Cardiorenal syndrome includes numerous conditions affecting the heart and kidney, and is a strong predictor of cardiovascular mortality.
MethodsAn analysis was performed on 157 consecutive patients admitted to the Coronary Care Unit of the Camilo Cienfuegos Hospital due to an ST-segment elevation myocardial infarction and heart failure, from January 2013 to December 2016. An analysis was made of the presence of cardiorenal syndrome and its relationship with epidemiological, clinical, and analytical variables, as well as complementary explorations. The relationship between cardiorenal syndrome and in-hospital mortality was assessed using binary logistical regression.
ResultsA total of 52 (33.1%) patients had a cardiorenal syndrome. The haemoglobin level was lower in the group of patients with cardiorenal syndrome (117.2±15.3 vs. 123.3±15.1, p=0.019), and in left ventricular ejection fraction (34.8±8 vs. 43.2±10.8). A positive correlation was found between the Killip class and the increase in serum creatinine after 48h. The serum creatinine was associated with left ventricular ejection fraction (r=0.166; p=0.038). The multivariate analysis showed that cardiorenal syndrome was an independent predictor of in-hospital mortality when adjusted for a history of ischaemic heart disease, diabetes mellitus status, atrial fibrillation, ventricular arrhythmias, left ventricular ejection fraction, age and systolic blood pressure.
ConclusionThe presence of cardiorenal syndrome has an influence on the prognosis of patients who suffer a cardiorenal syndrome. Its detection could be useful in the risk stratification.
El síndrome cardiorrenal incluye numerosas enfermedades que afectan el corazón y el riñón y empeora el pronóstico de los pacientes con síndrome coronario agudo.
MétodoSe estudiaron prospectivamente 157 pacientes que ingresaron de forma consecutiva con diagnóstico de infarto agudo de miocardio con elevación del segmento ST y clase Killip>I, de enero de 2013 a diciembre de 2016, en el Hospital General Docente Camilo Cienfuegos. Se recogieron datos clínicos, de laboratorio y ecocardiográficos en relación con la presencia de síndrome cardiorrenal y se determinó la implicación pronóstica del mismo en la mortalidad intrahospitalaria a través de la regresión logística binaria.
ResultadosEl síndrome cardiorrenal se presentó en 52 pacientes (33,1%). La hemoglobina mostró medias inferiores en el grupo de pacientes con síndrome cardiorrenal (117,2±15,3 vs. 123,3±15,1; p=0,019), al igual que la fracción de eyección del ventrículo izquierdo (34,8±8 vs. 43,2±10,8). Existió una correlación positiva entre la clase Killip y el aumento de la creatinina a las 48h y de esta con la fracción de eyección del ventrículo izquierdo (r=0,166; p=0,038). El síndrome cardiorrenal fue más frecuente en el infarto anterior extenso y resultó un predictor independiente de mortalidad (OR 4,1; IC 95% 1,2-13,9; p=0,022).
ConclusionesEl síndrome cardiorrenal en el curso de un infarto agudo del miocardio puede asociarse a una mayor mortalidad intrahospitalaria. Su detección sería de utilidad en la estratificación pronóstica del síndrome coronario agudo.
The prognosis of patients with ST-segment elevation myocardial infarction (STEMI) is related to the probability of developing an adverse event in the short or long term, and depends on multiple factors.1,2 Diseases which involve the heart and the kidney are manifestations of a systemic vascular disease resulting from the process of atherosclerosis, and share aetiological factors. Heart failure as a complication of acute coronary syndrome (ACS) often co-exists with kidney dysfunction. This is defined as cardiorenal syndrome (CRS).3 CRS type i is determined by an acute deterioration in heart and kidney function initiated by heart damage, and has prognostic complications in ACS.3,4 The main mechanism involved is related to the fall in minute volume which, added to systemic haemodynamic disturbances, alter renal perfusion with the consequent decrease in glomerular filtration rate.5,6
Kidney function deterioration (KFD) in the context of acute heart failure has a prevalence of 10–40%.7 It is known that patients admitted due to ACS and acute or chronic deterioration of kidney function present a worse prognosis in the short and long-term,1,8 in particular if they are associated with concomitant heart failure.7 Considering this issue, we propose as an objective to evaluate the involvement of CRS in the risk of in-hospital death of patients with STEMI who develop any degree of heart failure.
