Familial hypercholesterolaemia (FH) in children is under-detected and is difficult to diagnose in clinical practice. The aim of this study was to evaluate clinical, biochemical and vascular imaging variables in order to detect children and adolescents with FH.
MethodsA total of 222 children aged 4–18 years old were recruited to participate in a project for the early detection of FH (The DECOPIN Project). They were distributed into 3groups: FH, if genetic study or clinical criteria were positive (n=91); polygenic hypercholesterolaemia (PH) if LDL-cholesterol >135mg/dl without FH criteria (n=23), and control group (CG) if LDL-C <135mg/dl (n=108). Data were collected from family history, anthropometric data, and clinical variables. The usual biochemical parameters, including a complete lipid profile were analysed. The carotid intima-media thickness (cIMT) and thickness of Achilles tendons were determined using ultrasound in all participants.
ResultsA total of 91 children had a diagnosis of FH, 23 with PH, and 108 with CG. Children with FH had higher concentrations of total cholesterol, LDL-C, ApoB/ApoA1 ratio, and cholesterol-year score, than the other groups. HDL-C was lower in the FH group than in the CG. Thickness of the Achilles tendon and cIMT did not show any differences between groups, although a greater cIMT trend was observed in the FH group. ApoB/ApoA1 ratio >0.82 was the parameter with the highest sensitivity and specificity to predict the presence of mutation in children with FH.
ConclusionsAlthough LDL-C is the main biochemical parameter used to define FH, the ApoB/ApoA1 ratio (>0.82) may be a useful tool to identify children with FH and a positive mutation.
La hipercolesterolemia familiar (HF) infantil está infradiagnosticada y su diagnóstico no es fácil en la práctica clínica. El objetivo fue evaluar qué características clínicas, bioquímicas y de imagen vascular pueden ayudarnos a detectar a niños/as y adolescentes con hipercolesterolemia afectados de HF.
MétodosDoscientos veintidós niños y adolescentes de entre 4 y 18 años fueron reclutados para participar en un proyecto de detección precoz de HF (proyecto DECOPIN). La HF se diagnosticó por criterios genéticos o clínicos. Se definió hipercolesterolemia poligénica (HP) cuando el c-LDL >135mg/dl pero sin criterios clínicos ni genéticos de HF. Participantes con c-LDL <135mg/dl se incluyeron en el grupo control (GC). Se recogieron la historia familiar, los datos antropométricos y las variables clínicas. Se analizaron parámetros bioquímicos y lipídicos. Se determinó el grosor íntima-media carotídeo (GIMc) y los tendones de Aquiles por ecografía.
ResultadosNoventa y un niños fueron diagnosticados de HF y 23 de HP, y 108 como GC. El grupo HF presentó mayores concentraciones de CT, c-LDL, índice ApoB/ApoA1 e índice colesterol año. El c-HDL fue menor en grupo HF que en el GC. Si bien el c-LDL fue el parámetro más definitorio de HF, el índice ApoB/ApoA1 >0,82 fue el que de forma aislada mostró mayor sensibilidad y especificad para predecir la presencia de mutación en el grupo de niños HF. El grosor de los tendones de Aquiles no mostró diferencias entre grupos. El GIMc fue mayor en los niños HF sin diferencias significativas.
ConclusionesLos niveles de c-LDL son el marcador de HF. Un índice ApoB/ApoA1 >0,82 puede ser una herramienta útil para decidir el estudio genético en niños con sospecha de HF.
Familial hypercholesterolaemia (FH) is the most common monogenic disorder. It has 2 forms: the homozygous (HoFH) and the heterozygous (HeFH) form. HoFH has a prevalence of 1/160,000–300,000 according to data published for the European population.1 In Spain, recent data estimate a prevalence of 1/450,000.2 The latest studies in the European population, establish that the prevalence of HeFH ranges between 1/200–250.1 The prevalence of people under the age of 18 with phenotype HeFH in the Spanish population is 1/217.3 In a recent meta-analysis of the paediatric population under 19 years of age, a prevalence of HeFH of 1/279 was observed.4
FH is an autosomal dominant disorder, which means that it can be transmitted with a probability of 50% from parent to child, being detectable from birth due to the presence of elevated levels of total cholesterol (TC). Children affected by FH have low-density lipoprotein cholesterol (LDL-C) plasma concentrations of up to three times higher than children who are not affected.5 This disease is mainly caused by mutations in the gene encoding the LDL-C receptor (LDL-R). To a lesser extent, defects in the gene encoding apolipoprotein B (ApoB) and the gene encoding proprotein convertase subtilisin/kexin type 9 (PCSK9) have been described. Clinically, these are expressed in the same way and only genetic testing can allow us to tell them apart. Nevertheless, in around 5–30% of current cases with the FH phenotype, the gene responsible for this pathology has not been identified.6 Young adults (20–39 years old) diagnosed with FH have a risk 100 times greater of presenting with a premature coronary episode than the unaffected population. Arteriosclerosis in FH begins at an early age in life.7 For this reason, detection and diagnosis are a priority so that recommendations for healthy life habits can be implemented, and where appropriate, pharmacological treatment can be initiated, as early as possible. This will certainly contribute to the decrease of premature cardiovascular disease (PCVD), characteristic of this population. Despite considerable evidence of the benefits of an early diagnosis, the implementation of detection strategies is still an unresolved issue. Only in Slovenia is universal screening carried out in the paediatric population.8 Expert consensus does not always follow the same line. The ideal age for diagnosis is between 8 and 10 years of age; however, in our country [Spain] there is no established detection policy. The current reality is that, in most cases, detection is conducted through direct cascade screening or opportunistic detection.9
Suspected diagnosis in under-18 patients is established in the face of levels of LDL-C>130mg/dl (90th percentile, in the Spanish population),10 along with a history of severe hypercholesterolaemia in a parent or a history of PCVD in first- or second-degree relatives. Since it is not always possible to conduct a genetic test, in clinical practice it would be ideal if we had biomarkers that would allow us to differentiate hypercholesterolaemia of monogenic origin from that of polygenic hypercholesterolaemia.
