across tertiles of relative handgrip strength were 0.45 (0.33, 0.62) for tertile 2 and 0.13 (0.08, 0.20) for tertile 3 in comparison to the lowest tertile, after adjusting for demographic factors, calorie intake, and physical activity. This study proposed cutoff values of relative handgrip strength for the development of MetS, which were 0.52 and 0.40 for male and female participants, respectively.58 These relative HGS cut-off points are significantly different from those proposed in Brazilian adults aged 25–50 years59 and in Colombian college students aged 18–30 years.60 These results suggest that relative HGS cutoff points for metabolic risk vary in each population, and reference values stratified by sex and age should be calculated specifically for each country.
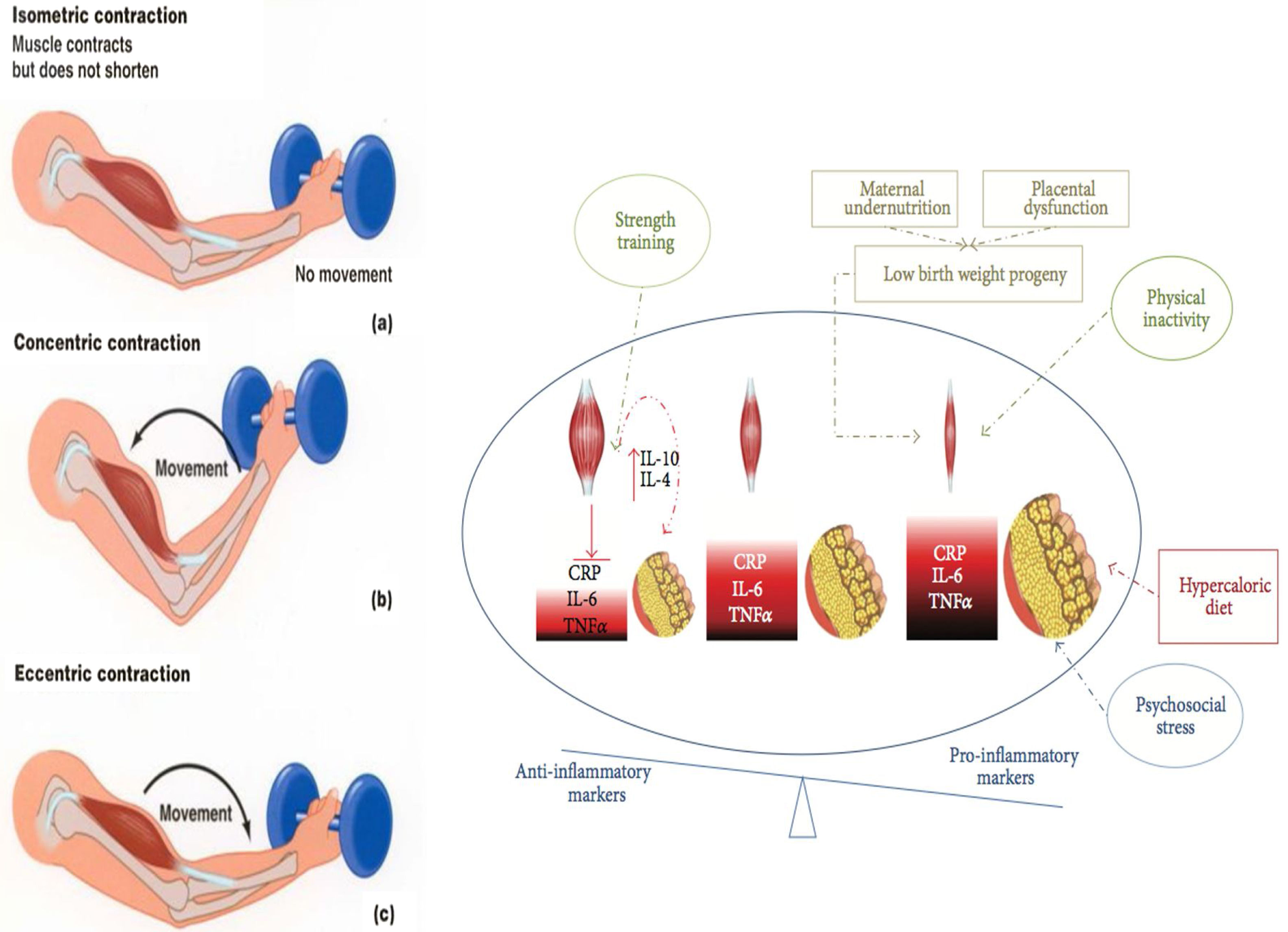
Interventions on muscular strength and impact in risk factorsResistance training (RT) is a type of exercise that can potentially result in muscle hypertrophy and increased strength, as it requires exertion against an external resistance (excluding the individual's own weight). The beneficial effects of resistance training have been well documented in adults, including old individuals.61 All types of RT can increase muscle power, strength, and mass, whilst dynamic resistance training can reduce overall mortality, cardiovascular-related death, and CVD incidence, independently of aerobic exercise and after adjusting for physical activity and BMI.61 Furthermore, RT improves blood pressure, glycosylated hemoglobin (HBA1C), and dyslipidemia.62–67 Recent studies demonstrate that exercise, particularly combined RT and endurance training (ET) can have significant anti-inflammatory effects that counteract harmful proinflammatory adipokines secreted by adipocyte.28 During exercise, skeletal muscle contractions stimulate the secretion of myokines, which act as autocrine, paracrine, and endocrine molecular messengers that regulate metabolic processes occurring in the muscle, liver, and adipose tissue and are mediating the crosstalk between these organs and the brain. Myokines have positive effects on the metabolism by increasing glucose uptake, glucose tolerance, fat oxidation, and muscle regeneration.28 Moreover, a long-term exercise intervention can have a large anti-inflammatory effect, and that combined training (aerobic plus resistance exercise) is more effective than aerobic training alone in reducing hs-CRP levels, despite similar caloric expenditure.68 Moreover, decreased adiposity accounted for only 71% of hs-CRP reductions, suggesting that exercise has a constitutional anti-inflammatory effect69 (Fig. 2).
