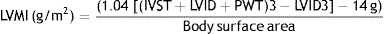Chronic hemodialysis (HD) patients are among the highest population risk for accelerated atherosclerosis. Osteopontin (OPN) is a multifunctional protein that is increased in chronic kidney disease that may play a role in vascular remodelling and intimal proliferation.
AimTo assess the relation between OPN levels and severity of carotid atherosclerosis among prevalent HD patients.
MethodsEighty chronic HD patients underwent serum OPN levels assessment and were further classified into 3 subgroups according to the OPN tertiles’ levels; sub-group 1 (lower tertile) subgroup 2 (middle tertile) and sub-group 3 (upper tertile), together with the carotid duplex and Transthoracic Doppler Echocardiography examination.
ResultsThe mean carotid intima-media thickness (CIMT) was 0.89±0.14mm. Out of the studied group, 50 (62.5%) patients had atheromatous plaques and 15 patients (18.8%). had significant stenosis. The 3rd group with the upper OPN tertile (78–270ng/dl) had the highest incidence of atherosclerosis. A significant correlation between the OPN levels and the CIMT (r=0.533, p=0.001). OPN values detect atherosclerosis with diagnostic sensitivity of 70%, specificity of 69%, positive predictive value (PPV) 73%, negative predictive value (NPV) 65% with area under the curve (AUC) 0.804 (95% CI: 0.711–0.897). Serum OPN detect carotid stenosis with sensitivity of 66%, specificity of 81%, PPV 45%, NPV 91% with AUC=0.769 and detect the presence of carotid atheroma with sensitivity 70%, specificity 66.67%, PPV 77.8%, NPV 57.1% and AUC=0.767 (p-value<0.001). Moreover, serum levels of OPN were significantly positively correlated with grade of diastolic dysfunction (r=0.312, p=0.005), E/A ratio (r=0.293, p=0.008) and inversely correlated with left ventricular ejection fraction (r=−0.304, p=0.006).
ConclusionsSerum Osteopontin is of clinical value as a predictor biomarker of the severity of carotid atherosclerosis, presence of atheroma and carotid stenosis with high diagnostic sensitivity and specificity in chronic hemodialysis patients. Increased Osteopontin level is associated with left ventricular diastolic and systolic dysfunction in those patients.
Los pacientes de hemodiálisis crónica (HD) son una de las poblaciones de mayor riesgo de aterosclerosis acelerada. La osteopontina (OPN) es una proteína multifuncional que se incrementa en la enfermedad renal crónica, y que puede jugar un papel en la remodelación vascular y la proliferación de la íntima.
ObjetivoEvaluar la relación entre los niveles de OPN y la gravedad de la aterosclerosis carotídea en los pacientes prevalentes en HD.
MétodosSe evaluó el nivel sérico de OPN en 80 pacientes de HD crónica. Seguidamente, se clasificó a dichos pacientes en tres subgrupos con arreglo a los niveles de los terciles de OPN: subgrupo 1 (tercil inferior) subgrupo 2 (tercil medio) y subgrupo 3 (tercil superior), junto con dúplex carotídeo y ecocardiografía Doppler transtorácica.
ResultadosEl espesor medio de la íntima-media carotídea (CIMT) fue de 0,89 ± 0,14 mm. En el grupo estudiado, 50 (62,5%) pacientes tenían placas ateromatosas y 15 pacientes (18,8%) tenían estenosis significativa. El tercer grupo, con tercil OPN (78-270 ng/dL) reflejó la mayor incidencia de aterosclerosis. Existió una correlación significativa entre los niveles de OPN y CIMT (r = 0,533, p = 0,001). Los valores de OPN detectaron aterosclerosis con una sensibilidad diagnóstica del 70%, especificidad del 69%, valor predictivo positivo (VPP) del 73%, valor predictivo negativo (VPN) del 65% y área bajo la curva (AUC) de 0,804 (intervalo de confianza [IC] 95%: 0,711-0,897). La OPN detectó estenosis carotídea con una sensibilidad del 66%, especificidad del 81%, VPP del 45%, VPN del 91%, y AUC = 0,769 y detectó la presencia de ateroma carotídeo con una sensibilidad del 70%, especificidad del 66,67%, VPP del 77,8%, VPN del 57,1% y AUC = 0,767 (valor p < 0,001). Además, los niveles séricos de OPN se correlacionaron positiva y significativamente con el grado de disfunción diastólica (r = 0,312, p = 0,005), ratio E/A (r = 0,293, p = 0,008) y guardaron una correlación inversa con la fracción de eyección ventricular izquierda (r = -0,304, p = 0,006).
ConclusionesLa osteopontina sérica tiene valor clínico como biomarcador predictivo de la severidad de la aterosclerosis carotídea, presencia de ateroma y estenosis carotídea con alta sensibilidad y especificidad diagnóstica en los pacientes de hemodiálisis crónica. Además, la OPN puede jugar un papel en el desarrollo de la disfunción diastólica ventricular izquierda y la disfunción sistólica en estos pacientes.
The prevalence of cardiovascular diseases (CVD) is about 70% among hemodialysis patients as reported by USRDS Annual Data Reports, CVD is a major cause of mortality among ESRD patients.1 The NEFRONA study confirmed an increased prevalence of atherosclerosis in chronic kidney disease (CKD) patients.2 Several factors in end-stage renal disease (ESRD) patients may contribute to atherosclerosis and arterial stiffness risk, for instance, inflammations, over-activity of the rennin-angiotensin system, volume overload, and CKD–mineral and bone disorder such as increased calcium-phosphate product and vascular calcification.3
Osteopontin (OPN) is a secreted multifunctional glycol-phospho-protein found widely in bone, kidney, and other tissues.4 Elevated serum levels are highly expressed in several pathologies with a chronic inflammatory component including CKD.5 OPN has been classified as a T-helper 1 cytokine and so believed to exacerbate inflammation including atherosclerosis.6 OPN is a highly negatively charged, extracellular matrix protein, It is composed of about 314 amino acids in humans and is expressed as a 33-kDa and molecular weight may be increased to about 44kDa as OPN can go through posttranslational modifications,7 so OPN is non-dialyzable protein. Moreover, serum OPN levels were noted significantly higher in hemodialysis (HD) groups than CKD patients pre-HD, and healthy volunteer groups which may be attributed to uraemia and the dialysis procedure itself.8
Despite the careful monitoring of CKD patients, there is an increased risk of cardiovascular diseases.9 Traditional risk factors for atherosclerosis include age, smoking, hypertension, diabetes, and dyslipidemia.10 Many new risk factors for atherosclerosis have emerged over the past years including OPN.
OPN serum levels in patients with cardiovascular disease are assumed to reflect the extent of atherosclerosis and may play a role in plaque formation and vascular disease progression. The objective of this study was to assess OPN levels and their correlation to the progression of atherosclerosis in patients with ESRD.