The aim of this study was to assess which clinical, biochemical and vascular imaging variables can help us to differentiate those children and adolescents with polygenic hypercholesterolaemia (PH) from those affected by FH.
Subjects and methodsStudy designTransversal Study of the Programme for the Detection of Familial Hypercholesterolaemia in the Paediatric Population (DECOPIN project). A total of 222 children and adolescents aged between 4 and 18 years were included. Three detection strategy types were applied: opportunistic, inverse cascade and direct cascade. To implement opportunistic screening, an information campaign was organised for paediatricians, inviting them to participate in the project. The detection criteria were applied by the Primary Healthcare paediatrician in children and adolescents aged 4 to 14 years. Children with suspected FH were re-evaluated by the Paediatric Endocrinology Department and in turn sent to the Unitat de Medicina Vascular i Metabolisme (UVASMET) of the Hospital Universitari Sant Joan (Reus, Tarragona) for genetic diagnosis. The inverse cascade study was applied to children for whom suspected FH was opportunistically detected if the following criteria were met: LDL-C >135mg/dl in at least 2 tests and a history of PCVD in first- or second-degree relatives or severe hypercholesterolaemia (TC >300mg/dl) in one parent or in unspecified relatives. When a child met the criteria for suspected FH, we proceeded to the study of their parents. The clinical criteria from the Dutch Lipid Clinic Network (DLCN) was applied to the parents for the diagnosis of FH, and a genetic test was carried out for those with a score of greater than or equal to 8. In the event of a positive result, the genetic test was conducted for the child1 and their siblings, if they had any, independently of LDL-C values.
Direct cascade was applied to children and adolescents between 4 and 18 years old, the children of patients diagnosed with FH in our department. When the genetic test was positive in the parent, it was also carried out for their children. If the genetic test result was negative in the parent, it was not carried out for the child.
The Hospital Universitari Sant Joan Ethics Committee approved the study. The parents or legal representatives gave their consent in writing. Their consent was also requested for the genetic test when applicable. The study protocol complied with the ethics regulations of the Declaration of Helsinki, 1975.
PatientsTwo hundred and twenty-two children and adolescents aged between 4 and 18 years were included in the DECOPIN Project between March 2013 and June 2017.
The study population was divided into 3 groups:
- -
Children with FH: mutation carriers or children with LDL-C >135mg/dl and parent with FH diagnosis by DLCN criteria and score ≥8, independent of genetic result.
- -
Children with PH: LDL-C >135mg/dl but without clinical or genetic diagnosis of FH in their parents.
- -
Control children (control group [CG]): children with LDL-C <135mg/dl.
Children with secondary hypercholesterolaemia: hypothyroidism, renal diseases, hepatic diseases or other chronic diseases and those affected by HoFH, were excluded.
Medical history and physical examinationA personal clinical history was carried out, including family history of PCVD and dyslipidaemia in first- and second-degree relatives. Anthropometric data were collected during the physical examination. The BMIScore (BMI in children−50th percentile of the Orbegozo growth curves/SD 50th percentile of the Orbegozo growth curves11) were used to calculate BMI. Dietary habits and physical activity were assessed. Specific signs of dyslipidaemia were sought in all children. Both parents of the children coming from opportunistic screening were examined for the presence of corneal arcus, tendon xanthomas and xanthelasma.
Biochemical analysis and lipid profileNone of the assessed children had been treated with lipid-lowering agents. Following 8h of fasting, all participants were subject to a blood extraction. The biochemical and lipid parameters and lipoproteins were determined through enzymatic, colorimetric and immunoturbidimetric methods, which were adapted to a Cobas Modular 700 self-analyser (Roche®, Basel, Switzerland).
LDL-C was calculated using the Friedewald formula. Cholesterol-year score was calculated (LDL-C×years), as well as ApoB/apoA1 ratio. The presence of proteinuria was ruled out.
Genetic testThe presence of mutations was studied using the Liponext genetic test, which selectively detects mutations in LDLR, APOB, PCSK9 and large rearrangements. A genetic sequencing tool (SEQPRO LIPO RS® [Progenika Pharma]) was used.
Ultrasound studyThis was carried out at UVASMET in the Hospital Universitari Sant Joan. It was performed with a MyLab 60-X Vision Ultrasound (Esaote, Genova, Italy) ultrasound machine. A 7.5–10MHz linear probe with semi-automatic software was used. The images were obtained by the same operator to reduce observer variation.12
Determination of the carotid intima-media thicknessThe carotid intima-media thickness (cIMT) was determined by a semi-automatic method with live images and by radiofrequency. Images were obtained from the posterior wall of the common carotid artery to 1cm proximal to the carotid bifurcation. The final cIMT was the result of the mean of both common carotid cIMTs.
Determination of the Achilles tendons’ thicknessFor the testing of this variable, the participants were in prone position with their legs at an angle of flexion of 90°, with a high-resolution probe placed perpendicular to the tendon at 2cm from the proximal calcaneal insertion site. 3 separate measurements were taken at the area of maximum tendon width with 0.5 between them. The width of the Achilles tendon of each leg is the result of the mean of the 3 measurements. Presence of xanthomas was also checked for.