Types of muscle contractions and their association with exercise modalities. Isometric resistance exercise is a type of strength training involving isometric contractions (a), during which muscle tension increases while muscle length remains relatively constant and limb movement is not produced. Concentric (b) and eccentric(c) muscle contractions (associated with muscle fiber shortening and lengthening, respectively) occur during dynamic resistance training, aerobic exercise, and combined forms of training. These contractions are isotonic (tension within the muscle remains constant) and result in limb movement. The strength trained increase the muscular mass and reestablish the inflammatory/anti-inflammatory equilibrium.
Furthermore, moderate and high-intensity RT result in significant increases in adiponectin concentrations, which ameliorates insulin sensitivity and affects carbohydrate and lipid metabolism. RT intensity is correlated with adiponectin levels after the intervention has ceased, suggesting that high-intensity protocols might yield a greater long-term benefit over IR and ultimately glucose disposal.69
Currently, the strongest evidence supporting a protective effect of muscle fitness against hypertension comes from several clinical trials assessing the effects of exercise on BP. These studies demonstrated that all types of exercise training result in significant reductions in blood pressure,70–72 although the magnitude of the effect differed substantially according to the specific training mode, being isometric resistance exercise the most effective.73 The benefits of isometric exercise over BP are independent of age, sex, or characteristics of the exercise sessions (e.g., frequency, duration), and BP classification. In fact, isometric training has proven to be useful in prehypertensive and hypertensive patients, yielding a greater benefit in the latter group.64
A recent metanalysis66 revealed that isometric resistance training (IRT) results in clinically meaningful reductions in office systolic (−6.97mmHg), office diastolic (−3.86mmHg), central systolic (−7.48mmHg), central diastolic (−3.75mmHg) and 24-h diastolic blood pressure (−2.39mmHg) (all P<0.05). In addition, isometric resistance training was deemed safe as it wasn’t associated with a significant risk of adverse events. However, high-quality trials are required to confirm these findings.
In addition, RT can unmask hidden cases of hypertension. A study showed that during exercise, patients with masked hypertension exhibit a markedly greater increase in SBP and DBP from the first minute of training, in comparison to normotensive individuals, like the BP response to exercise seen in people with known hypertension.67 However, it is important to note that some trials evaluating isometric resistance training at a very low intensity failed to show a significant decrease in SBP and DBP. For instance, a clinical trial evaluating the effects of IRT for 8 weeks at an intensity of 5% and 30% of the individual's maximum voluntary contraction showed that training at 30% intensity resulted in a 7-mmHg reduction of resting SBP (136±12 to 129±15; P=0.04) and 4mmHg in mean arterial pressure (MAP) (100±8 to 96±11; P=0.04), while there were no statistically significant reductions in BP in the 5% group.68
The mechanism by which resistance training decreases blood pressure is not completely understood. Previous research suggests that RT is associated with a transient BP peak during the training, which in turn improves the weight-bearing capacity of collagen and elastin in the tunica media, besides stimulating protective changes in arterial smooth muscle. These changes could potentially prevent resting BP from rising, even after the detraining period. Available evidence indicates that both dynamic and isometric resistance training might promote vascular adaptations, improving conductance and endothelial function, resulting in lower BP values in both prehypertensive and hypertensive individuals.72
Over the years, concerns about the safety of RT and its association with arterial stiffness have been raised. A recent metanalysis that included 16 clinical trials evaluating the acute and chronic effects of RT in adult men and women, reported that resistance training of at least four weeks duration, 2 times a week, does not alter arterial stiffness, regardless of the type of training, muscle groups involved, intensity, duration, and frequency. Remarkably, training focusing on lower extremities was often associated with a significant reduction of arterial stiffness parameters, even in older men. However, in young men, high-intensity RT focused on the upper body might increase arterial stiffness as a long-term effect.72 These results should be considered when prescribing RT, ensuring that the training is safe and beneficial for cardiovascular health.
