Disease nonalcoholic fatty liver disease (NAFLD) comprises a series of histologically similar to those induced by alcohol consumption in people with very little or no liver damage same. The importance of NAFLD is its high prevalence in our Western societies, from the point of view liver in its progressive evolution from steatosis to steatohepatitis, cirrhosis and liver cancer. During the last decade it has been observed that NAFLD leads to an increased cardiovascular risk with accelerated atherosclerosis and cardiovascular events, the leading cause of morbidity and mortality. This updated January 2016 revision consists of two parts. In this second part, the treatment of NAFLD and its influence on cardiovascular disease and drugs used in the control of cardiovascular risk factors showing a beneficial effect on the liver disease will be reviewed.
La enfermedad del hígado graso no alcohólico (EHGNA) comprende una serie de lesiones hepáticas histológicamente similares a las inducidas por el alcohol en personas con un consumo del mismo muy escaso o nulo. La importancia de la EHGNA radica en su alta prevalencia en nuestras sociedades occidentales y, desde el punto de vista hepático, en su progresiva evolución desde esteatosis a esteatohepatitis, cirrosis y cáncer de hígado. Durante la última década se ha observado que la EHGNA da lugar a un incremento del riesgo cardiovascular con aceleración de la arteriosclerosis y de los episodios cardiovasculares, principal causa de su morbimortalidad. Esta revisión actualizada a enero de 2016 consta de 2 partes. En esta segunda parte se revisarán el tratamiento de la EHGNA y su influencia sobre la enfermedad cardiovascular, así como los fármacos empleados en el control de los factores de riesgo cardiovascular que muestran un efecto beneficioso sobre esta hepatopatía.
In the first part of this review,1 the association of nonalcoholic fatty liver disease (NAFLD) with cardiovascular disease (CVD) was analysed. The second part reviews the treatment of NAFLD, its influence on CVD and the drugs used in the control of risk factors that show a beneficial effect on this liver disease.
IntroductionGiven that non-alcoholic fatty liver disease (NAFLD) comprises both progressive liver disease and the onset of cardiovascular disease (CVD), treatment objectives must seek to improve both complications. Measures taken to meet these objectives include improving the histological abnormalities of the liver with a subsequent fall in cardiovascular risk. This paper primarily focuses on tried and tested treatments to reverse the histological changes inherent in this disease, as well as their impact on treating the associated cardiovascular risk factors (CVRF) to reduce the accelerated atherosclerotic burden caused by the onset of this liver disease. In addition, the influence of those drugs that have been shown to be effective in controlling CVRF or CVD, which have also been found to have a positive effect on the clinical or histological characteristics of NAFLD, will also be examined.
Lifestyle changes to treat non-alcoholic fatty liver diseaseThe basic treatment for NAFLD is diet, exercise and weight loss. By adopting an appropriate lifestyle that includes these three components, the advance of liver disease may be slowed and cardiovascular risk is extremely likely to fall as an additional benefit.
Weight lossNumerous studies have clearly shown how even modest weight loss improves insulin resistance (IR), transaminase levels (ALT), fat infiltration and inflammatory phenomena.2–5 In morbidly obese patients who followed extremely low calorie diets, the amount of liver fat, as measured by magnetic resonance imaging, fell by up to 40% after losing 9kg in 6 weeks.6–8 Other studies achieved similar results by reducing daily intake to 500kcal for 6 months.7,9 From a histological point of view, it has been conclusively proven that weight loss improves steatosis, inflammation, ballooning and the histological severity of the disease, as assessed by the NAFLD activity score (NAS).10 However, whether or not it improves fibrosis remains open to debate.11,12
A meta-analysis of weight loss studies has shown that weight loss of 7% or more improves steatosis, ballooning and inflammation, but not fibrosis. However, this meta-analysis found that less than 50% of patients achieved weight loss of more than 7%, and that losing between 5% and 7% of body weight improves liver fat content.13 Another meta-analysis, which investigated the effects of lifestyle change in obese patients, showed that only 3.5% weight loss was achieved on average over a 2–3 period, which is below the optimum figure required to achieve histological change.14 Two recent studies that assessed the histological impact of weight loss found that the greater the weight loss, the greater the benefit, both in terms of the NAS and non-alcoholic steatohepatitis (NASH). Fibrosis also improved with weight loss in excess of 10%, regardless of the patient's starting weight.15,16 Although the rs738409[G]/I148 M polymorphism of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) causes an increased risk of developing steatosis, the response of carriers of this polymorphism is three times greater than the general population in terms of reduced intrahepatic fat content after lifestyle change.17 One evidence-based review concluded that dietary recommendations for patients with non-alcoholic fatty liver disease should include a reduction of 600–800 calories per day or a calorie intake limited to 25–30kcal/kg/day of ideal body weight.18 The combined Practice Guideline by the American Association for the Study of Liver Diseases, the American College of Gastroenterology, and the American Gastroenterological Association (AASLD/AGA/ACG) recommends weight loss of at least 3–5% to improve steatosis, and more than 10% to have an impact on necrosis and inflammation.19