MethodsStudy populationThis is a cross-sectional study that included clinically stable ESRD adult patients undergoing regular HD>6 months, 3 sessions per week, 4h per session by bicarbonate dialysate, low flux dialyzer and heparin was used as an anticoagulant.
Patients suffering from an active bacterial or viral infection, severe hepatic failure, autoimmune diseases and connective tissue diseases, malignancy, decompensated heart failure, history of surgery and trauma within 1 month, were excluded from the study.
The study group was further divided into three sub-groups according to the OPN tertiles’ levels; sub-group 1 (lower tertile) included 29 patients with OPN levels ranging from 12–38ng/dl, subgroup 2 (middle tertile) included 26 patients with OPN levels ranging from 40 to 75ng/dl and sub-group 3 (upper tertile) included 25 patients with OPN levels ranging from 78 to 270ng/dl.
Laboratory measurementsA total of 6ml venous blood was withdrawn from each subject before the dialysis session, in addition to another 2ml blood samples collected after the dialysis session to assay post-dialysis urea level. 2ml were collected in EDTA K3 vacutainers to perform CBC. The remaining 4ml were collected in sterile vacutainers with a Z Serum Sep Clot Activator (Greiner Bio-One). After clotting, samples were centrifuged at 1500xg for 15minutes; the serum was used for the assay of pre-dialysis renal functions, electrolytes, lipid profile, ALP, iPTH and viral hepatitis markers immediately. The rest of the serum was stored at −20°C for measurements of human osteopontin.
Serum creatinine, blood urea nitrogen, sodium, potassium, calcium, phosphorus, serum alkaline phosphatase and lipid profile: cholesterol, triglyceride, HDL-C was assayed on Beckman coulter AU 480 system(Beckman coulter, Inc. 250s.Kraemer Blvd. Brea, CA92821, USA.)
The LDL-C value was calculated according to the Friedwald equation. CBC was assayed on the Beckman coulter cell counter (Beckman coulter, Inc. 250s.Kraemer Blvd. Brea, CA92821, USA.)
Intact Parathyroid hormone (iPTH) and C-reactive protein (CRP) titre were assayed by electro-chemiluminescence on a Cobas e411 immunoassay autoanalyzer (Roche Diagnostic Gmbh), using the Parathyroid hormone and C-reactive protein immunoassay kits also provided by Roche.
Serum OPN assay was done by double-antibody sandwich enzyme-linked immune sorbent assay (ELISA) kit supplied by Sunredbio (No.6497, hutai Road, Baoshan District, Shanghai, China).
HD adequacy was determined by the urea reduction rate and the KT/V ratio (Dialyzer urea clearance (K), time (t) & (V) patient's urea distribution volume).
B-mode carotid duplexAll patients were examined in the supine position with the neck extended and rotated to the opposite side of the examination using an ultrasound system (GE vivid S5) equipped with a 5–10MHz linear probe. B-mode scanning protocol involved scanning the right and left common carotid arteries; 3cm before the carotid bifurcation, at the carotid bifurcation, as well as of the internal carotid artery 2cm distally from the carotid bifurcation. The average of the right and left CIMT were used to calculate the mean CIMT, considering IMT>0.9mm as a normal value.11 The presence of plaque was defined as a focal structure that invaded the arterial lumen of at least 0.5mm or >50% of the surrounding IMT or demonstrated a thickening>1.5mm measured from the media-adventitia interface to the intima-lumen interface.12–14
Transthoracic Doppler echocardiographyTransthoracic Doppler Echocardiography was performed on all of the participants in this study using a-vivid s5, Tokyo, Japan, Left ventricular internal dimension, septal and posterior wall thickness were measured at end-diastole and end-systole according to the American Society of echocardiography recommendations15 and Left ventricular mass was calculated using the Devereux equation.16 The presence of left ventricular hypertrophy was established when the left ventricular mass index (LVMI) was 111g/m2 for men and 106g/m2 in women.
(LVID) left ventricular internal diameter, (IVST) interventricular septal thickness, and (PWT) posterior wall thickness.The E/A ratio is calculated as a marker of the left ventricular function, It represents the ratio of peak velocity flow in early diastole (the E wave) to peak velocity flow in late diastole caused by atrial contraction (the A wave). Diastolic function grading from normal to IV grade left ventricular diastolic dysfunction (LVDD).17 LV ejection fraction (LVEF) was calculated according to Lang et al. (2015).18
Statistical analysis was performed using SPSS (version 20.0, IBM Corp.). Qualitative data were expressed as percentages. Quantitative data were expressed as a mean±SD. Skewed data were expressed as a median and inter-quartile range. Chi-square test was applied for categorical variables, to compare between different groups. F-test (ANOVA) was applied for normally distributed quantitative variables, to compare between more than two groups and Post Hoc test (LSD) for pairwise comparisons. The Kruskal–Wallis test (H test) was applied for statistical comparison between three or more sets of data if one or more of them had a skewed distribution. The Mann–Whitney U-test (Wilcoxon rank-sum test) was used to compare two independent sets of data if one or both of them had a skewed distribution. Spearman's rank correlation coefficient (rs) was used to assess the degree of correlation between two sets of variables if one or both of them showed a skewed distribution. Forward multiple linear regression analysis was performed with CIMT levels as the dependent variable and those determinants that correlated in the univariate analysis with p<0.05 as independent variables. p<0.05 was considered significant and p<0.01 was considered highly significant. Receiver operating characteristic curve (ROC) analysis was applied to assess the diagnostic performance of osteopontin.
Ethical considerations: All procedures performed in the study were following the ethical standards of the Ain-Shams University hospital research committee (Ethics committee reference number 000017585) and with the ethical standards laid down in the 1964 Declaration of Helsinki. This study was approved by our institutional review board and informed consent was obtained from all individuals enrolled in the study.
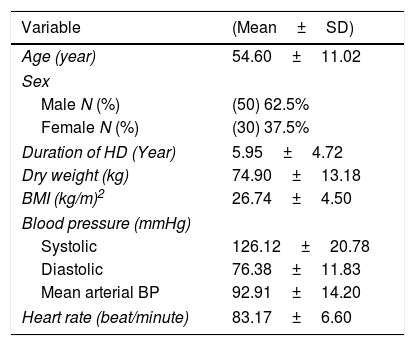
ResultsOut of 115 patients referred to the hemodialysis unit in the study period, 80 patients were enrolled in the study, 35 patients were excluded due to the presence of heart failure, malignancy and autoimmune diseases. The study included 80 clinically stable ESRD adult patients undergoing regular hemodialysis>6 months. The mean age of the study population was 54.60±11.02 years. The duration of hemodialysis was 5.95±4.72 years, the demographic characteristics of the studied group are presented in Table 1.