Physical activity is an essential component of the prevention and treatment of T2DM, and most clinical guidelines recommend that patients perform regular physical exercise, including endurance training (ET) and all RT subtypes.73,74 RT also has the potential to prevent up to 35% of new T2DM cases and up to 26% of incident CVD cases, experiencing the greatest benefit at a training frequency of 60–120min weekly.75 In diabetic patients, it has been demonstrated that RT improves glycemic control (HbA1c reductions) whilst increasing lean body mass and functional capacity.76,77 Despite initial concerns, high-intensity (75–80% of an individual's total capacity) RT has proven to be a safe alternative for older diabetic patients (60–80 yr), potentially improving HbA1c and lean body mass in the absence of weight loss.78
Moreover, the joined performance of RT and ET has shown to produce greater metabolic benefits over glycemic control and prevents a greater number of T2DM and CVD cases than either of those exercise modalities alone.79
Overall, available trials suggest that higher-volume and higher-intensity protocols may yield greater metabolic benefits compared with lower-volume and lower-intensity exercise programs, depending on the population being considered. There is a strong correlation between increases in lean body mass, post-training muscle cross-sectional area, and improvement of both glycemic control and insulin sensitivity in diabetic patients, with or without changes in adiposity.79 Differences in the magnitude of muscle size and fat-free mass changes after each exercise program could potentially explain discordant results in different trials. However, further research is needed to determine the specific characteristics of RT training needed to achieve the highest possible benefit in different groups of patients.
The mechanisms by which RT improves glycemic control are not fully understood, but it has been proposed that increased skeletal muscle mass leads to a greater expression of GLUT 4 cotransporters, which reduces plasma glucose concentrations. Moreover, some studies have also reported that IR can improve after RT inclusive in the absence of lean body mass changes, possibly due to improvements in muscle quality and upregulation of GLUT4 glucose cotransporters, insulin receptors, and enhanced enzymatic activity of protein kinase B-α/β and glycogen synthase, which leads to increased glycogen storage in the muscle, reducing blood glucose by utilizing it in glycogen synthesis. In addition, exercise might stimulate glucose by upregulating AMP-activated protein kinase (AMPK).39
Multiple interventional studies have established that in healthy individuals, RT leads to a significant decrease in serum levels of TC, TG and LDL, and increased HDL concentrations, even at low or moderate intensity.80–84 Furthermore, RT has to potential to improve the blood lipid profile of patients with baseline metabolic comorbidities, with only 4 months of RT resulted in decreased body fat mass, increased muscle strength and significant improvements in the atherogenic lipid profile in the absence of diet changes. However, the effects of endurance training on metabolic parameters and muscle strength were only modest, suggesting that the metabolic benefits derived from all exercise are linked to the improvement of muscle strength.84 Studies in older diabetic type 2 adults have failed to show an effect of RT on the participant's lipid profile and insulin levels, in spite of better glycemic control and increased lean mass.45 These findings suggest that the effects of RT might differ according to age groups, and older adults possibly require longer interventions to show further metabolic improvement. It's likely that RT can decrease TC, TG and LDL-C by increasing the production of myokines, which increases phosphorylation of AMPK and acetyl-CoA carboxylase-beta, enhancing fat oxidation.24
Although few trials have assessed the effect of resistance exercise on the metabolic syndrome perse, as previously discussed RT can improve each one of its individual components including hyperglycemia, elevated blood pressure, and dyslipidemia. In subjects with diabetes and MetS, RT can reduce the HOMA-IR index, HDL-cholesterol, waist circumference, insulin levels, and HbA1c and there is evidence indicating that RT can prevent MetS in obese individuals at increased risk of metabolic alterations. In obese subjects a 20-week resistance training program, resulting in a significant reduction of all MetS components, including waist circumference, SBP, DBP, fasting glucose, and triglycerides in both obese and morbidly obese individuals. Additionally, both groups showed improved muscle strength.84 As with diabetes, available evidence indicates that the combination of ET and RT yields a greater benefit than either of those types of training alone.82
ConclusionsLow muscular fitness, including low HGS, and obesity are intrinsically ligated, representing a keystone in the development of inflammation, metabolic disorders, and chronic diseases. Although the role of muscle strength in the progression toward CV risk factors, and the mechanisms mediating fetal programming are not completely understood, there seems to be a complex interaction between environmental factors as inadequate diet, lower levels of physical activity and epigenetic regulations, that ultimately determine the health of an individual. Both obesity and low grip strength are important risk factors for mortality and CV disease. Interestingly, in the presence of obesity increased muscular strength can provide protection against metabolic alterations and adverse outcomes, constituting a potential therapeutic target.
Financial disclosureFor the present study, the authors declare than have not received any specific financial aid from public or private institutions, nor from non-profit organizations.
Conflicts of interestThe authors declare no personal or financial conflicts of interest.