In terms of cardiovascular effects, a meta-analysis13 confirmed that weight loss largely reduces cardiovascular risk because it improves the obesity-related risk factors deriving from glucose and lipid metabolism. It should be noted that the influence of weight loss on cardiovascular prognosis in patients with ischaemic heart disease was only confirmed after a meta-analysis, which showed that intentional weight loss improved survival and reduced the risk of cardiovascular events by 33%. Unintentional weight loss, on the other hand, led to an increase in cardiovascular events.20
DietThe optimum diet to treat NAFLD has yet to be definitively established.8 Nevertheless, it is believed that a low-fat diet reduces hepatic lipid deposition,21 although a meta-analysis of three small controlled and randomised clinical trials that compared the effects of calorie-control with a low-carbohydrate diet versus a low-fat diet concluded that both diets yielded similar weight loss, similar liver fat content, as assessed by spectroscopic magnetic resonance imaging, and similar ALT serum levels, as well as similar improvements in insulin sensitivity as estimated by the Homeostasis Model Assessment (HOMA), plasma triglycerides and adiponectin levels. However, the low-carbohydrate diet led to a greater reduction in waist circumference and blood glucose compared to a low-fat diet, which more consistently improved the lipid profile.13 A recent review concluded that data on the effect of macronutrients in NAFLD are scarce, particularly in human models. A higher consumption of simple carbohydrates, sugary drinks and saturated fatty acids may exacerbate NAFLD and cause increased fat accumulation in the liver, while a higher intake of fibre and low-glycaemic index carbohydrates, monounsaturated fatty acids, omega-3, soya and whey protein tend to improve NASH. Furthermore, it is believed that a low-carbohydrate, low-fat and high-protein diet is of benefit to NAFLD patients.22 Whatever the diet, it seems that replacing saturated fat with polyunsaturated and monounsaturated fats improves both cardiovascular risk and NAFLD.23
Mediterranean dietThe Mediterranean diet deserves special mention for its beneficial effects on both the liver and CVD. Notwithstanding the fact that adhering to a diet of this type prevents or improves metabolic syndrome (MetS),24 its impact on reducing NAFLD severity has also been demonstrated. In this regard, a case–control study found that good adherence to the Mediterranean diet is associated with lower insulin resistance, lower ALT levels and a reduced risk of developing steatohepatitis.25 A study of NAFLD patients diagnosed by biopsy compared the Mediterranean diet with another high-carbohydrate, low-fat diet, and found that the Mediterranean diet significantly reduced steatosis as measured by MRI (39% versus 7%), despite no difference in weight loss between the two groups. There were also improvements to IR, as measured by HOMA, as well as blood pressure. This study suggests that the Mediterranean diet may benefit NAFLD patients to a much greater extent than just dyslipidaemia management.26
In terms of its cardiovascular effects, the Mediterranean diet has been shown to be useful in both primary prevention (PREDIMED study)27 and secondary prevention (LYON study).28 A systematic review confirmed that adherence to this diet reduces cardiovascular morbidity and mortality by 38%. According to the scoring system used, a two-point increase in adherence to the Mediterranean diet was associated with a 13% reduction in CVD risk.29
FructoseFructose, which is widely used as a sweetener in soft drinks, has been independently associated with the risk of onset of NAFLD and its severity in both population studies and a randomised trial.13 However, a meta-analysis of fructose studies found no direct correlation between fructose intake and NAFLD, apart from in terms of excess calorie intake that its consumption may involve. The meta-analysis also found that fructose intake contributed to increased intrahepatic fat, as measured by magnetic resonance imaging, as well as to higher transaminase levels.30
AlcoholData concerning whether or not alcohol may be consumed in NAFLD are conflicting. While some argue that alcohol consumption in small amounts may protect against development of the disease,31,32 others advise against its consumption once the disease has been diagnosed.33,34 A Japanese 10-year follow-up study found that 40–280g of alcohol per week protect from steatosis.35 Another study showed that moderate alcohol consumption in NAFLD reduces the likelihood of developing carotid plaques or stenosis.36 A recent meta-analysis of more than 40,000 subjects found that moderate alcohol consumption (<40g/day) has a protective effect on NAFLD irrespective of the body mass index (BMI), reducing its prevalence by 31% and with greater benefit to women (53% reduction) than to men (30%). An extremely surprising finding was that NASH progression fell by almost 50% with alcohol consumption.37 The AASLD/AGA/ACG guideline recommends a maximum alcohol consumption of four drinks per day for men and two for women.19 Nevertheless, recent data from large prospective cohort studies suggest that the combined effects of alcohol and obesity may synergistically increase the risk of liver damage, liver disease-related death and the onset of liver cancer. As such, this is an area that requires more clinical research.38–40
In terms of cardiovascular effects, very moderate consumption may be more beneficial than abstinence. In this regard, daily consumption of 1–2 drinks was found to reduce cardiovascular mortality by 25%, myocardial infarction by 29%, coronary mortality by 25% and total mortality by 13%.41
ExerciseWe will first consider how exercise may prevent NAFLD, and then how it may play a part in its treatment.