Demographic characteristics of the study group (total N=80).
| Variable | (Mean±SD) |
|---|---|
| Age (year) | 54.60±11.02 |
| Sex | |
| Male N (%) | (50) 62.5% |
| Female N (%) | (30) 37.5% |
| Duration of HD (Year) | 5.95±4.72 |
| Dry weight (kg) | 74.90±13.18 |
| BMI (kg/m)2 | 26.74±4.50 |
| Blood pressure (mmHg) | |
| Systolic | 126.12±20.78 |
| Diastolic | 76.38±11.83 |
| Mean arterial BP | 92.91±14.20 |
| Heart rate (beat/minute) | 83.17±6.60 |
| NO. | % | |
|---|---|---|
| Comorbidities | ||
| DM | 13.5 | 13 |
| IHD | 32.5 | 26 |
| HCV Ab | 58.8 | 47 |
| Hypertension | 41.3 | 13 |
| Cause of ESRD | ||
| HTN | 37.5 | 30 |
| ADPKD | 10 | 8 |
| DM | 7.5 | 6 |
| Obstructive uropathy | 17.5 | 14 |
| Unknown aetiology | 12.5 | 10 |
| Congenital atrophy | 2.5 | 2 |
| Glomerulonephritis | 12.5 | 10 |
BMI: body mass index, BP: blood pressure, HTN: hypertension, DM: Diabetes mellitus, ADPKD: Autosomal dominant polycystic kidney disease, HCV Ab: Hepatitis C virus antibodies, IHD: ischaemic heart disease.
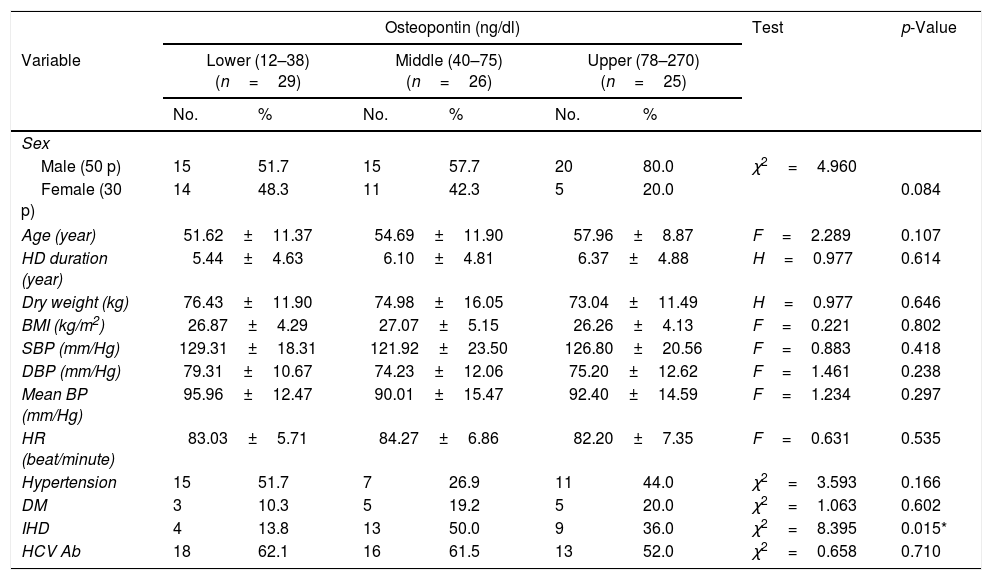
The 80 patients were further classified into three sub-groups according to the OPN tertile levels, the demographic characteristics of the 3 subgroups are presented in Table 2. There was no significant difference between the subgroups as regard age, sex, body mass index, duration of hemodialysis, hypertension and diabetes. A statistically significant difference was found between the 3 subgroups as regards the history of ischaemic heart disease (13.8% vs 50% vs 36.0%, p=0.015).
Comparison between the 3 OPN tertiles according to the demographic characteristics of the patients.
| Osteopontin (ng/dl) | Test | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Lower (12–38) (n=29) | Middle (40–75) (n=26) | Upper (78–270) (n=25) | |||||
| No. | % | No. | % | No. | % | |||
| Sex | ||||||||
| Male (50 p) | 15 | 51.7 | 15 | 57.7 | 20 | 80.0 | χ2=4.960 | |
| Female (30 p) | 14 | 48.3 | 11 | 42.3 | 5 | 20.0 | 0.084 | |
| Age (year) | 51.62±11.37 | 54.69±11.90 | 57.96±8.87 | F=2.289 | 0.107 | |||
| HD duration (year) | 5.44±4.63 | 6.10±4.81 | 6.37±4.88 | H=0.977 | 0.614 | |||
| Dry weight (kg) | 76.43±11.90 | 74.98±16.05 | 73.04±11.49 | H=0.977 | 0.646 | |||
| BMI (kg/m2) | 26.87±4.29 | 27.07±5.15 | 26.26±4.13 | F=0.221 | 0.802 | |||
| SBP (mm/Hg) | 129.31±18.31 | 121.92±23.50 | 126.80±20.56 | F=0.883 | 0.418 | |||
| DBP (mm/Hg) | 79.31±10.67 | 74.23±12.06 | 75.20±12.62 | F=1.461 | 0.238 | |||
| Mean BP (mm/Hg) | 95.96±12.47 | 90.01±15.47 | 92.40±14.59 | F=1.234 | 0.297 | |||
| HR (beat/minute) | 83.03±5.71 | 84.27±6.86 | 82.20±7.35 | F=0.631 | 0.535 | |||
| Hypertension | 15 | 51.7 | 7 | 26.9 | 11 | 44.0 | χ2=3.593 | 0.166 |
| DM | 3 | 10.3 | 5 | 19.2 | 5 | 20.0 | χ2=1.063 | 0.602 |
| IHD | 4 | 13.8 | 13 | 50.0 | 9 | 36.0 | χ2=8.395 | 0.015* |
| HCV Ab | 18 | 62.1 | 16 | 61.5 | 13 | 52.0 | χ2=0.658 | 0.710 |
χ2, p: χ2 and p values for Chi square test. MCp: p value for Monte Carlo for Chi square test, F, p: F and p values for ANOVA test. H, p: H and p values for Kruskal–Wallis test.