Statistical analysisThe description of the variables is presented using standard statistics, including mean and standard deviation (SD) for the normal variables, and median and interquartile range (IQR) for non-normal variables. The frequencies are presented as percentages. The normal distribution of continuous variables was studied using the Kolmogorov–Smirnov test. For the categorical variables and to compare frequencies between groups, the Chi-square test was used. The Student's t-test or ANOVA test with Bonferroni post hoc test was used to compare and analyse the continuous variables between groups with normal distribution, and for non-normal, the Mann–Whitney U test and Kruskall–Wallis tests were used. To be able to connect the variables, the Pearson or Spearman correlations were carried out between continuous variables of normal or non-normal distribution, respectively. The multivariate analysis was carried out with logistic or linear regression, depending on whether the variables were dichotomous or continuous. ROC curves were generated (acronym of the initials: receiver operating characteristic curve) and the area under the curve (AUC) was calculated to assess the capability of identifying children with FH with or without mutation. Statistical significance was considered to be p<0.05.
Statistical analysis was carried out with the SPSS 22.0 for Windows statistical analysis package (SPSS, IBM®, Chicago, IL).
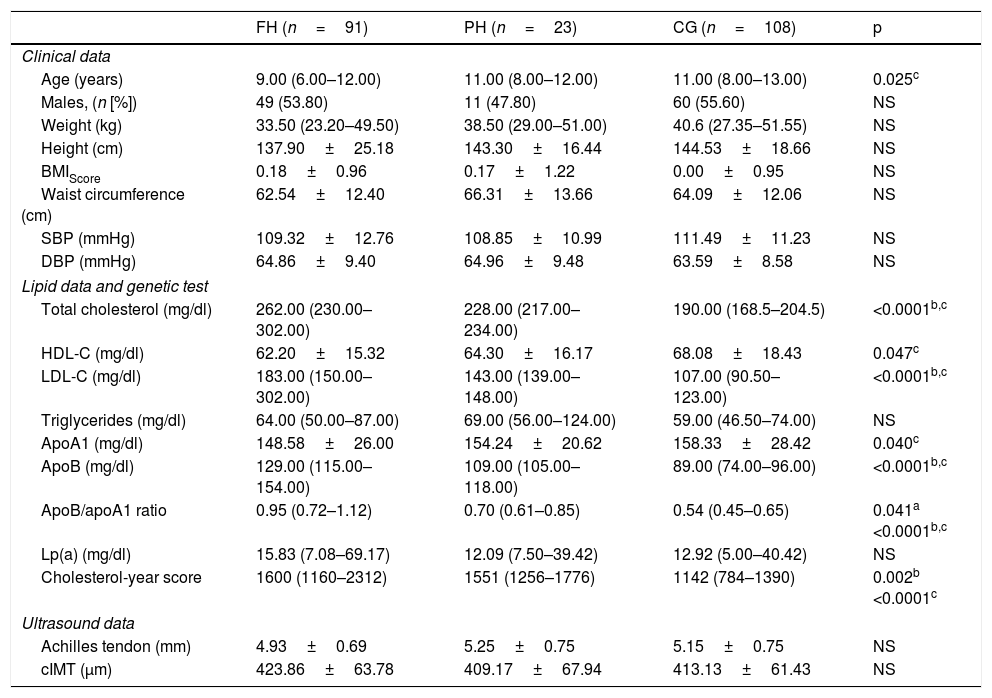
ResultsA total of 222 children and adolescents were included through the DECOPIN Project, of whom 91 were diagnosed with FH (53 through direct cascade and 38 by inverse cascade), 23 PH and 108 CG. In Table 1, the baseline characteristics of the population studied are shown distributed according to diagnosis. The clinical variables studied do not demonstrate statistically significant differences, except for age between the FH and CG groups (p=0.025). Statistically significant differences were observed in the lipid profile between the CG with respect to the FH and PH group with TC, LDL-C, ApoB and cholesterol-year score (p<0.0001), which also demonstrated differences between FH and PH (p=0.002). A lower concentration of HDL-C was observed in the FH group in relation to the CG (p=0.047). The Achilles tendon measurement and the cIMT did not demonstrate statistically significant differences between groups. However, in the cIMT there was a superior tendency in the FH group with respect to the other 2 groups. In 66 children with FH, the genetic test was positive (92.5% with mutation in LDLR, 3.0% in APOB and 4.5% in PCSK9). The remainder of the biochemical parameters analysed in blood and urine did not demonstrate significant differences between groups (data not shown).
Data on the population studied distributed according to diagnosis.
| FH (n=91) | PH (n=23) | CG (n=108) | p | |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 9.00 (6.00–12.00) | 11.00 (8.00–12.00) | 11.00 (8.00–13.00) | 0.025c |
| Males, (n [%]) | 49 (53.80) | 11 (47.80) | 60 (55.60) | NS |
| Weight (kg) | 33.50 (23.20–49.50) | 38.50 (29.00–51.00) | 40.6 (27.35–51.55) | NS |
| Height (cm) | 137.90±25.18 | 143.30±16.44 | 144.53±18.66 | NS |
| BMIScore | 0.18±0.96 | 0.17±1.22 | 0.00±0.95 | NS |
| Waist circumference (cm) | 62.54±12.40 | 66.31±13.66 | 64.09±12.06 | NS |
| SBP (mmHg) | 109.32±12.76 | 108.85±10.99 | 111.49±11.23 | NS |
| DBP (mmHg) | 64.86±9.40 | 64.96±9.48 | 63.59±8.58 | NS |
| Lipid data and genetic test | ||||
| Total cholesterol (mg/dl) | 262.00 (230.00–302.00) | 228.00 (217.00–234.00) | 190.00 (168.5–204.5) | <0.0001b,c |
| HDL-C (mg/dl) | 62.20±15.32 | 64.30±16.17 | 68.08±18.43 | 0.047c |
| LDL-C (mg/dl) | 183.00 (150.00–302.00) | 143.00 (139.00–148.00) | 107.00 (90.50–123.00) | <0.0001b,c |
| Triglycerides (mg/dl) | 64.00 (50.00–87.00) | 69.00 (56.00–124.00) | 59.00 (46.50–74.00) | NS |
| ApoA1 (mg/dl) | 148.58±26.00 | 154.24±20.62 | 158.33±28.42 | 0.040c |
| ApoB (mg/dl) | 129.00 (115.00–154.00) | 109.00 (105.00–118.00) | 89.00 (74.00–96.00) | <0.0001b,c |
| ApoB/apoA1 ratio | 0.95 (0.72–1.12) | 0.70 (0.61–0.85) | 0.54 (0.45–0.65) | 0.041a <0.0001b,c |
| Lp(a) (mg/dl) | 15.83 (7.08–69.17) | 12.09 (7.50–39.42) | 12.92 (5.00–40.42) | NS |
| Cholesterol-year score | 1600 (1160–2312) | 1551 (1256–1776) | 1142 (784–1390) | 0.002b <0.0001c |
| Ultrasound data | ||||
| Achilles tendon (mm) | 4.93±0.69 | 5.25±0.75 | 5.15±0.75 | NS |
| cIMT (μm) | 423.86±63.78 | 409.17±67.94 | 413.13±61.43 | NS |
Data expressed as mean±SD for the variables that follow a normal distribution, mean (IQR) for the variables that do not follow a normal distribution and n (%) for categorical variables.