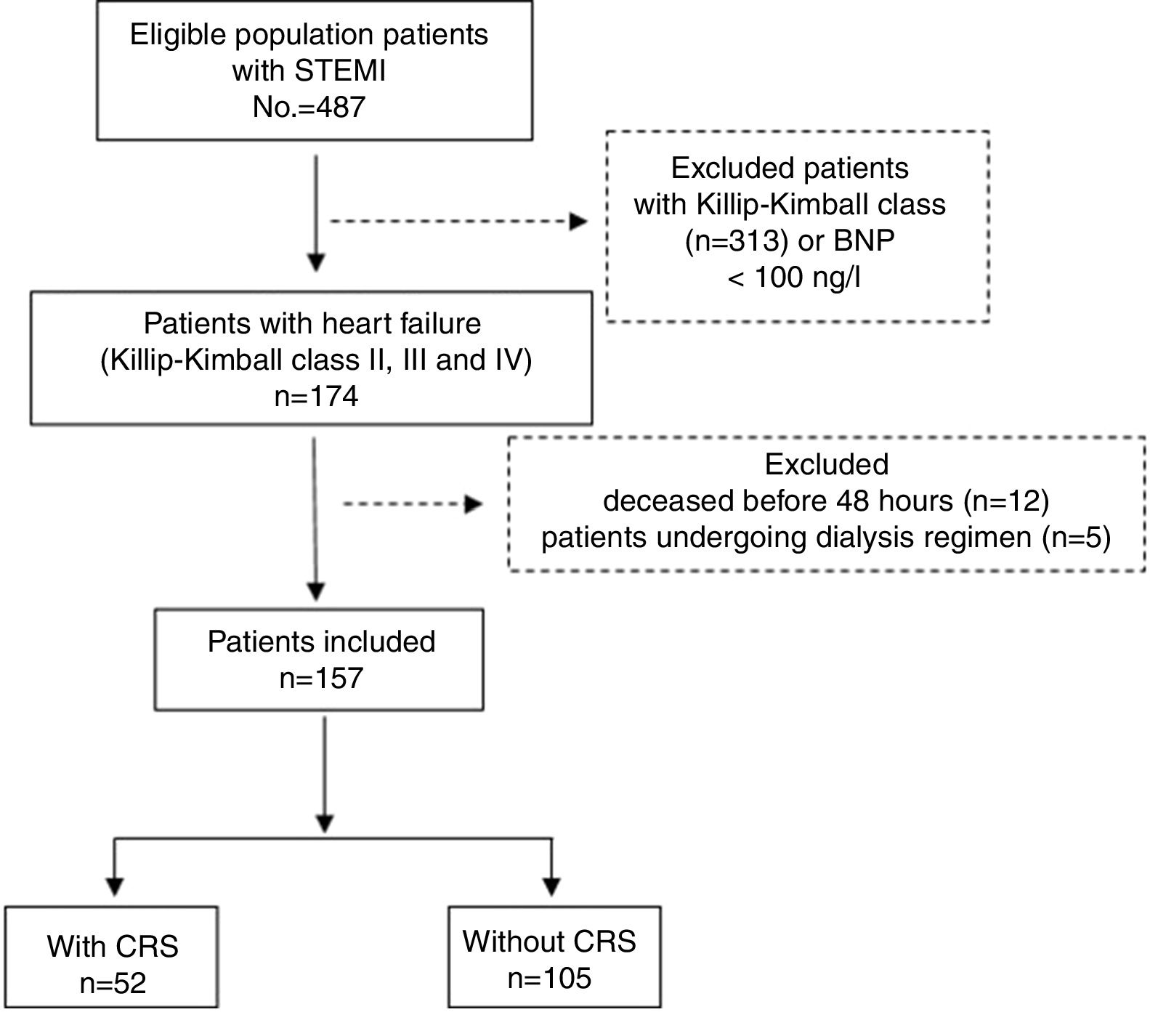
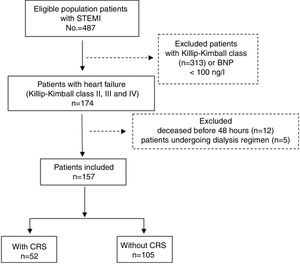
Material and methodStudy designA prospective observational study was conducted which included all patients with STEMI admitted consecutively to the Coronary Care Unit of the Hospital General Docente Camilo Cienfuegos de Sancti Spíritus (Cuba), in the period from 1 January 2013 to 31 December 2016. The sample selection diagram is described in Fig. 1.
The diagnosis of STEMI was defined by pain typical of coronary insufficiency with new ST-segment elevation >0.2mV, measured from the J point in two or more precordial leads or 0.1mV in two or more standard leads or a new onset of left bundle branch block.1,9
Procedure and study variablesThe initial assessment of the patient and follow-up were carried out by specialist cardiologists and nephrologists, who verified compliance with the study protocol. Data collection was performed by means of record formats which included demographic data (age, gender and skin colour); medical history and cardiovascular risk factors hypertension, previous ischaemic heart disease, dyslipidaemia, smoking, diabetes mellitus and obesity (considering obese patients to be those with a body mass index greater than 30kg/m2); clinical data, such as systolic and diastolic blood pressure, and also heart rate at admission. Thrombolysis was performed with recombinant streptokinase and infarct topography was determined by the admission electrocardiogram and was classified in accordance with the Bayés de Luna criteria.10
Venous blood samples were taken when the patient was admitted. The tests performed were haemoglobin, blood sugar, leukogram, creatinine, B-type natriuretic peptide and creatine phosphokinase. The latter was repeated after 6, 12, 24 and 48h, and the peak value was taken. The creatinine test was repeated 48h after admission, and patients with an increase greater than or equal to 26.2μmol/l (0.3mg/dl), or an increase up to a value greater than or equal to 150% of their baseline value, were diagnosed with CRS, in accordance with the criteria of the Acute Kidney Injury Network.11 The glomerular filtration rate (GFR) of the kidneys was calculated using the MDRD-4 formula.3
The blood test was processed in an automated Cobas c311 analyser in a sample of venous blood taken in the first three hours of the patient's admission, and was repeated after 48h of admission.