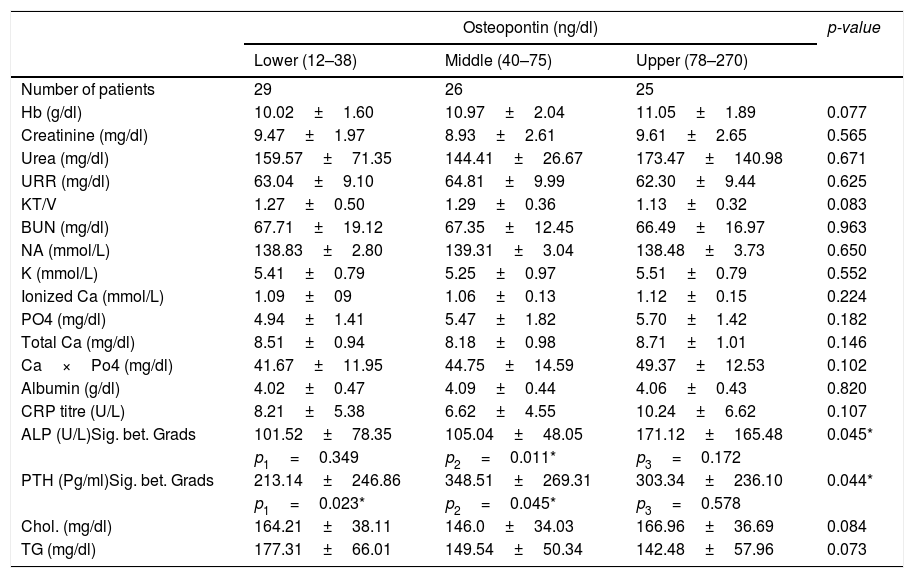
Concerning the routine laboratory tests, there were no significant differences between the 3 subgroups regarding haemoglobin, serum creatinine, urea, sodium, potassium, total and ionized calcium, phosphorus, albumin, cholesterol, triglycerides and CRP (Table 3). However, there was a significant difference in serum alkaline phosphatase (p=0.045) and serum parathyroid hormone (p=0.044) between the 3 tertiles. Correlation analyses were performed between OPN and the studied laboratory tests, and it revealed a positive significant correlation between OPN and PTH (r=0.295, p=0.008), OPN and alkaline phosphatase (r=0.298, p=0.007), as well as, OPN and Ca×Po4 (r=0.234, p=0.037).
Comparison between the 3 OPN tertiles as regard laboratory results.
| Osteopontin (ng/dl) | p-value | |||
|---|---|---|---|---|
| Lower (12–38) | Middle (40–75) | Upper (78–270) | ||
| Number of patients | 29 | 26 | 25 | |
| Hb (g/dl) | 10.02±1.60 | 10.97±2.04 | 11.05±1.89 | 0.077 |
| Creatinine (mg/dl) | 9.47±1.97 | 8.93±2.61 | 9.61±2.65 | 0.565 |
| Urea (mg/dl) | 159.57±71.35 | 144.41±26.67 | 173.47±140.98 | 0.671 |
| URR (mg/dl) | 63.04±9.10 | 64.81±9.99 | 62.30±9.44 | 0.625 |
| KT/V | 1.27±0.50 | 1.29±0.36 | 1.13±0.32 | 0.083 |
| BUN (mg/dl) | 67.71±19.12 | 67.35±12.45 | 66.49±16.97 | 0.963 |
| NA (mmol/L) | 138.83±2.80 | 139.31±3.04 | 138.48±3.73 | 0.650 |
| K (mmol/L) | 5.41±0.79 | 5.25±0.97 | 5.51±0.79 | 0.552 |
| Ionized Ca (mmol/L) | 1.09±09 | 1.06±0.13 | 1.12±0.15 | 0.224 |
| PO4 (mg/dl) | 4.94±1.41 | 5.47±1.82 | 5.70±1.42 | 0.182 |
| Total Ca (mg/dl) | 8.51±0.94 | 8.18±0.98 | 8.71±1.01 | 0.146 |
| Ca×Po4 (mg/dl) | 41.67±11.95 | 44.75±14.59 | 49.37±12.53 | 0.102 |
| Albumin (g/dl) | 4.02±0.47 | 4.09±0.44 | 4.06±0.43 | 0.820 |
| CRP titre (U/L) | 8.21±5.38 | 6.62±4.55 | 10.24±6.62 | 0.107 |
| ALP (U/L)Sig. bet. Grads | 101.52±78.35 | 105.04±48.05 | 171.12±165.48 | 0.045* |
| p1=0.349 | p2=0.011* | p3=0.172 | ||
| PTH (Pg/ml)Sig. bet. Grads | 213.14±246.86 | 348.51±269.31 | 303.34±236.10 | 0.044* |
| p1=0.023* | p2=0.045* | p3=0.578 | ||
| Chol. (mg/dl) | 164.21±38.11 | 146.0±34.03 | 166.96±36.69 | 0.084 |
| TG (mg/dl) | 177.31±66.01 | 149.54±50.34 | 142.48±57.96 | 0.073 |
F and p values for ANOVA test, Sig. bet. grps was done using Post Hoc Test (LSD), p1: p value for comparing between low and moderate, p2: p value for comparing between low and high, p3: p value for comparing between moderate and high.
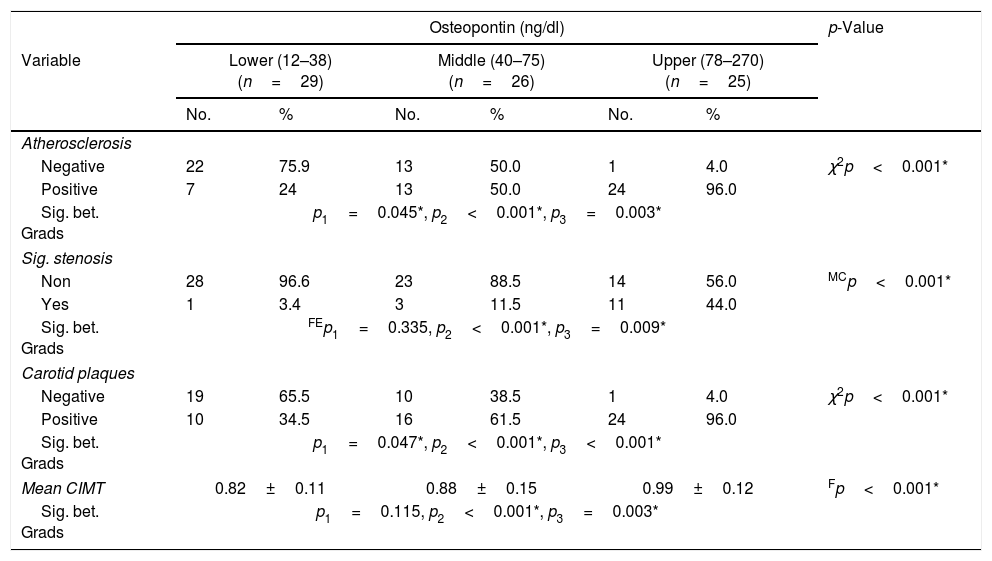
All patients in the study group underwent carotid duplex examination, the mean CIMT was 0.89±0.14mm. Among the studied group, 44 patients (55%) had atherosclerosis, 50 patients (62.5%) had atheromatous plaque and 15 patients (18.8%). showed significant stenosis. Table 4 illustrates the carotid duplex parameters measured in the 3 subgroups.