The statistical test used was ANOVA (normal distribution), Kruskal–Wallis (non-normal distribution) or Chi-square (for categorical data).
A p value of <0.05 was considered to be statistically significant.
ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; BMIScore: body mass index score; CG: control group; cIMT: carotid intima-media thickness; DBP: diastolic blood pressure; FH: familial hypercholesterolaemia; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; Lp(a): lipoprotein a; PH: polygenic hypercholesterolaemia; SBP: systolic blood pressure.
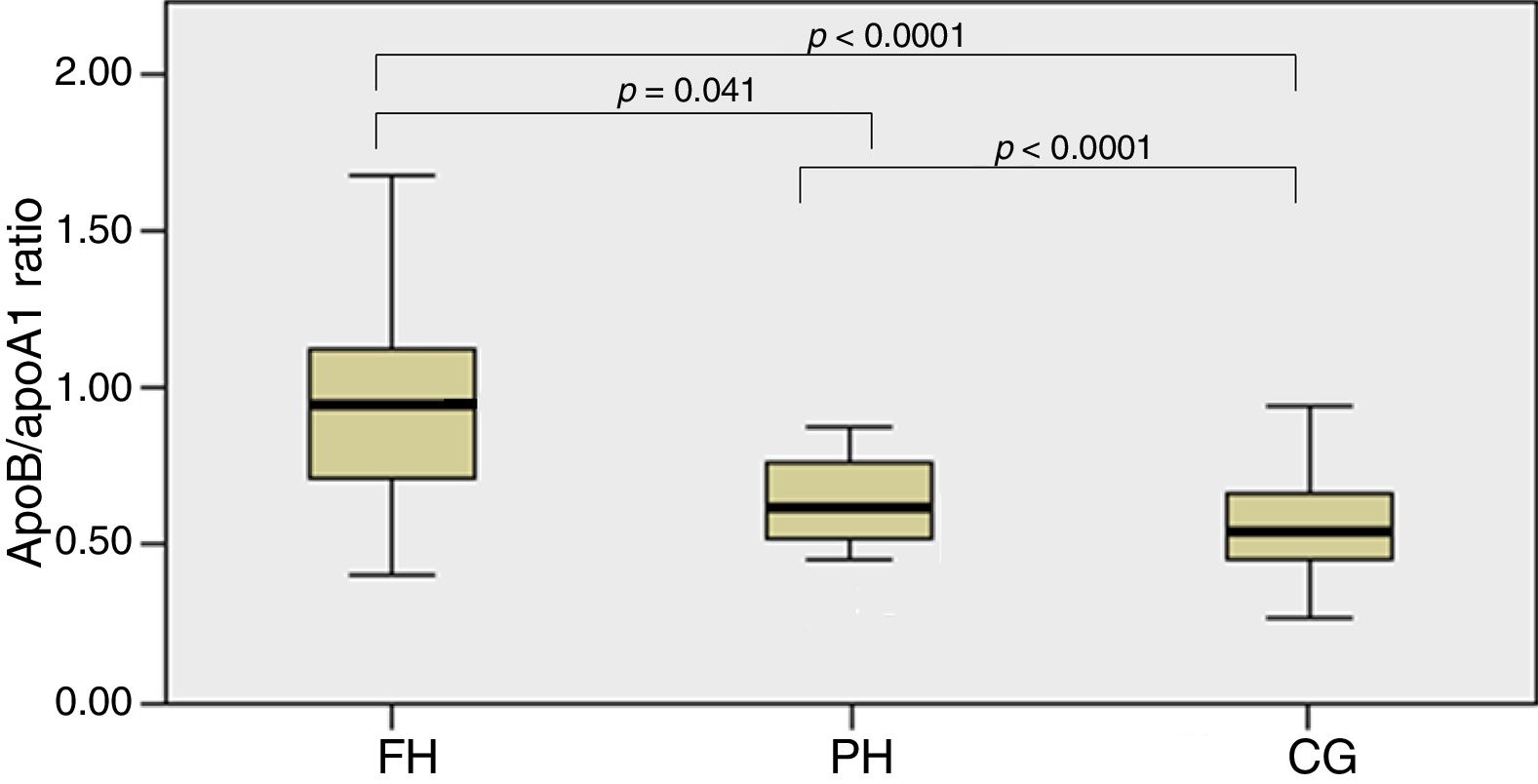
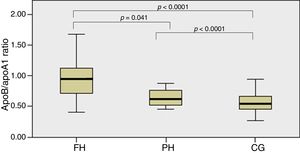
In Fig. 1, the ApoB/apoA1 ratio is shown between groups which detected statistically significant differences between the FH and PH groups (p=0.041) and between these two groups and the CG (p<0.0001).