Once haemodynamic stability was reached, a transthoracic echocardiogram was performed at the patient's bedside with a PHILIPS EPIQ 5 machine, and the left ventricular ejection fraction (LVEF) was determined using the biplane Simpson's method.
Outcome and follow-upPatient follow-up was performed during their hospital stay, and the main outcome analysed was mortality during admission. Morbidity was also studied during patient follow-up: Killip and Kimball class, atrial fibrillation, atrioventricular block, recurrence of myocardial infarction, cardiorespiratory arrest and presence of tachycardia/ventricular fibrillation.
Ethical provisionsThe study protocol was designed in accordance with the Declaration of Helsinki and was approved by the hospital's Ethics Committee. Patient identification data was not published. However, confidentiality was respected during the handling of the data.
Statistical analysisA database created in the statistical package SPSS v.17.0 for Windows was used.
Continuous data were presented as their mean and standard deviation, and categorical data as numbers and percentages.
Normal distribution of the variables was checked using the Kolmogorov–Smirnov test (p>0.05). The comparison of the quantitative variables between groups, if they followed a normal distribution, was performed with the Student's t-test for independent samples; if they did not follow a normal distribution, the non-parametric Mann–Whitney U test was used. The non-parametric Pearson's chi-square test was used to check the strength of the association between qualitative variables, and, in situations in which more than 20% of the expected frequencies presented values less than 5, the Fisher's exact test was used.
To determine the independent role of CRS in the prediction of mortality, a multivariate analysis was performed with a binary logistic regression model, with the dependent (dichotomous) variable being in-hospital mortality. The estimated coefficients were expressed as odds ratio with their respective 95% confidence intervals (95% CI). Variables that had a p<0.05 in the univariate analysis were included in the multivariate analysis.
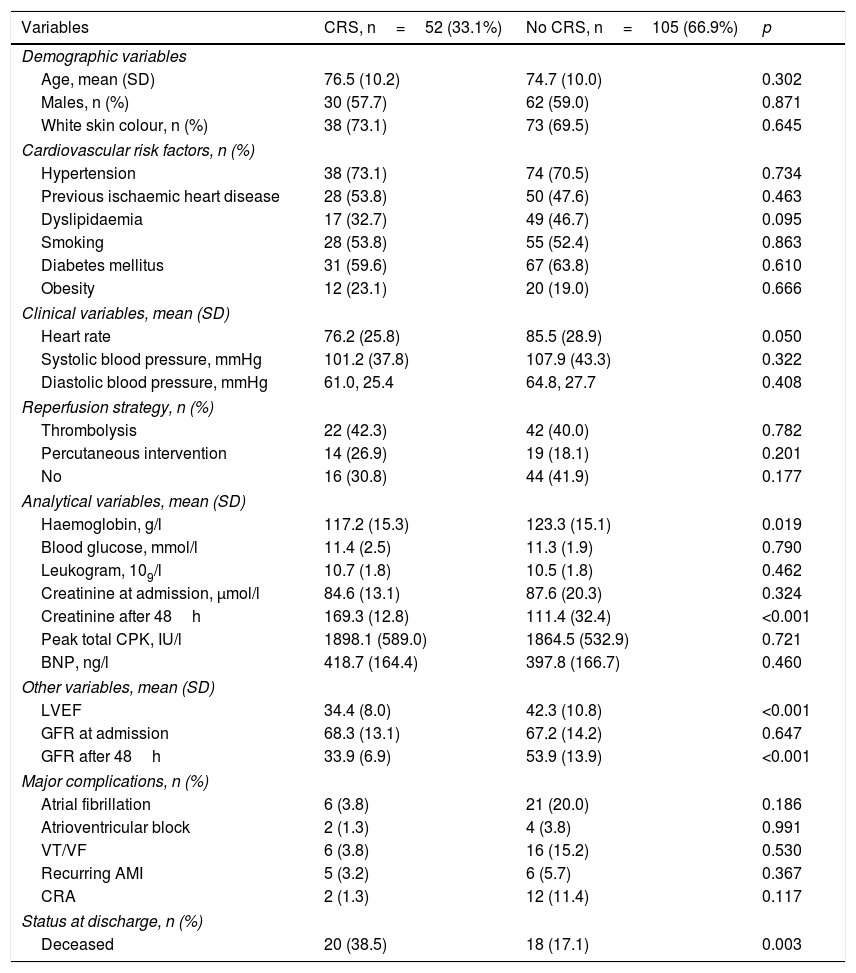
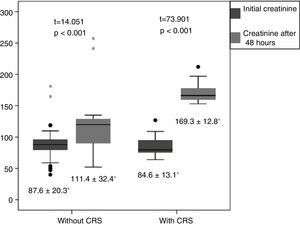
ResultsA total of 157 patients with heart failure were studied, of whom 52 (33.1%) developed CRS. The baseline characteristics of the population are shown in Table 1. Patients with CRS were more often men with a median age of 76.5±10.2. There were no differences regarding the history of diabetes mellitus, hypertension and previous ischaemic heart disease. The reperfusion strategies used were similar in both groups, as were the clinical variables. The haemoglobin level presented lower means in the group of patients with CRS (117.2±15.3 vs. 123.3±15.1; p=0.019). Creatinine and GFR deteriorated after 48h compared to their baseline value (p<0.001).