Exercise and NAFLD preventionA number of epidemiological studies have confirmed an inverse correlation between NAFLD and physical activity.42 The National Health and Nutrition Examination Survey (NHANES) 2003–200643 showed that NAFLD patients tend to be less physically active, both in terms of time invested and actual activity as measured by an accelerometer. In another cross-sectional study of 72,359 healthy Korean adults, those who exercised regularly had a 28–53% lower risk of NAFLD across almost all the BMI deciles.44 Numerous studies have found an inverse correlation between different types of physical activity and NAFLD prevalence, proportional to exercise intensity and irrespective of visceral obesity, IR and MetS.45,46 Doing regular exercise in one's free time prevents the onset of steatosis fundamentally by reducing abdominal fat, with anaerobic exercise appearing to have a more pronounced effect.47 Daily individual lifestyle has a significant impact on fat accumulation in the liver. It has been proven that sitting for a long time is a risk factor for NAFLD, regardless of total daily physical activity.48 Time spent watching television is also associated with NAFLD, irrespective of other parameters such as exercise, although this appears to be attenuated if BMI is taken into account.49
Exercise and benefits to the liver in non-alcoholic fatty liver diseaseA significant number of research studies have shown that exercise reduces liver fat and potentially transaminase levels in patients with NAFLD, with or without weight loss. One study found that three 30–45-min weekly sessions on a bike led to a significant fall in liver triglyceride levels and visceral adipose tissue in patients with NAFLD, with no changes to body weight.50 Similarly, doing 30–60min of exercise five times a week for 16 weeks reduced liver fat by 10% without changes to body weight, but had no effect on liver triglyceride secretion, unless the patient also experienced weight loss.51 In another study, 60min of aerobic exercise a week was found to reduce liver triglyceride content and ALT levels in NAFLD patients.52 A meta-analysis of controlled trials – excluding exercise and diet versus diet alone – concluded that exercise reduces liver fat content by 37%, but in no case improves transaminase levels. This benefit was still seen with minimal or no weight loss and with an exercise level below the current recommendations for obesity treatment.53 The AASLD/AGA/ACG guideline suggests that in adults with NAFLD, exercise may only reduce hepatic steatosis, while its capacity to improve other aspects of liver histology remains unknown.19 The effect of exercise on falling liver triglyceride levels is supported by the first randomised and controlled study to investigate these aspects, which found that any type of aerobic exercise, regardless of frequency, intensity or duration, reduced liver fat without significant weight loss.54 Although scarce data are available on histological improvements as a result of exercise, a systematic review found a trend towards reduced inflammation, while its effect on fibrosis was inconclusive.8 In NAFLD diagnosed by biopsy, it would appear that only intense exercise reduces the likelihood of developing NASH by 35% and fibrosis by 47%, with mild or moderate exercise having no preventive effect.55 Resistance training also improves steatosis, as measured by MRI, regardless of weight loss.56 A randomised and controlled study also found that 3 months of resistance training improved fat content as quantified by the hepatorenal-ultrasound index.57 However, it has yet to be established whether aerobic exercise or resistance training offers greater benefits. One study found that both forms of exercise reduced liver fat in NAFLD patients in equal measure.58
All of the studies referred to above suggest that doctors should actively recommend regular exercise to their NAFLD patients. This is particularly applicable to non-obese patients, in whom it is not feasible to prescribe slimming diets.59 Although there is still no clear consensus on the frequency, duration or intensity of exercise, or indeed on the combination of anaerobic exercise with resistance training or the need to follow a calorie-controlled diet,60 overall lifestyle change should encompass these elements. When faced with the question of how much exercise to recommend, it is probably safe to say as much as possible and the more the better. Some experts recommend 150–300min of exercise per week spread over at least 3 days.61 A recent review extols the hepatic physiopathological benefits of exercise.62
In terms of cardiometabolic effects, there is substantial evidence to suggest that exercise improves vascular and metabolic comorbidity associated with NASH, including IR, dyslipidaemia, inflammation, hypertension63 and endothelial dysfunction,64 thereby reducing the risk of coronary, cardiovascular and hepatic morbidity and mortality.65 A dose-response relationship concerning these preventive effects of exercise has been identified.66 In fact, an improvement of one metabolic equivalent in cardiorespiratory fitness (3.5ml/kg/min) was associated with a 13% reduction in all-cause mortality and a 15% fall in the risk of cardiovascular events, respectively.66,67 Conversely, time spent sitting is correlated to total mortality.68
Bariatric surgeryNon-randomised studies have shown that bariatric surgery improves the components of metabolic syndrome (MetS), with a drastic reduction in hepatic steatosis, as well as reduced ballooning and inflammation that may manifest with NAFLD, while its effect on fibrosis remains unclear.69,70 Nevertheless, steatohepatitis and fibrosis may be exacerbated if the surgery leads to excessively rapid weight loss of more than 1.6kg per week.71 A meta-analysis of 15 studies that investigated the influence of bariatric surgery on NAFLD found improved steatosis, steatohepatitis and fibrosis, as well as a fall in BMI.72 A review conducted by Cochrane on bariatric surgery as a treatment for NASH did not identify any randomised clinical trials or quasi-randomised controlled trials but examined 21 cohort studies. Although consistent improvements in steatosis and inflammation scores were found, four studies reported fibrosis deterioration 5 years after surgery.73 The American guidelines19 specify that: (1) bariatric surgery is not contraindicated in obese subjects with NAFLD and NASH with no established cirrhosis; (2) the safest, most effective and best form of bariatric surgery in NASH-induced cirrhosis has not yet been established, and (3) it is premature to consider surgery as a specifically established option in the treatment of NASH. Another more recent systematic review identified significant improvements in steatosis (50%), ballooning (68%), inflammation (50%), fibrosis (12%) and a fall in liver enzyme levels: ALT (11U/l), AST (4U/l), GGT (18U/l). Concerning point 2 of the aforementioned guidelines, surgery does not appear to exacerbate cirrhosis. As with the reviews cited above, the high heterogeneity between the studies must be taken into account as this prevents definitive conclusions from being drawn.74 In terms of type of surgery, the Roux-en-Y gastric bypass seems to be more effective than the gastric band in achieving a better NAFLD activity score as it improves all NAFLD-related parameters.75 Although the most influential factor was greater weight loss, this does not fully explain the benefits achieved.