Comparison between the 3 tertiles of OPN according to carotid duplex parameters.
| Osteopontin (ng/dl) | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Lower (12–38) (n=29) | Middle (40–75) (n=26) | Upper (78–270) (n=25) | ||||
| No. | % | No. | % | No. | % | ||
| Atherosclerosis | |||||||
| Negative | 22 | 75.9 | 13 | 50.0 | 1 | 4.0 | χ2p<0.001* |
| Positive | 7 | 24 | 13 | 50.0 | 24 | 96.0 | |
| Sig. bet. Grads | p1=0.045*, p2<0.001*, p3=0.003* | ||||||
| Sig. stenosis | |||||||
| Non | 28 | 96.6 | 23 | 88.5 | 14 | 56.0 | MCp<0.001* |
| Yes | 1 | 3.4 | 3 | 11.5 | 11 | 44.0 | |
| Sig. bet. Grads | FEp1=0.335, p2<0.001*, p3=0.009* | ||||||
| Carotid plaques | |||||||
| Negative | 19 | 65.5 | 10 | 38.5 | 1 | 4.0 | χ2p<0.001* |
| Positive | 10 | 34.5 | 16 | 61.5 | 24 | 96.0 | |
| Sig. bet. Grads | p1=0.047*, p2<0.001*, p3<0.001* | ||||||
| Mean CIMT | 0.82±0.11 | 0.88±0.15 | 0.99±0.12 | Fp<0.001* | |||
| Sig. bet. Grads | p1=0.115, p2<0.001*, p3=0.003* | ||||||
χ2, p: χ2 and p values for Chi square test for comparing between the three studied grades and each two grades. FEp: p value for Fisher Exact for Chi square test for comparing between the each two grade. MCp: p value for Monte Carlo for Chi square test for comparing between the three studied grades. p1: p value for comparing between low and moderate, p2: p value for comparing between low and high, p3: p value for comparing between moderate and high.
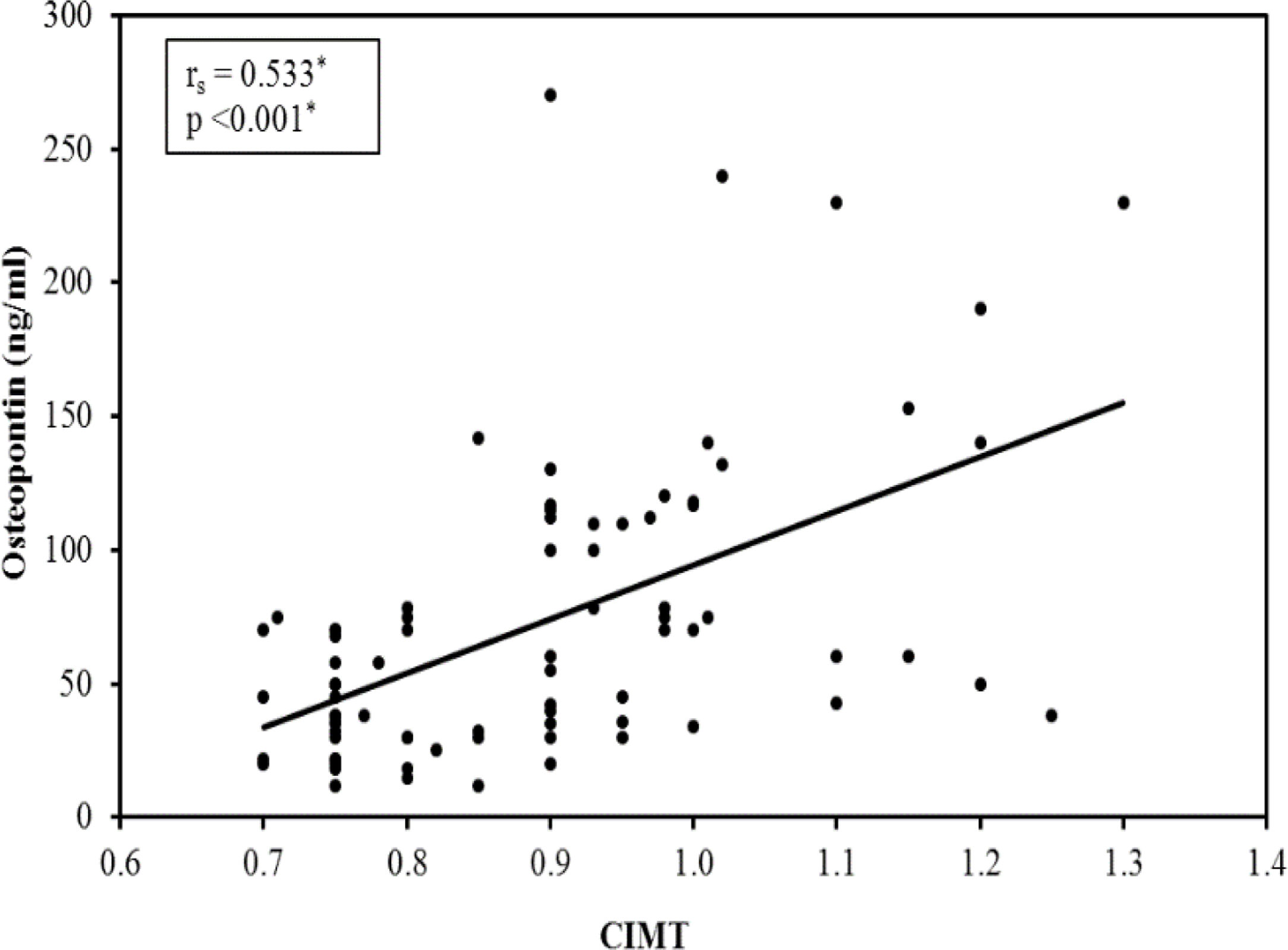
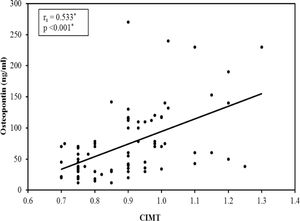
A significant difference was found between the subgroups as regard carotid atherosclerosis, carotid significant stenosis, presence of carotid plaques and mean CIMT (Table 4). The 3rd subgroup with the upper tertile showed a higher incidence of atherosclerosis with 24 patients (96%) having carotid plaques and 11 patients (44%) having significant carotid artery stenosis. Comparing the CIMT among the 3 subgroups revealed that CIMT increased significantly in patients with higher OPN levels. The CIMT in the 1st, 2nd and 3rd subgroups was 0.82±0.11 vs 0.88±0.15 vs 0.99±0.12mm, respectively, p=0.001. Moreover, there was a highly significant positive correlation between the mean OPN serum level and mean CIMT (r=0.533, p=0.001) (Fig. 1).
Multivariate regression analysis of the predictors of carotid intima-media thickness after age adjustment showed that CIMT value was independently positively associated with OPN levels (beta=0.553, p<0.001), as well as serum phosphate (beta=0, 304, p=0.002).