Baseline characteristics of the population in accordance with the presence or absence of cardiorenal syndrome.
| Variables | CRS, n=52 (33.1%) | No CRS, n=105 (66.9%) | p |
|---|---|---|---|
| Demographic variables | |||
| Age, mean (SD) | 76.5 (10.2) | 74.7 (10.0) | 0.302 |
| Males, n (%) | 30 (57.7) | 62 (59.0) | 0.871 |
| White skin colour, n (%) | 38 (73.1) | 73 (69.5) | 0.645 |
| Cardiovascular risk factors, n (%) | |||
| Hypertension | 38 (73.1) | 74 (70.5) | 0.734 |
| Previous ischaemic heart disease | 28 (53.8) | 50 (47.6) | 0.463 |
| Dyslipidaemia | 17 (32.7) | 49 (46.7) | 0.095 |
| Smoking | 28 (53.8) | 55 (52.4) | 0.863 |
| Diabetes mellitus | 31 (59.6) | 67 (63.8) | 0.610 |
| Obesity | 12 (23.1) | 20 (19.0) | 0.666 |
| Clinical variables, mean (SD) | |||
| Heart rate | 76.2 (25.8) | 85.5 (28.9) | 0.050 |
| Systolic blood pressure, mmHg | 101.2 (37.8) | 107.9 (43.3) | 0.322 |
| Diastolic blood pressure, mmHg | 61.0, 25.4 | 64.8, 27.7 | 0.408 |
| Reperfusion strategy, n (%) | |||
| Thrombolysis | 22 (42.3) | 42 (40.0) | 0.782 |
| Percutaneous intervention | 14 (26.9) | 19 (18.1) | 0.201 |
| No | 16 (30.8) | 44 (41.9) | 0.177 |
| Analytical variables, mean (SD) | |||
| Haemoglobin, g/l | 117.2 (15.3) | 123.3 (15.1) | 0.019 |
| Blood glucose, mmol/l | 11.4 (2.5) | 11.3 (1.9) | 0.790 |
| Leukogram, 109/l | 10.7 (1.8) | 10.5 (1.8) | 0.462 |
| Creatinine at admission, μmol/l | 84.6 (13.1) | 87.6 (20.3) | 0.324 |
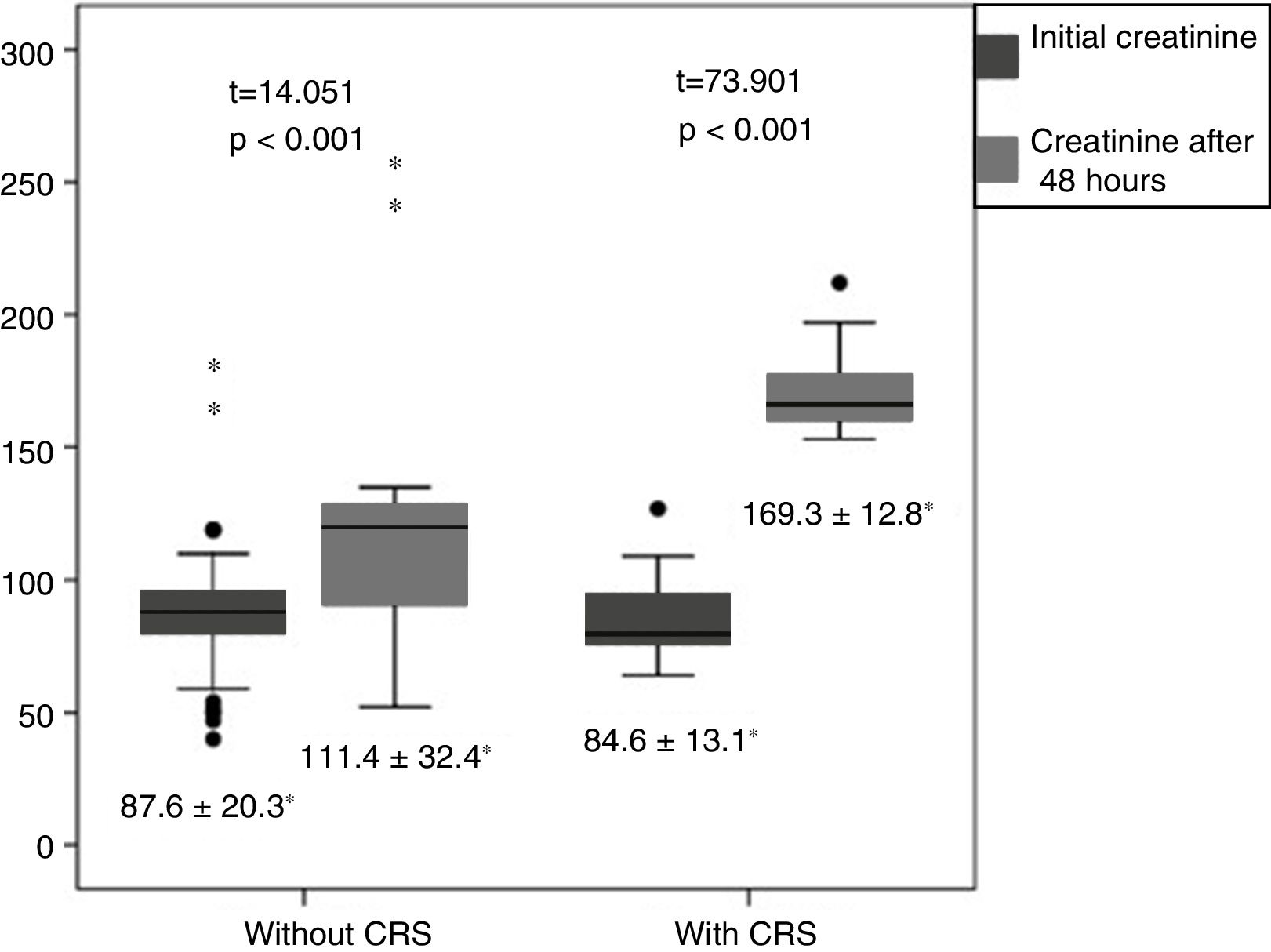