In terms of cardiovascular effects, the metabolic changes resulting from bariatric surgery that may lead to reduced cardiovascular risk are multiple and complex. IR tends to improve instantly, and, if it fails to do so, NAFLD usually persists.76 Bariatric surgery results in diabetes remission in 80% of patients,77 a 65% improvement in dyslipidaemia—defined as HDL cholesterol <40mg/dl, or LDL cholesterol >160mg/dl, or TG >200mg/dl—,78 and a 25% fall in oxidised LDLs.79 Blood pressure has been reported to drop by up to 38%, with an 8mmHg fall in systolic blood pressure and a 5mmHg fall in diastolic blood pressure. However, other authors have not found any differences in blood pressure before and after surgery, leading some to conclude that the role of bariatric surgery on hypertension has yet to be determined.80 On the other hand, it has been found to improve left ventricular hypertrophy and diastolic dysfunction.81 A meta-analysis on observational studies showed that weight loss surgery reduces total mortality by 50%, CVD by 46%, myocardial infarction by 54% and stroke by 51%.82 Another 10-year follow-up case–control study of more than 800 patients found that gastric bypass surgery reduces mortality by 52%.83 The pathophysiological explanation for the beneficial effects of bariatric surgery on the cardiovascular system was examined in a recent publication.80
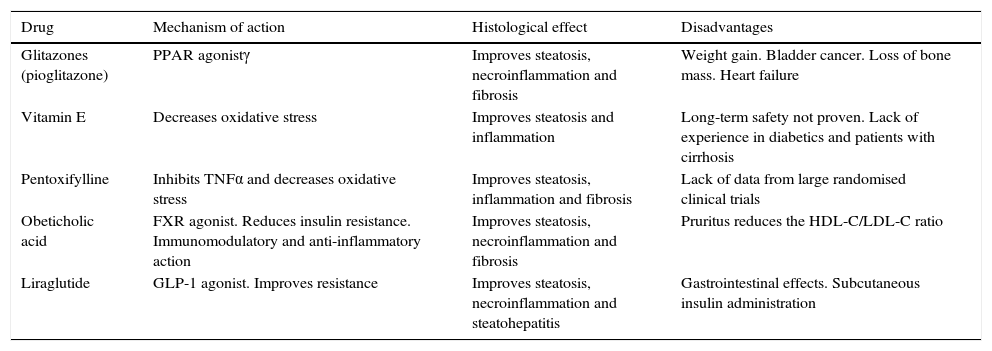
Pharmacological treatment of non-alcoholic fatty liver diseaseA multitude of drugs to treat NAFLD that act on different levels of the sequence of pathophysiological pathways, ranging from simple steatosis to advanced disease, have been investigated in the last 10 years. However, none of which to date has been found to completely reverse liver damage or to reduce associated morbidity and mortality. This review shall present the results of randomised clinical trials assessing the effects of five drugs on hepatic histology in NAFLD: thiazolidinediones, vitamin E, pentoxifylline, obeticholic acid and liraglutide (Table 1). It will also review a small group of drugs administered to control CVRFs, which in turn have a beneficial effect on NAFLD.
Primary drugs that histologically improve non-alcoholic fatty liver disease.
| Drug | Mechanism of action | Histological effect | Disadvantages |
|---|---|---|---|
| Glitazones (pioglitazone) | PPAR agonistγ | Improves steatosis, necroinflammation and fibrosis | Weight gain. Bladder cancer. Loss of bone mass. Heart failure |
| Vitamin E | Decreases oxidative stress | Improves steatosis and inflammation | Long-term safety not proven. Lack of experience in diabetics and patients with cirrhosis |
| Pentoxifylline | Inhibits TNFα and decreases oxidative stress | Improves steatosis, inflammation and fibrosis | Lack of data from large randomised clinical trials |
| Obeticholic acid | FXR agonist. Reduces insulin resistance. Immunomodulatory and anti-inflammatory action | Improves steatosis, necroinflammation and fibrosis | Pruritus reduces the HDL-C/LDL-C ratio |
| Liraglutide | GLP-1 agonist. Improves resistance | Improves steatosis, necroinflammation and steatohepatitis | Gastrointestinal effects. Subcutaneous insulin administration |
FXR: farnesoid X nuclear receptor; GLP-1: glucagon-like peptide 1; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; PPARγ: peroxisome proliferator-activated receptor gamma; TNFα: tumour necrosis factor-α.