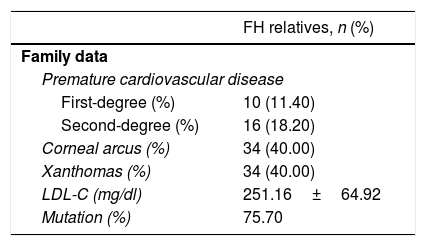
In Table 2, data from 81 families with FH is collected, including history of PCVD in first- and second-degree relatives, and lipid deposits in the affected parents, as well as their mean baseline LDL-C and the percentage of those in whom the mutation was detected.
Premature cardiovascular disease in first- and second-degree relatives in FH children from a total of 81 families and physical examination of the parent affected by FH.
| FH relatives, n (%) | |
|---|---|
| Family data | |
| Premature cardiovascular disease | |
| First-degree (%) | 10 (11.40) |
| Second-degree (%) | 16 (18.20) |
| Corneal arcus (%) | 34 (40.00) |
| Xanthomas (%) | 34 (40.00) |
| LDL-C (mg/dl) | 251.16±64.92 |
| Mutation (%) | 75.70 |
Data expressed as mean±SD for the variables that follow a normal distribution, and n (%) for categorical variables.
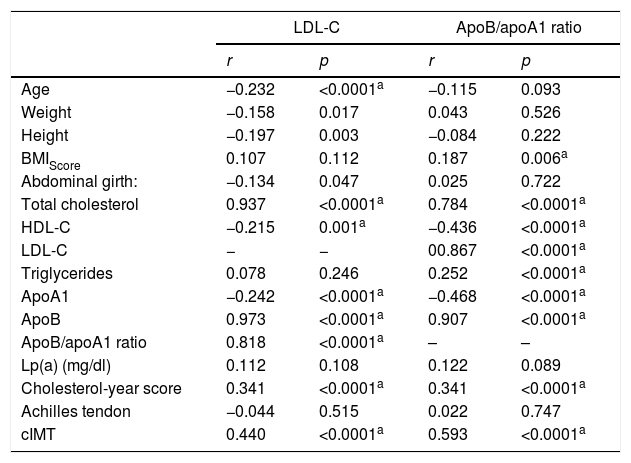
Bivariate correlation was analysed between LDL-C and the ApoB/apoA1 ratio with the anthropometric variables, lipid profile and ultrasound study in the studied populations (Table 3).
Correlations of the anthropometric variables, lipid profile and ultrasound study with LDL-C and ApoB/apoA1 ratio in the population studied (n=222).
| LDL-C | ApoB/apoA1 ratio | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −0.232 | <0.0001a | −0.115 | 0.093 |
| Weight | −0.158 | 0.017 | 0.043 | 0.526 |
| Height | −0.197 | 0.003 | −0.084 | 0.222 |
| BMIScore | 0.107 | 0.112 | 0.187 | 0.006a |
| Abdominal girth: | −0.134 | 0.047 | 0.025 | 0.722 |
| Total cholesterol | 0.937 | <0.0001a | 0.784 | <0.0001a |
| HDL-C | −0.215 | 0.001a | −0.436 | <0.0001a |
| LDL-C | − | − | 00.867 | <0.0001a |
| Triglycerides | 0.078 | 0.246 | 0.252 | <0.0001a |
| ApoA1 | −0.242 | <0.0001a | −0.468 | <0.0001a |
| ApoB | 0.973 | <0.0001a | 0.907 | <0.0001a |
| ApoB/apoA1 ratio | 0.818 | <0.0001a | – | – |
| Lp(a) (mg/dl) | 0.112 | 0.108 | 0.122 | 0.089 |
| Cholesterol-year score | 0.341 | <0.0001a | 0.341 | <0.0001a |
| Achilles tendon | −0.044 | 0.515 | 0.022 | 0.747 |
| cIMT | 0.440 | <0.0001a | 0.593 | <0.0001a |
The statistical test used was the Pearson correlation (normal distribution), and Spearman (non-normal distribution) or Chi-square (for categorical data).
A p value of <0.05 was considered to be statistically significant.
ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; BMIScore: body mass index score; cIMT: carotid intima-media thickness; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; Lp(a): lipoprotein a.
It is worth noting the statistically significant positive correlations between the ApoB/apoA1 ratio and the complete lipid profile, as well as the cIMT (p≤0.0001).