| Creatinine after 48h | 169.3 (12.8) | 111.4 (32.4) | <0.001 |
| Peak total CPK, IU/l | 1898.1 (589.0) | 1864.5 (532.9) | 0.721 |
| BNP, ng/l | 418.7 (164.4) | 397.8 (166.7) | 0.460 |
| Other variables, mean (SD) | |||
| LVEF | 34.4 (8.0) | 42.3 (10.8) | <0.001 |
| GFR at admission | 68.3 (13.1) | 67.2 (14.2) | 0.647 |
| GFR after 48h | 33.9 (6.9) | 53.9 (13.9) | <0.001 |
| Major complications, n (%) | |||
| Atrial fibrillation | 6 (3.8) | 21 (20.0) | 0.186 |
| Atrioventricular block | 2 (1.3) | 4 (3.8) | 0.991 |
| VT/VF | 6 (3.8) | 16 (15.2) | 0.530 |
| Recurring AMI | 5 (3.2) | 6 (5.7) | 0.367 |
| CRA | 2 (1.3) | 12 (11.4) | 0.117 |
| Status at discharge, n (%) | |||
| Deceased | 20 (38.5) | 18 (17.1) | 0.003 |
AMI: acute myocardial infarction; BNP: B-type natriuretic peptide; CPK: creatine phosphokinase; CRA: cardiorespiratory arrest; CRS: cardiorenal syndrome; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; SD: standard deviation; VT/VF: ventricular tachycardia and ventricular fibrillation.
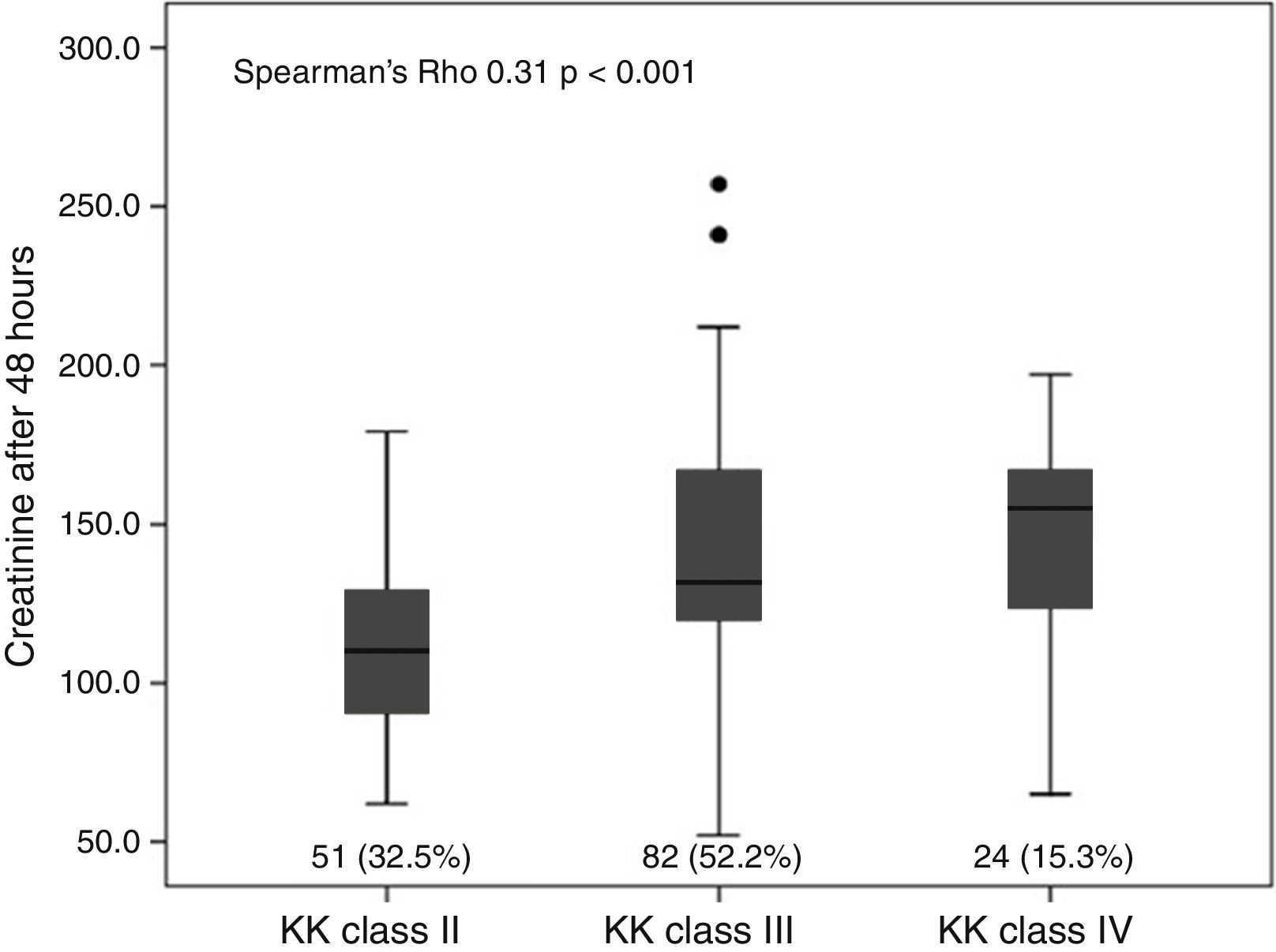
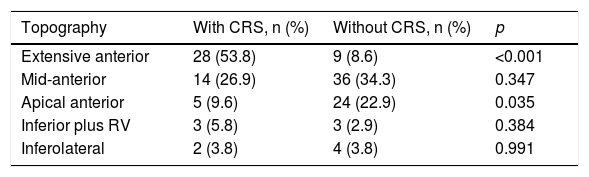
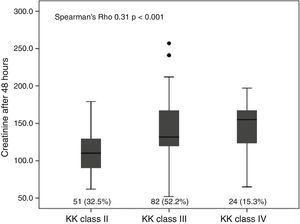
In patients with CRS, the mean LVEF was lower (34.8±8) compared to patients without CRS (43.2±10.8). There were no differences in terms of non-life-threatening complications. A positive correlation was found between the degree of heart failure (Killip and Kimball class) and the increase in serum creatinine after 48h of admission (Spearman's Rho 