They act as peroxisome proliferator-activated receptor gamma (PPARγ) agonists to improve insulin sensitivity, increase the peripheral uptake and the oxidation of free fatty acids (FFA) and decrease their intrahepatic synthesis. They also increase adiponectin levels. An analytical and histological improvement was found in short-term studies with thiazolidinediones,84,85 particularly with pioglitazone, which may even improve fibrosis.86,87 In a 1-year follow-up study, rosiglitazone reduced ALT levels and steatosis, but had no effect on inflammation or fibrosis.88 Extending the treatment to 2 years did not lead to a greater improvement than that seen in the first year in the majority of patients in this series.89 The largest meta-analysis of studies assessing the effects of thiazolidinediones in patients with NAFLD incorporated 11 randomised trials and 862 participants (38% diabetic). Findings included an improvement in steatosis, hepatocyte ballooning and necroinflammation, as well as delayed liver fibrosis progression. The insulin resistance of adipose and muscle tissue also improved.14 Two further meta-analyses corroborate these results90 and also found that pioglitazone leads to an improvement in fibrosis.91 The effects of thiazolidinediones tend to subside shortly after they are withdrawn.92 The AASLD/AGA/ACG guideline only permits pioglitazone administration to treat patients with biopsy-proven steatohepatitis. Nevertheless, the guideline also expresses concerns about its long-term safety and efficacy due to the increased risk of coronary events with rosiglitazone and pioglitazone's association with bladder cancer, loss of bone mass, weight gain, painful swelling of the legs and congestive heart failure.19 Pioglitazone reduces arteriosclerosis progression, as measured both by changes to the carotid artery intima-media thickness (IMT),92 as well as by analysis of the atheromatous plaque by intravascular ultrasound of the coronary arteries.93 The post hoc analyses of the Prospective pioglitazone clinical trial in macrovascular events (PROACTIVE) found the cardiovascular preventive effect of pioglitazone to be most pronounced in secondary prevention.94 This trial found that patients treated with pioglitazone who had previously had a myocardial infarction had a 28% lower risk of infarction, a 39% lower risk of acute coronary syndrome (ACS), a 19% fall in the combined objective comprising infarction, coronary revascularisation, ACS and cardiac death, as well as a 47% reduction in the risk of suffering a stroke.95 It was also found that pioglitazone reduced the likelihood of experiencing another cerebrovascular accident by 47% in patients who had already suffered a stroke.96 Furthermore, pioglitazone reduced the likelihood of death, infarction and stroke by 16% in high-risk diabetic patients.94 Finally, a meta-analysis of 19 randomised controlled trials confirmed that patients treated with pioglitazone were at a significantly lower risk of death, infarction and stroke.97 In addition, the publication on the 6-year follow-up of the patients enrolled in the PROACTIVE study reported that pioglitazone reduced the number of cardiovascular events compared to patients who did not receive the drug, irrespective of duration of treatment.98
Vitamin EAs an antioxidant, a series of preliminary in vitro and in vivo studies indicated that vitamin E (α-tocopherol) may be useful in improving certain aspects of NAFLD. The following were the two most significant clinical trials. In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) study, which recruited 247 biopsied patients with NASH, it was shown that a dose of 800IU/day improves ALT levels, steatosis and inflammation, but not fibrosis.99 The Treatment of NAFLD in Children (TONIC) study found that only ballooning reduced in children treated with 800IU/day of vitamin E versus placebo, with no improvements in other histological parameters or transaminase levels. However, the NAFLD Activity Score (NAS) improved, as did the percentage of resolved NASH cases.100 Given that numerous meta-analyses and systematic reviews have found that administration of vitamin E increases total mortality (39 deaths per 10,000 patients who take 400IU/day13,101,102), raises the risk of haemorrhagic stroke103 and increases the likelihood of developing prostate cancer in patients over the age of 50,104 some authors advise against its use. Despite this, the AASLD/AGA/ACG guideline argues that 800IU/day of vitamin E improves hepatic histology in non-diabetic adults with steatohepatitis diagnosed by biopsy, and as such it should be considered a first-line pharmacological treatment for this patient population. Having said that, pending new data, its use is advised against for the treatment of NASH in diabetic patients, NAFLD without a liver biopsy, cirrhosis or cryptogenic NASH or cirrhosis.19 A recent meta-analysis of five studies and 401 patients indicated that administering 400IU/day of vitamin E improves NASH histology (steatosis, inflammation and ballooning) and fibrosis, while also improving transaminase and alkaline phosphatase concentrations. The authors of the meta-analysis observed that these findings should improve the grading quality of evidence currently laid out by the guidelines.105 Another smaller meta-analysis of three articles produced similar results.106
In terms of the cardiovascular system, vitamin E offers no preventive or therapeutic effect.107,108
PentoxifyllinePentoxifylline inhibits multiple pro-inflammatory cytokines, such as tumour necrosis factor-α (TNFα), and acts as an antioxidant, limiting the production of oxygen free radicals and increasing liver glutathione synthesis.109 The in vitro studies conducted also suggest that pentoxifylline has an antifibrogenic effect on hepatic stellate cells activated by the degradation of extracellular collagen, while also reducing fibrogenic cytokine levels.109,110 However, evidence supporting the use of pentoxifylline is only of moderate quality. A meta-analysis on five small randomised, double-blind and placebo-controlled studies of 145 patients with NAFLD found that pentoxifylline improves weight, transaminase and TNFα levels, the NAS and lobular inflammation, but has no effect on ballooning or fibrosis.111 Another meta-analysis of these same studies confirmed most of these results, finding that pentoxifylline may reduce transaminase activity and the NAS while improving certain histological parameters—steatosis, inflammation and fibrosis. It was also found to reduce BMI and baseline blood glucose, but not TNFα levels. Further well-designed, randomised placebo-controlled studies with a significant sample size should be conducted to confirm these results.112
In terms of its application in the cardiovascular setting, a meta-analysis of six studies with 221 patients with a systolic ejection fraction below 40% found that mortality due to heart failure reduced by 71% in the cohort of patients that took 1200mg/day of pentoxifylline for 6 months.113 A Cochrane review114 concluded that a lack of data prevents any definitive conclusion being reached on whether or not pentoxifylline improves intermittent claudication, which is the primary cardiovascular treatment objective for which this drug is prescribed in Spain.