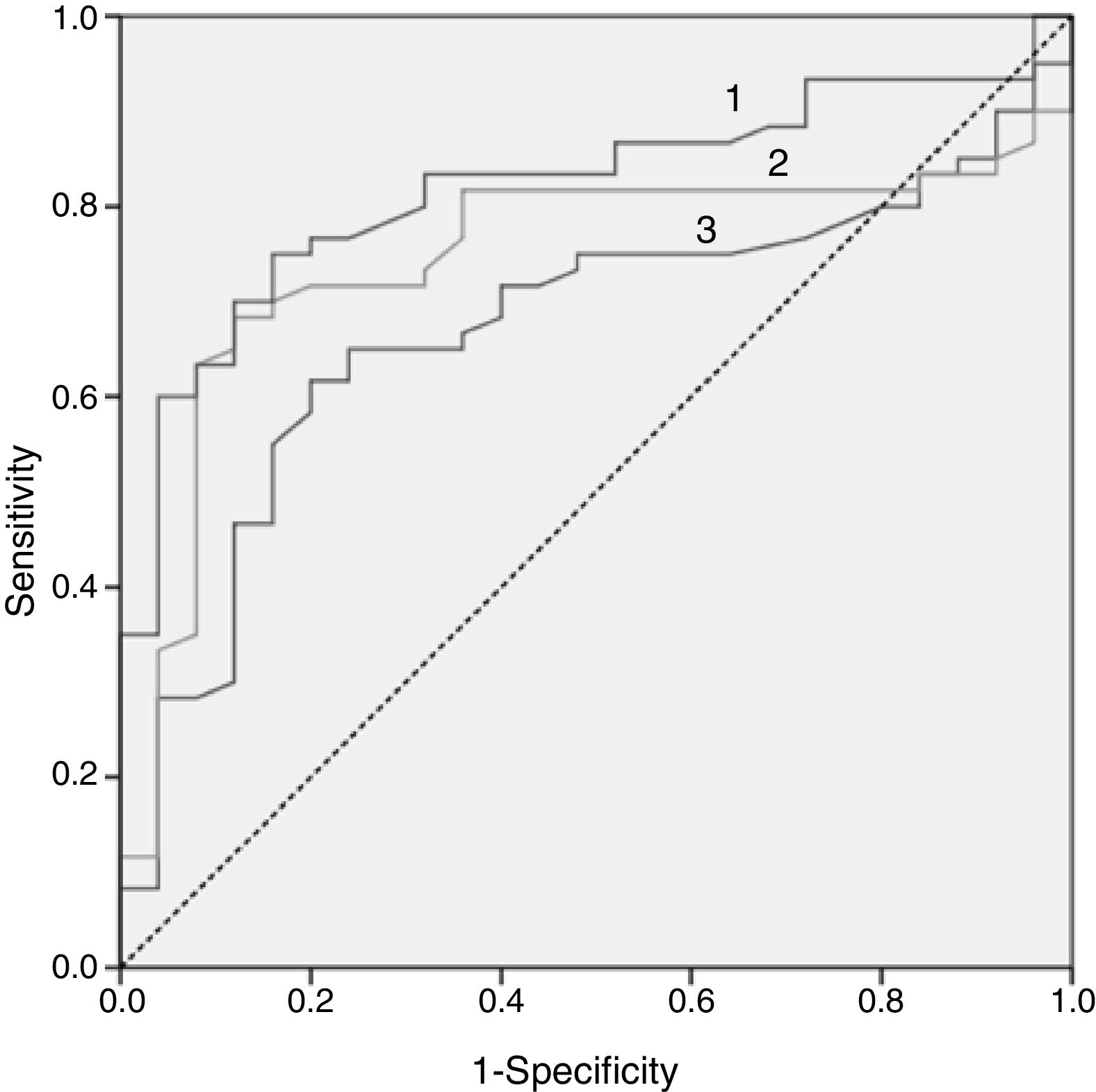
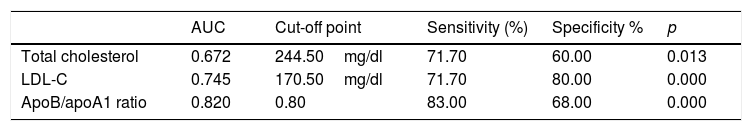
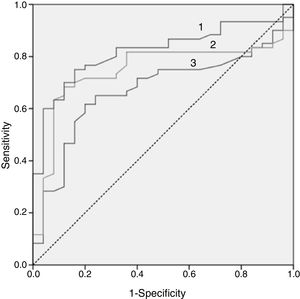
In Fig. 2, the ROC curve of the 3 lipid biomarkers that best allow for discrimination of the paediatric population with FH with positive and negative mutation, the best predictor being the ApoB/apoA1 ratio, can be observed. In Table 4, the cut-off point with its sensitivity and specificity can be observed.
Cut-off points for the 3 best biomarkers for their discriminative capacity (sensitivity and specificity) to differentiate between the genetically positive and negative FH population.
| AUC | Cut-off point | Sensitivity (%) | Specificity % | p | |
|---|---|---|---|---|---|
| Total cholesterol | 0.672 | 244.50mg/dl | 71.70 | 60.00 | 0.013 |
| LDL-C | 0.745 | 170.50mg/dl | 71.70 | 80.00 | 0.000 |
| ApoB/apoA1 ratio | 0.820 | 0.80 | 83.00 | 68.00 | 0.000 |
To analyse these data, the ROC curve was used.
A p value of <0.05 was considered to be statistically significant.
ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; AUC: area under the curve; LDL-C: LDL cholesterol.
In the hypercholesterolaemia study in the paediatric age group, the TC test is the most utilised tool between 1 and 9 years of age for detecting FH.13 In real clinical practice, in the face of all children or adults with hypercholesterolaemia, once secondary causes were discarded, we had to posit the diagnosis of FH. However, since the clinical criteria could not be applied in the paediatric age group and it is not always possible to conduct a genetic test, diagnosing FH is not easy. In this study, we included a total of 222 children, 114 with hypercholesterolaemia detected following the application of direct cascade or opportunistic detection by the Primary Healthcare paediatrician with subsequent application of inverse cascade. Eight children with normal cholesterol were included as a CG originating from the application of direct cascade on having a parent with FH or opportunistic detection. Our objective was to look for lipid or vascular biomarkers that would help us to distinguish those children with a higher probability of being affected by FH.