0.31; p<0.001), as well as with the decrease in GFR (Spearman's Rho −2.55; p=0.001) (Fig. 2). The infarction topography which was associated to the greatest extent with CRS was the extensive anterior infarction (28; 53.8%). In the inferior and lateral infarction topographies, there were no cases with KFD (Table 2).
Presence of cardiorenal syndrome in accordance with the infarction topography.
| Topography | With CRS, n (%) | Without CRS, n (%) | p |
|---|---|---|---|
| Extensive anterior | 28 (53.8) | 9 (8.6) | <0.001 |
| Mid-anterior | 14 (26.9) | 36 (34.3) | 0.347 |
| Apical anterior | 5 (9.6) | 24 (22.9) | 0.035 |
| Inferior plus RV | 3 (5.8) | 3 (2.9) | 0.384 |
| Inferolateral | 2 (3.8) | 4 (3.8) | 0.991 |
CRS: cardiorenal syndrome; RV: right ventricular.
The difference between the initial creatinine value and the value after 48h in patients with and without CRS presented significant differences which were much more evident in the first group (t=73.90 and t=14.05, respectively) in both cases with p<0.05 (Fig. 3).
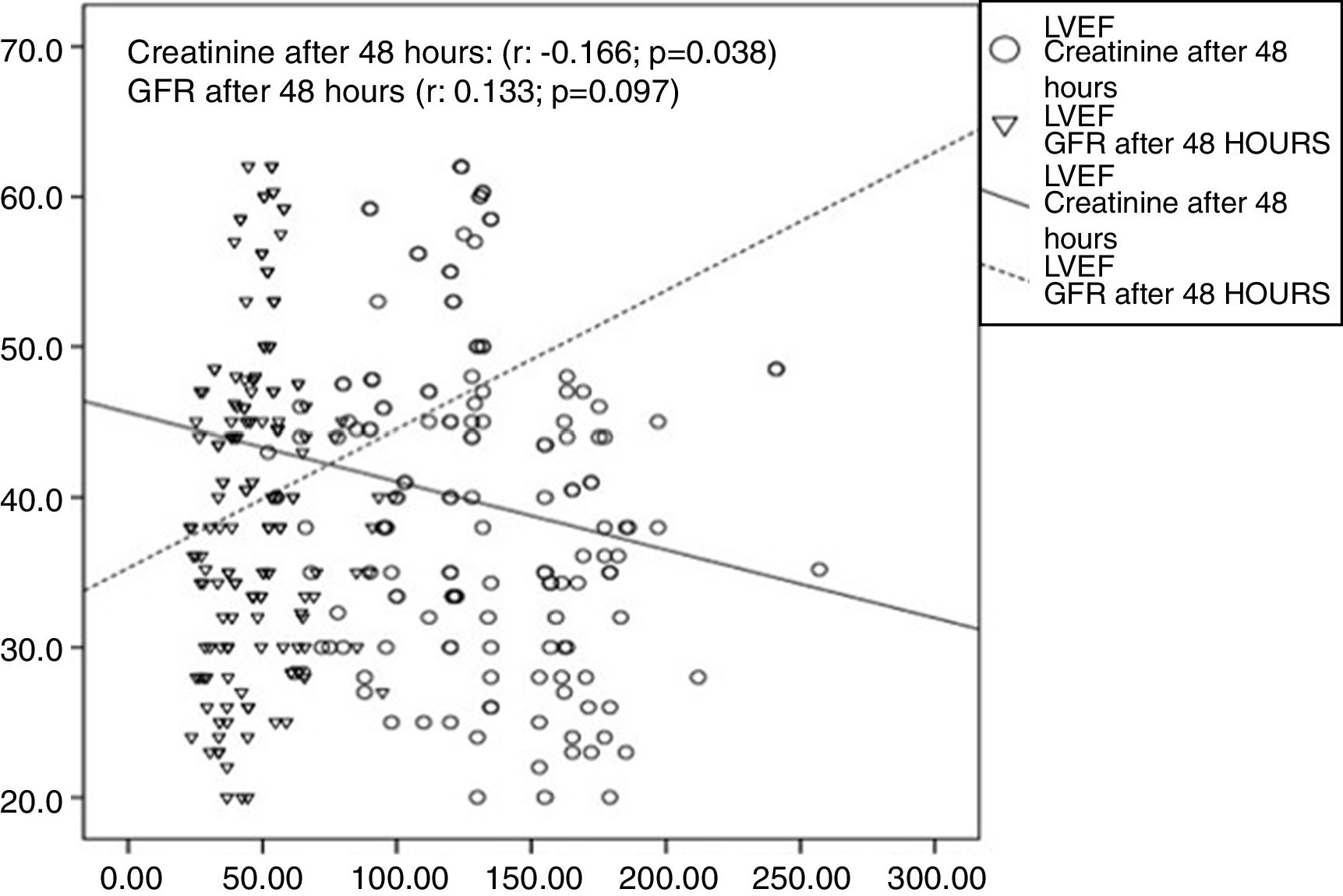
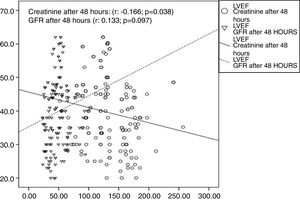
Fig. 4 shows the linear relationship of the degree of reduction of the LVEF with the increase in creatinine after 48h (r=−0.166; p=0.038), as well as with the reduction in GFR (r=0.133; p=0.097), although the latter did not show statistical significance.
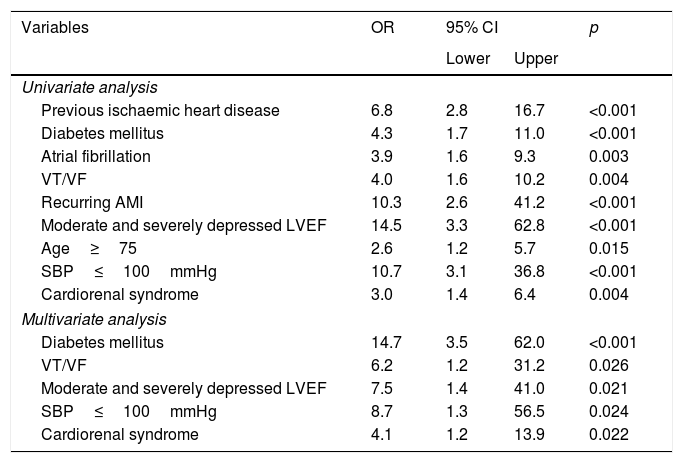