Obeticholic acidThis is a derivative of chenodeoxycholic acid, a natural FXR agonist, which is a farnesoid X nuclear receptor that regulates glucose and lipid metabolism. In animal models, obeticholic acid (OCA) reduces gluconeogenesis, glycogenolysis and IR,115,116 controls lipid homeostasis and leads to a fall in hepatic steatosis by reducing de novo lipogenesis.117,118 It has been shown that OCA possesses immunomodulatory and anti-inflammatory properties, inhibiting nuclear factor κβ (NF-κβ).119 In an initial phase 2, double-blind, controlled trial, 25 and 50mg/day of OCA was administered to patients with type 2 diabetes and NAFLD for 6 weeks. The drug was well tolerated, insulin sensitivity increased while liver enzyme levels, inflammatory markers and fibrosis all fell.120 A phase IIb multicentre, randomised, double-blind study sponsored by the US administration (FLINT),121 which treated 147 NASH patients with OCA for 72 weeks versus 142 patients treated with placebo, has recently been conducted. Although the study was suspended prematurely, an interim analysis planned in advance found that more patients in the OCA treatment arm achieved the primary objective of a two-point histological improvement on the NAFLD activity score without worsening of fibrosis (45% versus 21%; relative risk: 1.9; 95% CI: 1.3–2.8). Treatment with OCA doubled the likelihood of histological improvement – steatosis, ballooning and inflammation –, as well as improved fibrosis. In terms of its side effects, OCA led to a fall in the HDL/LDL cholesterol ratio and the onset of pruritus in more than 20% of patients. Although these resolved upon withdrawal of the drug, its discontinuation also led to increased transaminase levels. Given that it was a single study, other larger and longer studies are required to confirm its long-term benefits and safety. This is particularly pertinent given that the FLINT study found that OCA improves the NAS more than histological parameters, with a significant non-responder rate, and that its effect is severely curtailed in non-diabetic patients.122–125
No data are available concerning the effect of OCA on cardiovascular risk.
LiraglutideIncretins, such as glucagon-like peptide 1 (GLP-1), are hormones released by the gastrointestinal tract after meals in response to the activation of the G protein-coupled receptor by bile acids (TGR5). GLP-1 improves insulin sensitivity, promotes fatty acid oxidation and inhibits the release of fibroblast growth factor 21, which is secreted by the liver to regulate the glucose and lipid metabolism.126 Secretion of GLP-1 and hepatic GLP-1 receptors falls in NAFLD and NASH patients, while serum activity and the hepatic expression of dipeptidyl peptidase-4 (DPP-4) increases in NAFLD.127 Liraglutide is a GLP-1 receptor agonist indicated for the treatment of type 2 diabetes and obesity. In experimental trials, liraglutide was found to reduce liver fat content and the inflammatory component of steatohepatitis.128,129
In human studies, a meta-analysis of the LEAD programme, which investigated the effect of liraglutide on 4422 diabetic patients administered 1.8mg/week for 26 weeks, found that this drug improves transaminase levels, liver fat content, as measured by computed axial tomography, and the NAS.130 These findings led to the conduct of a prospective, randomised, placebo-controlled study (LEAN)131 that recruited 52 patients with biopsy-diagnosed NASH, 30% of which were diabetic, who were administered 1.8mg of liraglutide per week for 48 weeks. The drug achieved significant histological resolution of NASH with reduced steatosis and ballooning (39% in the liraglutide group versus 9% in the control group; p=0.019). Improved fibrosis was also observed, although this was not statistically significant. A LEAN substudy,132 which adopted a sophisticated methodology, demonstrated that liraglutide's action in NAFLD is based on: the reduction of de novo hepatic triglyceride synthesis; improved insulin action with reduced adipose tissue lipolysis irrespective of weight loss; reduced adipocytokine levels (leptin, resistin and monocyte chemoattractant protein-1 [MCP1]); increased adiponectin concentrations, which could explain the improvement in NASH and reduced fibrosis due to a reduction in proinflammatory factors; and finally, the fall in hepatic glucose production during fasting and fasting hyperglycaemia by improving insulin sensitivity. Liraglutide's gastrointestinal side effects, particularly nausea, and its subcutaneous route of administration, constitute the drug's main drawbacks.
In terms of its cardiometabolic effects, as well as improving glycaemic control and inducing weight loss, liraglutide may reduce LDL cholesterol and triglyceride concentrations,133 in addition to waist circumference and the percentage of patients with metabolic syndrome.134 Although its clinical significance is unknown, it has been reported that liraglutide may increase heart rate.135 This is being investigated in the trial entitled Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results—A Long-Term Evaluation (LEADER),136 which was completed in December 2015 and the results of which are due to be published.
Effect of cardiovascular preventive drugs on non-alcoholic fatty liver diseaseThis section assesses the few drugs that, as well as having demonstrated reduced cardiovascular risk or CVRF control, exert some beneficial effect on the clinical or histological characteristics of NAFLD.
Lipid-lowering drugsStatinsHMG-CoA reductase inhibitors play a well-established role in both dyslipidaemia treatment136 and cardiovascular disease prevention by reducing total and cardiovascular morbidity and mortality.137,138
Despite potential hepatotoxicity, it has been known for some time that statins pose no hepatic risk to NAFLD patients, even with discretely elevated baseline transaminases.139,140
In terms of hepatic benefits, statins may reduce transaminase and TNFα concentrations in patients with NAFLD, as well as the level of radiologically-determined steatosis.141 They also improve the histological degree of inflammation, but not fibrosis.142 These effects are irrespective of their lipid-lowering characteristics. Two meta-analyses found no evidence supporting the efficacy of statins in improving “hard” hepatic histological endpoints,143,144 although the findings of a recent study suggest that statins may improve NASH and fibrosis.145 Statins have been shown to reduce the likelihood of developing hepatocellular carcinoma in various observational studies and in two meta-analyses of observational studies.146,147 Given that the design of the studies and the origins of the populations are heterogeneous, more specific studies are required to be able to extrapolate these results.