We detected a statistically significant difference between the 3 OPN tertiles regarding the left ventricular hypertrophy (=0.018), left ventricular ejection fraction (p=0.011), and left ventricular mass index (p=0.004). Subsequently, post hoc test revealed that patients in the lower tertile have higher ejection fraction than both upper and middle OPN tertile (p>0.05). Moreover, serum levels of OPN were significantly positively correlated with grade of diastolic dysfunction (r=0.312, p=0.005), E/A ratio (r=0.293, p=0.008) and inversely correlated with left ventricular ejection fraction (r=−0.304, p=0.006).
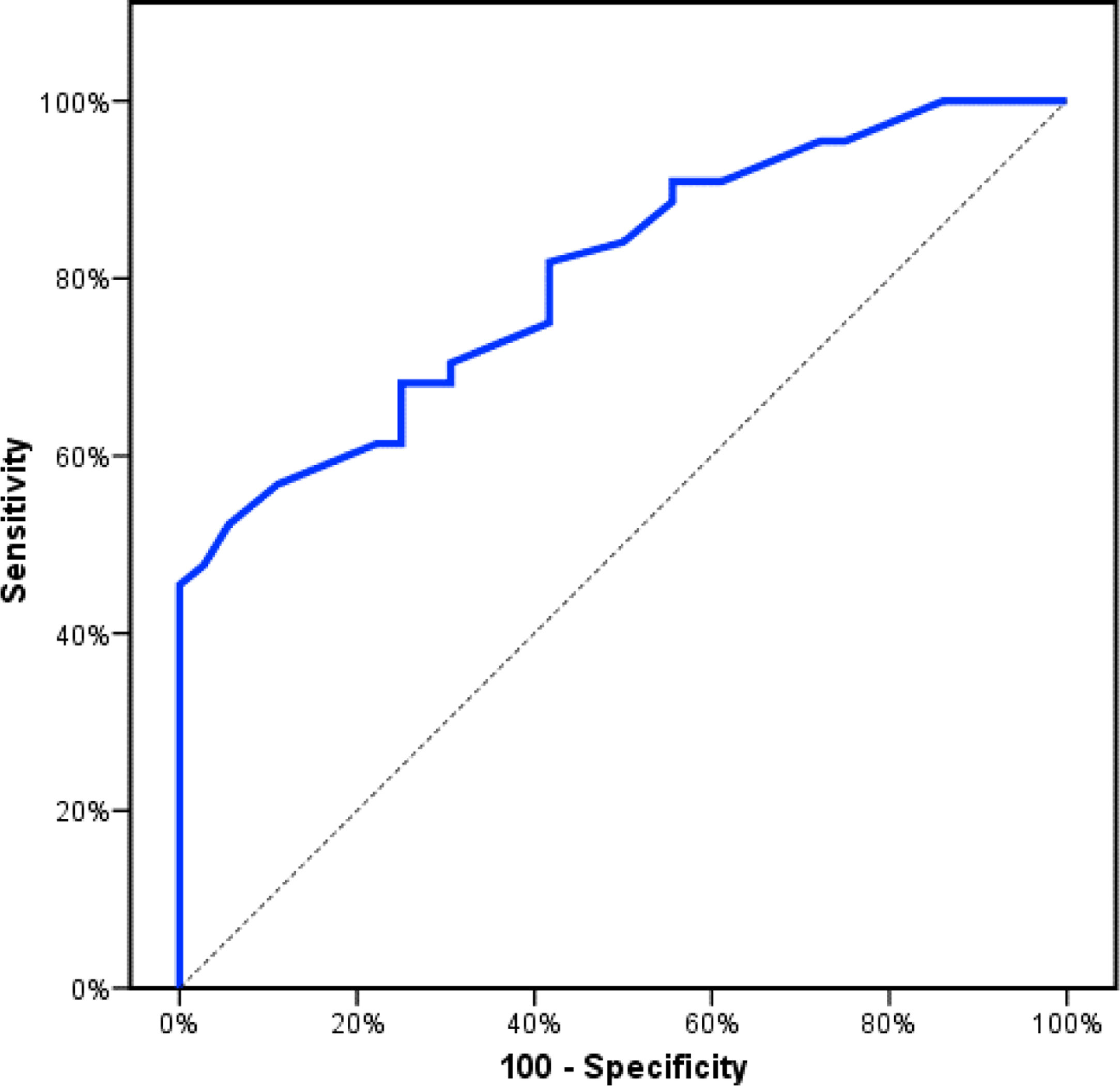
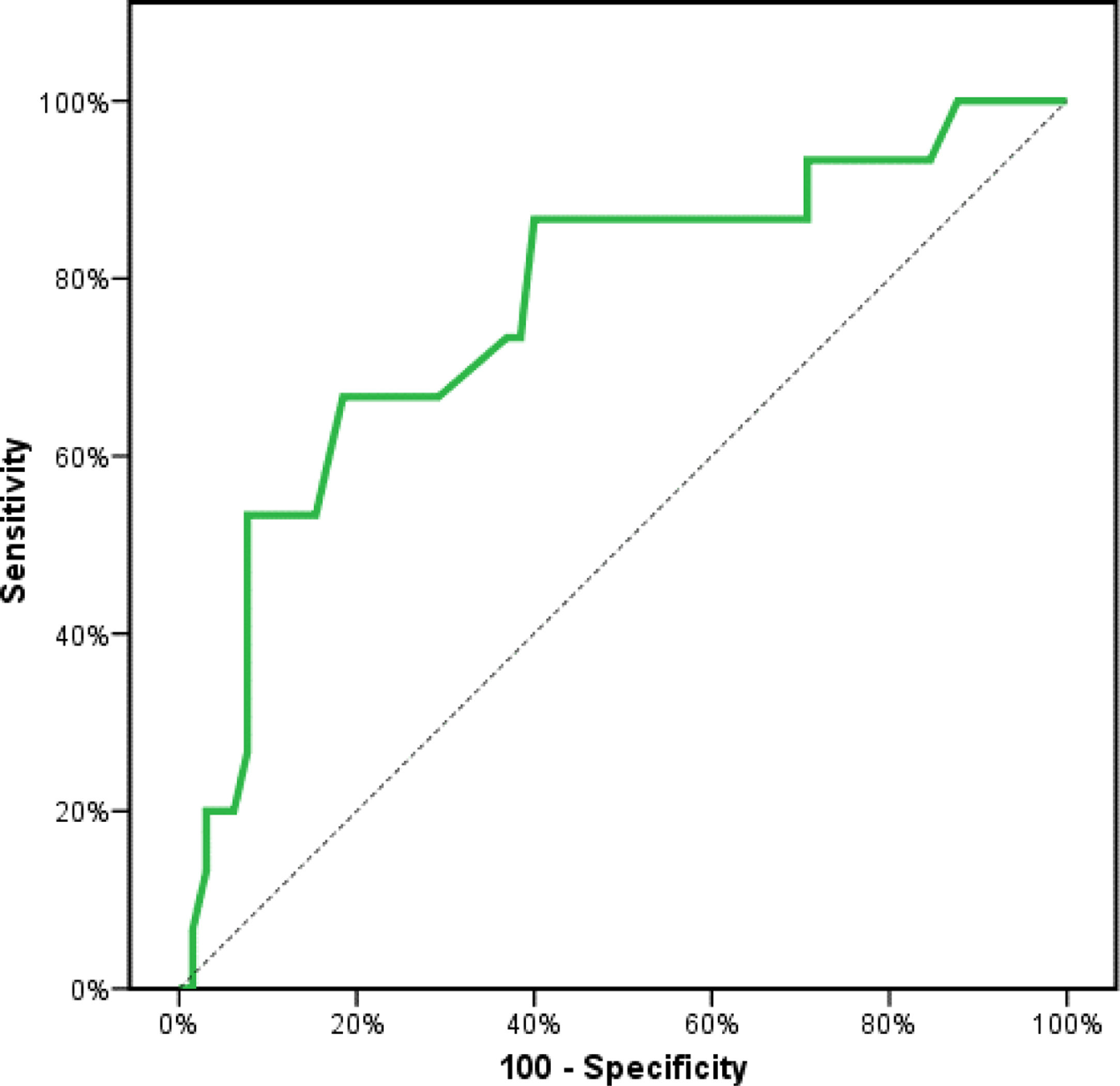
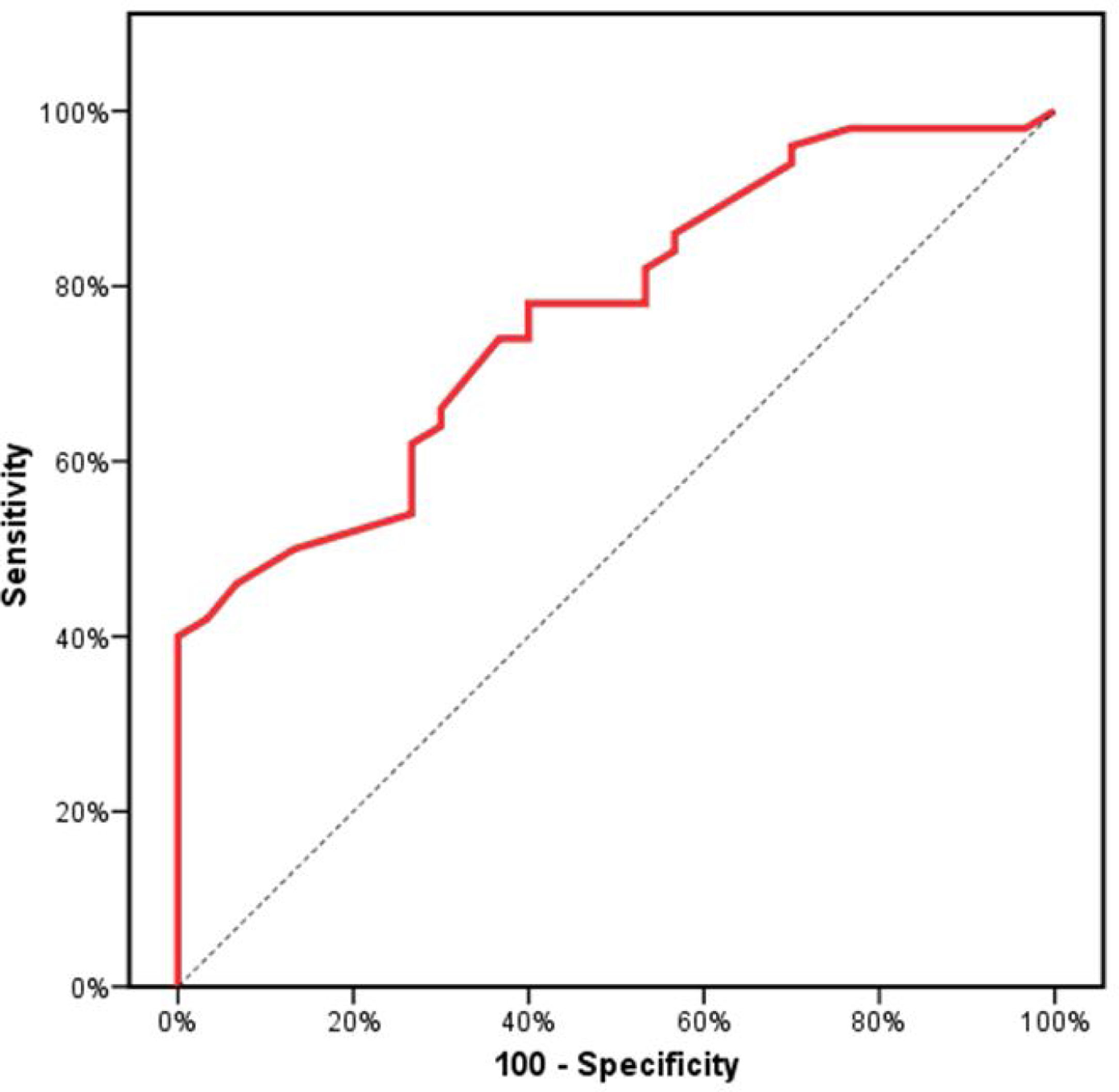
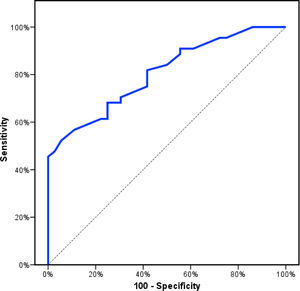
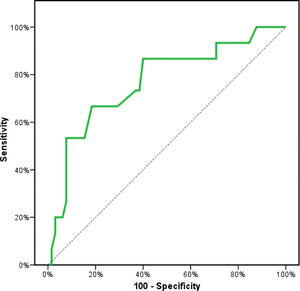
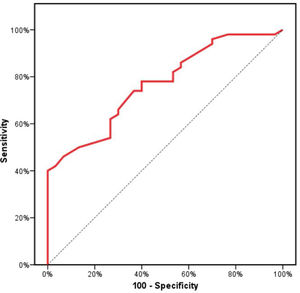
Receiver operating characteristics (ROC) curve analysis was constructed to examine the diagnostic performance of serum OPN for detection of atherosclerosis among the studied patients, and it showed that the best cut off value was 50ng/dl. This had a diagnostic sensitivity of 70%, specificity of 69%, positive predictive value (PPV) 73%, negative predictive value (NPV) 65% with area under the curve (AUC) 0.804 (95% CI: 0.711–0.897) (Fig. 2). In addition, at a cut off value of 78ng/dl, the OPN can detect stenosis with a diagnostic sensitivity of 66%, specificity of 81%, PPV 45%, NPV 91% with area under the curve (AUC) 0.769 (95% CI: 0.629–0.908) (Fig. 3) and at cut off value>45ng/dl, OPN biomarker can detect the presence of carotid atheroma with diagnostic sensitivity of 70%,specificity of 66.67%,PPV 77.8%, NPV 57.1% with AUC 0.767 (95% CI: 0.665–0.869) (Fig. 4) p-value<0.001.