FH is completely asymptomatic in children and adolescents. For this reason, family history is of great interest where applicable. Similarly, the examination of the parents, in search of stigmas of genetic dyslipidaemia (corneal arch, xanthomas), are elements of great interest for the diagnosis, although it should be taken into account that in young parents, these characteristic clinical signs are not usually present. Another relevant detail is knowledge of the family history of PCVD, taking into account the age of the parents. It is usually more common to find this history in second-degree relatives such as grandparents.14 In our population, 18.2% of the second-degree relatives had presented with PCVD, while only 10.4% of parents had that history. Klančar et al. reported that only a third of the relatives of children with FH with a genetic diagnosis had a history of PCVD, for which reason they concluded that the detection of FH in the child cannot be based on a family history of PCVD.7 In the same sense, O’Loughlin et al. reported that the detection of FH in the child, based on a family history of cardiovascular disease, had a low sensitivity and specificity.15 Current clinical diagnosis is based on LDL-C levels. We know that the phenotype of children with FH diagnosed genetically is very diverse, from presenting with very severe hypercholesterolaemia (LDL-C >190mg/dl) to normal LDL-C values, albeit with the majority of cases demonstrating figures ranging between 130 and 190mg/dl. In our study, the LDL-C mean was 183mg/dl, while in the children with PH it was 143mg/dl. Variability of the phenotype in children with FH has been objectified in a universal screening study for the detection of FH conducted in 10,095 children between 1 and 2 years of age through genetic analysis. Children with genetically confirmed FH showed mean LDL-C figures of 155mg/dl, although in some cases the figures were lower than 130mg/dl. This indicates that detection based solely on phenotype is insufficient.10 It has been indicated that an ApoB/apoA1 ratio of greater than 0.68 is a more sensitive and specific marker for the detection of FH.16 This ratio is used to distinguish those patients affected by myocardial infarction in the InterHeart study.17 More recently, the data from the Pure study18 have indicated that the lipid parameter associated with cardiovascular risk mediated by an atherogenic diet is also the apoB/apoA1 ratio. The apoB/apoA1 ratio is an estimate of the relationship between the pro- and anti-atherogenic lipoprotein particles. The measurement of these apolipoproteins could have dual significance when assessing cardiovascular risk and increasing detection of FH in children,19 although it is true that testing of lipoproteins is not commonplace in clinical health practice, a fact that limits its use in Primary Healthcare. However, it is a standard test in the specialised departments. We should highlight the positive correlation and statistical significance between the ApoB/apoA1 ratio and the cIMT observed in the group of participants in our study. Similar data have been described before by other groups, which indicates that a pro-atherogenic lipoprotein profile in children and adolescents predisposes the development of subclinical arteriosclerosis in adults.19
In our population of children diagnosed with FH, an apoB/apoA1 ratio of greater than 0.82 showed greater sensitivity and specificity in the detection of carriers of a genetic mutation, compared with LDL-C or CT. This fact is of great interest as it has not been described before.
In our study, the cholesterol-year score is higher in children with hypercholesterolaemia in relation to the CG. The load of accumulated LDL-C in the arterial wall throughout life, along with the presence of other cardiovascular risk factors, will determine the development of arteriosclerosis. In children, one way of measuring the LDL-C load is the cholesterol-year score test (LDL-C per year) without treatment. Torsade et al. observed that children with FH with a greater cholesterol-year score presented with a greater number of plates at the carotid artery level.20
In our study, the HDL-C concentration was significantly lower in relation to the CG. Current data indicate that it may be due to less activity in reverse cholesterol transport, as a consequence of a disturbance in the concentration of lipid content of small HDLs.21
On the other hand, our group has participated in a study, observing that patients with untreated FH presented with disturbances in the concentrations of enzymes that participate in the remodelling of HDLs; this would cause dysfunctional HDLs, which would bring about a defect in the ability to eliminate cholesterol from macrophages.22
The cIMT in children with FH was greater than the group with PH but with no significant difference. These data differ from those described previously by other groups, in those that differences in children have been observed with respect to their unaffected siblings before 8 years of age.23 Currently, the most recent guidelines on cardiovascular prevention from 2016 do not recommend the cIMT test as a marker of subclinical arteriosclerosis in the general population. However, in children, the cIMT has been demonstrated as having played an acceptable discriminatory role and we believe that it continues to be an appropriate tool in highly selected populations with a high cardiovascular risk, such as children with FH.
In this study, we only measured the Achilles tendons’ thickness; neither the deposits of cholesterol nor myofibril network distortion were assessed. We proposed that their determination could help us in the detection and diagnosis of children with FH, as has been described in the adult population.24 Both by palpation and their measurement by ultrasound, although they seemed to be thicker than expected, there were no significant differences between the 3 populations studied.
As a particular strength of the study, it is worth mentioning that it is the first one conducted in Spain in children for the detection and diagnosis of FH, with the collaboration and involvement of different healthcare areas. The application of opportunistic screening by Primary Healthcare paediatricians and the application of direct and inverse cascade in the specialist department.
As study limitations, the sample size was small, especially for the group of children with PH, but we should also remember that these were children detected in families with FH and not in the general population. Also, the age range studied included the pubertal stage, and it was difficult to assess the hormonal impact on the results obtained.
Conclusions and recommendationsThe FH detection strategies via direct and indirect screening allowed for an increase in childhood diagnosis of FH. Concentrations of TC elevated above 245mg/dl or of LDL-C greater than 170mg/dl is associated with the presence of FH with a positive genetic mutation. However, the parameter with the best relationship between sensitivity and specificity is an ApoB/apoA1 ratio of greater than 0.82. Educating the children and their families about following the recommended lifestyle changes and initiating pharmacological treatment in a timely manner will have a clear impact on the prevention of PCVD characteristic of this population.
FundingThis study was sponsored by the Marato project, with identification code 201524 30 31 and by the Sociedad Española de Arteriosclerosis [Spanish Society of Arteriosclerosis] and the Fundación Española de Arteriosclerosis por la Beca SEA/FEA [Spanish Arteriosclerosis Foundation for the SEA/FEA Scholarship] for clinical research in 2013 entitled “Early detection of heterozygous familial hypercholesterolaemia in the paediatric population”, presented at the XXVI Congress of Zaragoza.