In terms of cardiovascular effects, a post hoc analysis of the GREACE study148 not only showed that statins are safe in subjects with abnormal liver function, presumably due to NAFLD, but also that patients with steatosis who received atorvastatin at a mean dose of 24mg/day for 3 years experienced a significant reduction in cardiovascular events versus those who were not administered the drug (10% versus 30%; p<0.0001), as well as a 36% fall in transaminase levels. The study found that statins were more effective in patients with NAFLD than in patients without this condition. Subjects with ischaemic heart disease, suspected NAFLD and elevated ALT levels experienced a 39% fall in cardiovascular events compared to those with normal transaminase levels. Just five patients needed to be treated to identify this benefit. The post hoc analysis of a further two studies—Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT)149 and Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL)150—which administered various doses of atorvastatin in primary and secondary prevention, respectively, demonstrated improved transaminase levels in patients with elevated baseline transaminases. The ATTEMPT study also identified steatosis resolution as determined by ultrasound, while in the IDEAL study, the administration of 80mg/day of atorvastatin versus 20–40mg/day of simvastatin to patients with elevated ALT, probably caused by NAFLD, significantly reduced the rate of cardiovascular events by 44%. In light of the above, it would appear that moderately abnormal transaminase levels not only improve when higher statin doses are administered, but they could also be a useful marker for identifying subjects at particularly high cardiovascular risk who specifically need aggressive pharmaceutical treatment151.
FibratesFibrates act on the PPARα, regulating the intrahepatic metabolism of lipids, reducing IR and improving the lipid profile. Despite this, neither clofibrate nor fenofibrate were found to improve NAFLD-related analytical or histological abnormalities in preliminary studies.152 However, a prospective randomised study in non-diabetic patients with MetS and NAFLD, established due to the combination of elevated ALT levels and ultrasonographic evidence, found that 42% of patients treated with fenofibrate and 70% with the combination fenofibrate plus atorvastatin no longer presented these two NAFLD markers at the end of the trial.153 A meta-analysis of 145 patients13 found that fibrates do not improve transaminase levels, steatosis or histological parameters. However, as shown by two meta-analyses of trials that investigated these drugs,154,155 it must be remembered that fibrates reduce cardiovascular morbidity and mortality in patients with atherogenic dyslipidaemia, the most typical lipid abnormality associated with NAFLD, characterised by hypertriglyceridaemia and low HDL cholesterol. Because of its inferior myopathic potential, fenofibrate may be prescribed concomitantly with statins to improve atherogenic dyslipidaemia, especially in patients with metabolic syndrome or type 2 diabetes.156,157 Collectively, although it has not been proven that fibrates improve the histology of NAFLD, they are a safe and effective treatment for patients with dyslipidaemia and hepatic steatosis.158
EzetimibeEzetimibe reduces plasma cholesterol by inhibiting its intestinal absorption by means of the NPC1L1 receptor. It has been shown to reduce cardiovascular morbidity in secondary prevention in combination with statins.159
In terms of hepatic physiopathology, the presence of free cholesterol influences progression from simple steatosis to NASH. Ezetimibe blocks the NPC1L1 receptor in the liver, thereby improving insulin sensitivity and intrahepatic fat in obese mice.160 Two in-human studies suggest that ezetimibe leads to an improvement in liver enzymes. Although one did not find relevant ultrasonographic changes,161 the other reported an improvement in inflammatory phenomena.162 The addition of ezetimibe to a weight-loss diet reduces liver fat content, as determined by magnetic resonance spectroscopy, by 18%.163 Data concerning hepatic histology are conflicting. One study164 that investigated the administration of ezetimibe 10mg/day for 24 months to 45 patients with biopsy-diagnosed NASH found that IR, the lipid profile, liver damage markers and inflammation all improved. Fibrosis progressed in 9% of patients, was unchanged in 88% and regressed in 3%. Another study administered ezetimibe to 32 patients with NAFLD for 6 months. Cholesterol levels fell, while ballooning and fibrosis improved.165 Other authors have observed improved fibrosis and ballooning but no improvement in steatosis, inflammation or the NAS.166
ω3 polyunsaturated fatty acidsAccording to several meta-analyses of controlled and randomised trials, omega-3 (ω3) polyunsaturated fatty acid supplements reduced the risk of fatal coronary heart disease and sudden death by 10% in patients with a history of CVD, although the fall in the risk of sudden death was not significant.167 As well as sudden death, long-term cardiac mortality and myocardial infarction also appeared to fall with the use of these supplements in secondary prevention patients.168 Other potential beneficial cardiovascular effects of ω3 polyunsaturated fatty acids include reduced triglyceridaemia and improved vascular and cardiac haemodynamics, endothelial function, inflammation, thrombosis and arrhythmia, as well as autonomic control.169 Its role in primary prevention is less clear.170 Several studies171–174 and a meta-analysis175 have confirmed that the effect of ω3 polyunsaturated fatty acids on the liver is clear whether consumed through the diet or as supplements, as they improve transaminase levels and liver fat content in patients with steatosis, albeit with little effect on histological parameters. The AASLD/AGA/ACG guideline19 finds it premature to recommend ω3 in NAFLD but advocates their use in patients with steatosis if they have hypertriglyceridaemia. The main dietary ω3 polyunsaturated fatty acids from fish are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). However, their role in NAFLD treatment as purified agents is far from being well established. DHA seems to reduce liver fat content176,177 but does not improve transaminase levels in either the adult or the paediatric population.178,179 Data concerning the use of purified EPA also appear to be contradictory, with one study finding that it improves steatosis, inflammation and fibrosis,180 while another study found no beneficial effect from its administration in steatohepatitis.181