OPN was first described in 1985 by Franzen and Heinegard as a type of sialoproteins derived from the bovine bone matrix.19 It is a secreted adhesive molecule that functions in part as an inflammatory cytokine; it is thought to aid in the recruitment of monocytes-macrophages and regulate cytokine production in macrophages, dendritic cells and T-cells.6 OPN plays a role in the migration of endothelial cells, proliferation, and/or differentiation of vascular smooth muscle cells along with plaque progression and dystrophic calcification.20 It also can promote macrophage and vascular smooth muscle cells accumulation contributing to vasculature remodelling both in vivo and in humans,21 and thus play an important part in the inflammatory process of atherosclerosis.
As a secreted protein, OPN is of particular interest as a biomarker because of its detectability in body fluids that include plasma, urine, breast, milk, and cerebrospinal fluid, OPN is measurable by minimally invasive means, and this allows for rapidly repeated measures over time and may prove to be a useful prognostic for disease activity and severity.22 And so we sought to assess OPN value and its correlation to the progression of atherosclerosis in patients with chronic inflammatory disease being ESRD.
Out of 80 ESRD patients, 44patients (58.8%) had carotid atherosclerosis with increased CIMT>0.9mm, 50 patients (62.5%) showed carotid plaques, and significant carotid stenosis was present in 15 patients (18.8%).
This was in accordance with Falaknazi et al.23 and Abbasi et al.,24 who reported increased CIMT in ESRD patients. In addition to Collado et al., who studied 110 patients undergoing hemodialysis, and found that 83% of them had atherosclerotic plaques.25
Moreover, in 2011, Barreto DV et al.26 studied OPN levels in 94 chronic kidney disease patients, OPN levels were high in CKD patients compared to controls. Although OPN levels were reported to be increased in ESRD patients; however, there were no data on the relationship between OPN levels and the rate of progression of atherosclerosis.
Moreover, our results were in agreement with Druck et al.,27 who also found a positive correlation between OPN and both PTH and alkaline phosphatase. This correlation was attributed to the complex relationship between OPN and the Ca2+-P-vitamin D-PTH axis.
Among the studied group, the 3rd sub-group (upper tertile) with OPN levels ranging from 78 to 270ng/dl was found to have the largest CIMT of 0.99±0.12mm compared to the 1st and 2nd groups, 24 patients (96%) of the 3rd group were found to have carotid plaques and 11 patients (44%) were found to have significant carotid stenosis. Also, the frequency of atherosclerosis and carotid plaques was higher in the 2nd compared to the 1st group.
Not only the CIMT was greatest but also there was a significant correlation between the OPN levels and CIMT and to the best of our knowledge, this paper is the first in the literature to correlate the OPN levels to the severity of atherosclerosis among ESRD patients on HD.
In the current study, we found that the history of coronary heart disease in the 3rd and 2nd groups was more than the 1st group. In 2015, Mohamadpour et al. studied 304 subjects and found a positive association between circulating OPN concentrations and the presence of coronary artery disease,28 Chen et al. studied the relationship between plasma OPN levels and creatinine level and the severity of coronary artery lesions and concluded that plasma OPN level is associated with renal failure, both of which are correlated with the severity of coronary artery lesions.29
Analysis of the relation between OPN and the other predictors of CVD revealed that the presence of left ventricular hypertrophy (LVH) and diastolic dysfunction (DD) were significantly associated with higher serum OPN levels. Moreover, the left ventricular mass index (LVMI) in the upper and middle tertiles was significantly higher than in the lower tertile, and vice versa regarding the left ventricular ejection fraction (EF). Our results also demonstrated that the serum OPN was significantly positively correlated with the E/A ratio and grade of DD but significantly negatively correlated with EF.
These findings were with the results of Harada, M., Tabako, 2016 study, which found that decreased early diastolic mitral annular velocity relates to the maximum IMT, reflecting the severity of carotid atherosclerosis. They concluded that the presence of severe carotid atherosclerosis may affect LV diastolic dysfunction.30 Furthermore, Yang et al. found that circulating OPN was an independent risk factor for both LV hypertrophy and LVDD in essential hypertensive patients. However, OPN was not related to LV dimension and systolic function.31
Functional polymorphism (156del/G), present on the promoter region of the OPN gene upregulates the transcription of OPN in human beings. Nakayama et al., 2011, studied the association between diastolic dysfunction and this functional polymorphism; and concluded that the prevalence of 156del/G polymorphism was an independent factor for lowering E/A ratio, and suggested that OPN has a functional role in the development of diastolic dysfunction in hypertensive hearts.32
Studies on experimental animal models showed that conditions associated with increased mechanical stress lead to hypertrophied myocardium with upregulated OPN. Moreover, this was associated with overexpression of OPN in fibroblasts and macrophages in hypertrophied myocytes that mediate systolic and diastolic dysfunction.33
Assessment of the diagnostic performance of OPN revealed its ability for detection of atherosclerosis as evidenced by an AUC of 0.804 at a cutoff of 50ng/dl. In addition to the detection of stenosis with an AUC of 0.769 at a cutoff of 78ng/dl. Chaitanya et al.34 also observed a significant increase in the serum OPN level with the progression of CKD, in addition to a significant positive correlation with CIMT. Thus; OPN could be a potential target for atherosclerosis progression modification.
ConclusionSerum Osteopontin is of clinical value as a predictor biomarker of the severity of carotid atherosclerosis, presence of atheroma and carotid stenosis with high diagnostic sensitivity and specificity in chronic hemodialysis patients. Increased Osteopontin level is associated with left ventricular diastolic and systolic dysfunction in those patients.
LimitationsCross-sectional study and a small number of patients as no fund was received.
Authors’ contributionMaha A. Behairy: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Sahar Mahmoud Shawky: Supervision.
Reham Abd Elaziz Hamed: Data curation; Formal analysis; Investigation.
Somia Abd El Hamid Bawady: Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Hoda A. Abdelsattar: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Walid Ahmed Bichari: Data curation; Formal analysis; Supervision; Writing – review & editing.
Ethics approvalAll procedures performed in the study were following ethical standards of the Ain-Shams University hospital research committee (Ethics committee reference number 000017585) and with the ethical standards laid down in the 1964 Declaration of Helsinki.
Consent to participateAn informed written consent was obtained from all patients enrolled in the study.
Consent for publicationAll copyright ownership for the article is transferred to the journal upon acceptance.
Funding/supportNo source of funding was obtained to support the study.
Conflicts of interestNo conflict of interest.