The prognosis of in-hospital mortality was significantly worse in patients with CRS. In the multivariate analysis, CRS proved to be an independent predictor of mortality (odds ratio 4.1; 95% CI 1.2–13.9; p=0.022), when adjusted for history of ischaemic heart disease, diabetes mellitus, presence of atrial fibrillation, history of tachycardia/ventricular fibrillation, LVEF, age and systolic blood pressure (Table 3).
Predictive variables of in-hospital mortality. Univariate and multivariate analysis.
| Variables | OR | 95% CI | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Univariate analysis | ||||
| Previous ischaemic heart disease | 6.8 | 2.8 | 16.7 | <0.001 |
| Diabetes mellitus | 4.3 | 1.7 | 11.0 | <0.001 |
| Atrial fibrillation | 3.9 | 1.6 | 9.3 | 0.003 |
| VT/VF | 4.0 | 1.6 | 10.2 | 0.004 |
| Recurring AMI | 10.3 | 2.6 | 41.2 | <0.001 |
| Moderate and severely depressed LVEF | 14.5 | 3.3 | 62.8 | <0.001 |
| Age≥75 | 2.6 | 1.2 | 5.7 | 0.015 |
| SBP≤100mmHg | 10.7 | 3.1 | 36.8 | <0.001 |
| Cardiorenal syndrome | 3.0 | 1.4 | 6.4 | 0.004 |
| Multivariate analysis | ||||
| Diabetes mellitus | 14.7 | 3.5 | 62.0 | <0.001 |
| VT/VF | 6.2 | 1.2 | 31.2 | 0.026 |
| Moderate and severely depressed LVEF | 7.5 | 1.4 | 41.0 | 0.021 |
| SBP≤100mmHg | 8.7 | 1.3 | 56.5 | 0.024 |
| Cardiorenal syndrome | 4.1 | 1.2 | 13.9 | 0.022 |
AMI: acute myocardial infarction; 95% CI: 95% confidence interval; LVEF: left ventricular ejection fraction; OR: odds ratio; SBP: systolic blood pressure; VT/VF: ventricular tachycardia and ventricular fibrillation.