Antidiabetic drugsMetforminThis biguanide has proven to be effective both in treating diabetes and in slowing the development of carbohydrate intolerance.182 The UKPDS 34 study also found that it reduced total mortality by 36%.183 Metformin may also exert a cardioprotective effect by reducing arterial stiffness and improving endothelial function markers.184,185
Numerous studies conducted in patients with biopsy-diagnosed NAFLD initially asserted that the administration of metformin is associated with reduced transaminase levels and liver volume, as determined by ultrasound, as well as improved fatty infiltration and necroinflammatory phenomena.186,187 However, both the largest randomised trial conducted in children (TONIC100), as well as two systematic reviews188,189 concluded that, irrespective of the dose or the presence of diabetes, metformin does not improve transaminase levels or liver histology any more than lifestyle change, although transaminase levels did fall in the review by Li et al.189 On this basis, the AASLD/AGA/ACG guideline does not recommend metformin as a specific treatment for NAFLD or NASH, although it can be administered to diabetic patients with these liver diseases.19 In this regard, two separate meta-analyses found that metformin administered to diabetic patients was associated with a 62% fall in the risk of developing liver cancer190 and that it improves survival in diabetic patients with cirrhosis.191 In fact, in the cirrhosis phase of diabetic patients with NAFLD, metformin reduces total mortality by 53% without causing lactic acidosis.192
Dipeptidyl peptidase-4 inhibitorsAccording to a meta-analysis of small controlled and randomised trials, DPP-4 inhibitors do not appear to reduce the risk of cardiovascular events, but have also not been associated with a greater risk of their onset.193 The administration of saxagliptin may increase hospital admissions due to heart failure.194
DPP-4 activity has been found to increase by 30% in NASH,195 with a correlation between this activity and the degree of steatohepatitis, thereby providing a rational basis for its clinical application. However, experience with these drugs is limited and broader studies need to be conducted. Even so, a small 4-month study in patients with NAFLD found that sitagliptin improved ALT levels, steatosis as determined by resonance, ballooning and the NAS.196 A meta-analysis of two studies and very few patients showed that sitagliptin reduced transaminase levels.197 In similar vein, linagliptin has been shown to improve insulin sensitivity and steatosis in obese patients.198
Antihypertensive drugsAngiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs) are important modulators of the renin-angiotensin-aldosterone system used to treat hypertension and proteinuria. They have been widely proven to reduce cardiovascular mortality, myocardial infarction and stroke and to improve clinical outcomes in chronic heart failure.199 They also increase insulin sensitivity,200 which enhances their cardioprotective effect by reducing the likelihood of developing diabetes, as highlighted by two meta-analyses.201,202
ARBsIn initial trials, ARBs appeared to reduce hepatic fibrosis.203 Later, a small study of seven patients found that losartan improved steatosis, inflammation and necrosis. However, a 12-month, open-label, randomised study found that losartan combined with rosiglitazone did not improve liver histological parameters.91 A small trial on 54 hypertensive patients with biopsy-diagnosed NASH compared the effect of telmisartan with valsartan after 20 months of treatment. The results for both drugs were similar, but plasma lipids, insulin sensitivity, hepatic steatosis, necroinflammation and fibrosis improved to a greater extent in the telmisartan group.204 Another study conducted in patients being treated with simvastatin concluded that losartan was superior to amlodipine in improving hepatic steatosis indices.205 Based on the above evidence, it is difficult to assess the actual efficacy of ARBs in treating NAFLD and NASH. Nevertheless, they are recommended by the latest guidelines issued by the Japanese Society of Gastroenterology for patients with steatosis and hypertension.206
Effect of improved non-alcoholic fatty liver disease on vascular riskTo date, it has not been shown that improved NAFLD severity improves associated cardiovascular risk. A pre-specified sub-study of the WELCOME trial177 was the first to demonstrate that an improvement in NAFLD severity reduces carotid IMT, an indirect marker of CVD. This study, which administered 4g/day of a combination of DHA and EPA for 18 months, found that improved NAFLD severity, defined as a lower percentage of liver fat, as determined by magnetic resonance spectroscopy, and necroinflammation determined by cytokeratin 18 (CK-18), a highly sensitive and specific serum marker of hepatocyte apoptosis in NASH diagnosis, was associated with slower IMT progression. ω3 administration had no influence on IMT, although DHA tissue concentration was associated with a lower percentage of liver fat. Further studies must be conducted to ascertain whether an improvement in NAFLD severity has any cardiovascular benefit.
ConclusionThis review has assessed aspects of lifestyle and pharmacological treatments that may improve some of the phases of NAFLD. Despite the availability of five active substances that may offer some histological improvement, none is backed by sufficient clinical evidence to advocate its use in all patients with NAFLD. Numerous drugs are currently being trialled for potential use in NAFLD. For the time being, weight loss and exercise are the most appropriate and cost-effective strategies for this highly prevalent and far-reaching condition.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Brea Á, Pintó X, Ascaso JF, Blasco M, Díaz Á, González-Santos P, et al. Enfermedad del hígado graso no alcohólico, asociación con la enfermedad cardiovascular y tratamiento (II). Tratamiento de la enfermedad del hígado graso no alcohólico. Clin Invest Arterioscler. 2017;29:185–200.