AuthorshipDr. Núria Plana: conception and design of the study, clinical monitoring, data collection, monitoring of the collected data, interpretation of the data, draft and final approval of the version presented.
Dr. Cèlia Rodríguez-Borjabad: has contributed to the coordination of the study, obtaining data, analysis and interpretation of the data, critical review of the intellectual content.
Dr. Daiana Ibarretxe: has contributed to obtaining data, analysis and interpretation of the data, critical review of the intellectual content.
Dr. Raimon Ferré: carried out the ultrasound study, critical review of the intellectual content.
Dr. Albert Feliu: conception and design of the study, recruitment of patients, review of inclusion and exclusion criteria, critical review of intellectual content.
Dr. Alejandra Caselles: collaboration with recruitment, critical review of intellectual content.
Dr. Luís Masana: conception and design of the study, interpretation of the data, draft and final approval of the version presented.
Conflicts of interestFor this study, the authors declare that there was no interference in the attaining or interpretation of the results and that they therefore have no conflicts of interest. Some of the authors have received fees for conferences or advice, as detailed below.
Núria Plana has received conference fees from Alexion, Amgem, Ferrer, MSD, Rubio and Sanofi.
Luis Masana has received conference and scientific advice fees from Amgen, MSD, Recordati and Sanofi.
Daiana Ibarretxe has received conference fees from Sanofi, MSD, Rubio and Esteve.
The authors declare that they have no conflicts of interests.
To Carmen Buixadera for her invaluable coordination work. To Núria Aguilera and Anna Varela, for their golden hands as project nurses.
We would like to thank all participants and their families for their participation in this project.
We would also like to thank all the Primary Healthcare Paediatricians (DECOPIN Group) for the recruitment of participants and families with FH; their involvement has been crucial to the success of the project.
Researchers who participated in recruitment: Aguado, Fèlix (CAP Marià Fortuny, Reus); Amigó, Elisabeth (Hospital Sant Pau i Santa Tecla, Tarragona); Andrés, Patricia (CAP Riudoms, Riudoms); Barrio, Mercedes (CAP El Morell, Morell); Bilbao, José Ángel (CAP Riudoms, Riudoms); Bosch, Montserrat (CAP Salou, Salou); Cabedo, José Lluís (CAP Marià Fortuny, Reus); Calvo, Josefa (Hospital Sant Pau i Santa Tecla, Tarragona); Campillo, Carmen (CAP Torreforta-La Granja, Tarragona); Castejón, Emma (CAP La Selva del Camp, Selva del Camp); Castillejo, Gemma (Hospital Universitari Sant Joan, Reus); Castro, María (Hospital Sant Pau i Santa Tecla, Tarragona); Cliville Rosa (CAP Sant Pere, Reus); de Gotardo, Enrique (Hospital Sant Pau i Santa Tecla, Tarragona); Doménech, Vanesa (CAP Amposta, Amposta); Domínguez, Dolores (CAP Muralla, Tarragona); Duràn-Ballén, Marta (CAP Sant Pere, Reus); Escolà, Maria (CAP Roquetes, Les Roquetes); Fernández, Marta (Hospital Universitari Joan XXIII, Tarragona); García, Joan (CAP Sant Pere, Reus); Girona, Raquel (CAP El Pla de Santa Maria, Pla de Santa Maria); Gutiérrez, M. Antonia (CAP Constatí, Tarragona); Iglesias, Dolores (CAP Torreforta-La Granja, Tarragona); Miquel Salsas, Jaume (CAP Santa Bárbara, Santa Bárbara); Luque, Verónica (Hospital Universitari Joan XXIII, Tarragona); Machado, Pilar (Torreforta-La Granja, Tarragona); Maixé, Jordi (Hospital Sant Pau i Santa Tecla, Tarragona), Mallafré, Marta (CAP Cambrils, Cambrils); Martin, Ramona (CAP Marià Fortuny, Reus); Jiménez, Milagros (CAP Horts de Miro, Reus); Monne, Raquel (Hospital Universitari Joan XXII, Tarragona); Morillo, Susana (CAP Llibertat, Reus); Naranjo, Àngels (CAP L’Espluga de Francolí, Espluga de Francolí); Pérez, Cristina (CAP Llibertat, Reus); Planelles, Montserrat (CAP M. Fortuny, Reus); Querol, Cecilia (CAP de Sant Pere, Reus); Rabadà, M. José (CAP La Selva del Camp, Selva del Camp); Remedi, Ayelen (Hospital Mora d’Ebre, Mora d’Ebre); Riquelme, Carmen (Hospital Sant Pau i Santa Tecla, Tarragona); Rodríguez, Neus (Hospital Verge de la Cinta, Tortosa); Rosell, Laura (CAP Llibertat, Reus); Salvado, Maria (CAP Riudoms, Riudoms); Salvador, Olga (CAP Llibertat, Reus); Santos, Alicia (Hospital Universitari Joan XXIII,Tarragona); Sanz, Núria (CAP Les Borges del Camp, Les Borges del Camp); Segura, Sandra (CAP Montroig del Camp); Subirana, Gloria (CAP Rambla Nova, Tarragona); Tarrades, Pilar (Pius Hospital, Valls); Vendrell, Montserrat (ABS de Vandellós-Hospitalet del Infant, Hospitalet de l’Infant); Vilella, Mireia (CAP Rambla Nova, Tarragona), and Zalaba, Eduardo (CAP Riudoms, Riudoms).
The names of the members of the DECOPIN group are given in Appendix 1.
Please cite this article as: Plana N, Rodríguez-Borjabad C, Ibarretxe D, Ferré R, Feliu A, Caselles A, et al. Valor de los parámetros lipídicos y apoproteicos para la detección de hipercolesterolemia familiar en la infancia. Proyecto DECOPIN. Clin Invest Arterioscler. 2018;30:170–178.