The results found in our study reflect how KFD, assessed by the increase in baseline creatinine in accordance with the Acute Kidney Injury Network criteria,11 is associated with a greater risk of in-hospital death. The population studied showed similar baseline characteristics; however, patients with CRS showed lower haemoglobin means and LVEF. Anaemia in patients with ACS was reported in 15–43%12 and may be associated with CRS.6 One of the possible adaptive responses to the reduction in haemoglobin following ACS is left ventricular dilatation, with the consequent increase in parietal stress, which may cause myocyte necrosis and fibrosis.13
LVEF following an acute myocardial infarction has become an independent predictor of sudden death with a high predictive capacity.14 In a risk stratification model based on echocardiographic variables, LVEF proved to be an independent predictor in the multivariate analysis (hazard ratio 1.45, 95% CI 1.02–2.08; p=0.040) and the prognosis proved to be inversely proportional to the LVEF when this was below 40%.15
The anterior infarction topography was the most prevalent in our series, which is justified as patients who developed any degree of heart failure were included. CRS was presented more often in patients with an extensive anterior infarction topography which, consequently, had a greater involvement of myocardial mass. Janardhanan et al.16 observed in a large cohort that the majority of patients with ACS and heart failure at the time of admission presented with heart disease to a larger extent.
The progression of heart failure according to the Killip class was related to the increase in creatinine after 48h and provides evidence of the process of acute kidney damage established after myocardial dysfunction. Cháfer et al.,17 in their study which included 426 patients with STEMI, found that a Killip class>I was associated with a greater number of fatal episodes (10.9 vs. 3.9 per 100 patients per year of follow-up, p<0.001).
The difference between the initial creatinine value and the value after 48h in patients with and without CRS presented significant differences in both groups, and demonstrates some degree of kidney dysfunction in those who develop acute heart failure, although it does not manage to reach the cut-off values established for the diagnosis of CRS. Vavalle et al.,18 in a study carried out in 5244 patients with STEMI, demonstrated that KFD after a coronary intervention is related to baseline kidney dysfunction. Patients with GFR>90, from 60 to 90, from 30 to 59 and <30 deteriorated at 2.5, 4.1, 8.1 and 1.6%, respectively (p<0.0001). The best predictors of KFD were age and Killip class III and IV.
Furthermore, it is not always easy to establish which organ is initially responsible for overall deterioration. As it deals with patients with STEMI complicated with some degree of heart failure and with no significant differences with regard to initial GFR, presumably cardiac dysfunction has led the process. The degree of heart failure in our study was related to KFD, corresponding with the data from a multicentre study which included 947,012 patients in whom a percutaneous coronary intervention (PCI) had been performed, and revealed chronic kidney disease and cardiogenic shock as the main predictors of KFD.19
In our case study, a linear correlation was found between LVEF and creatinine after 48h. Our results are in line with those of a prospective cohort which included 604 Japanese patients with ACS, in which it was demonstrated that KFD was associated with a reduced LVEF and increased mortality.20 In another recent study which included patients treated with a primary coronary intervention, heart failure proved to be the only independent predictor of KFD.21
Our results reveal the presence of CRS as an independent predictor of in-hospital mortality. These data are in line with those of Cabrerizo-García et al.,22 who found a link between mortality and CRS (hazard ratio 3.08, 95% CI 1.13–8.40; p=0.029) and severely depressed LVEF. In another study carried out in 1260 patients with STEMI, KFD was linked to a higher number of complications during hospitalisation and after 30 days (13 vs. 1%; p<0.001), as well as a higher mortality after 5 years (28 vs. 5%; p<0.001). KFD proved to be an independent predictor of all-cause mortality (hazard ratio 6.68, 95% CI 2.1–21.6; p=0.002).23
The prevalence of CRS in our study was higher than that reported in other series,23,24 which would be justified by the inclusion of patients with Killip class greater than I. Shacham et al.,24 in a study of 842 patients with STEMI following PCI, reported 6.2% of KFD and odds ratio proved to be an independent predictor of mortality (2.64, 95% CI 1.25–5.56; p=0.01). In a meta-analysis conducted by Damman et al.,25 KFD was found in 25% of patients and was associated with higher rates of mortality and hospitalisation. A subsequent analysis, performed with the data from the Saudi Project for Assessment of Coronary Events registry, demonstrated that KFD is linked independently to an increase in-hospital mortality (odds ratio 28.02, 95% CI 13.2–60.28; p<0.0001),26 just like that reported in our series.
Our results highlight the importance of assessing kidney function at various points during hospitalisation, as kidney function deterioration can be linked to a higher number of complications. In view of the foregoing, it can be concluded that CRS in the course of a complicated STEMI with any degree of heart failure may be linked to higher in-hospital mortality, and it is more common in infarctions involving the anterior side. It would be wise to assess creatinine after 48h of admission to detect the development of CRS, which would be useful in the risk stratification and therapeutic decision-making in the course of ACS.
Limitations of the studyOne limitation of the study would be the reduced sample size and the consequent low number of patients with KFD who concomitantly showed some degree of heart failure (Killip class II–IV). Another significant limitation is that it is an observational study and the follow-up period was limited to the hospital stay.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Jiménez AE, Negrín-Valdés T, Cruz-Inerarity H, Machural-de la Torre PJ. Síndrome cardiorrenal como predictor de mortalidad intrahospitalaria en el síndrome coronario agudo con elevación del segmento ST. Clin Invest Arterioscler. 2018;30:163–169.