Statins have been associated with an increased risk of new-onset diabetes mellitus (NODM), as confirmed in previous observational studies and meta-analyses. Controversy exists as to whether this risk varies depending on statin type or dose. However, there appears to be unanimity regarding the different associated factors that raise this risk. Furthermore, diverse pathophysiologic mechanisms have been described that could explain the increased risk of diabetes in patients with statin treatment. These fundamentally cause a rise in insulin resistance together with a decrease in insulin secretion. The present review aimed to describe the relationship between statin treatment and the presence of diabetes and provide an update of previous published evidence and the possible mechanisms involved.
Las estatinas se han asociado con un incremento en el riesgo de aparición de diabetes mellitus de novo, siendo esto confirmado en estudios previos observacionales así como metaanálisis. Sin embargo, existe cierta controversia sobre si este riesgo varia en función de la dosis o tipo de estatina. Por otro lado, parece que hay unanimidad con respecto a los factores asociados a este incremento de riesgo. Asimismo, se han descrito diversos mecanismos fisiopatológicos que podrían explicar el aumento de riesgo de padecer diabetes en los pacientes tratados con estatinas. Estos mecanismos se basan fundamentalmente en un incremento en la resistencia a la insulina así como un descenso en su sensibilidad. La presente revisión está enfocada en describir la relación entre el tratamiento con estatinas y el riesgo de padecer diabetes y revisar los datos publicados hasta las fecha así como los posibles mecanismos fisiopatológicos involucrados en este aumento de riesgo.
Statins have been widely used for the treatment of lipid disorders. Their main role relies in lowering low-density lipoprotein (LDL) cholesterol levels, thereby reducing the risk of cardiovascular disease (CVD) in primary and secondary prevention settings. In this respect, current clinical guidelines recommend the use of statins for the prevention of atherosclerotic CVD, even in individuals with suboptimum lipid levels.1
Although statin therapy is well tolerated, several side effects have been associated with its use, including an increased diabetes risk. Since the incidence of this adverse effect is not negligible, especially for specific populations, the present review aimed to examine the relationship between statin treatment and the presence of diabetes. A review of previous clinical evidence and the possible pathophysiologic mechanisms involved will also be described.
Statins and diabetes riskAs stated previously, the risk of new-onset diabetes mellitus (NODM) in subjects receiving statin treatment has been a matter of debate in recent years.2 In 2001, Freeman et al.3 published one of the first papers speculating on the possible relationship between statin treatment and diabetes. In that sub-analysis of the West of Scotland Coronary Prevention Study (WOSCOPS), pravastatin was surprisingly shown to lower the risk of diabetes by 30%. However, further trials yielded opposite results to this first one, showing an increased risk of type 2 diabetes mellitus secondary to statin therapy. The first trial in this line was Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER).4 Seventeen thousand eight hundred and two subjects were randomized to 20mg of rosuvastatin daily or placebo. The treatment with rosuvastatin for almost 2 years was associated with a 44% decrease in cardiac events but a significant 26% increase (odds ratio 1.26, 95% CI 1.04–1.51) in NODM. These results have since been further confirmed in other studies, which suggests an excess risk of NODM of around 9–13% in individuals treated with statins.5–8 Based on these results, in 2012 the Food and Drug Administration added information on statin labels concerning the increased incidence of diabetes and possible rise in blood glucose levels secondary to this treatment.
A relationship also appears to exist between the excess risk of NODM observed in individuals receiving statins and the presence of other comorbidities, mainly the well-known cardiovascular risk factors, which include a higher body mass index, fasting blood glucose levels, triglycerides, blood pressure levels or low high-density lipoprotein (HDL) cholesterol levels.6,9 Thus, as the number of metabolic syndrome components in an individual treated with statins rises, the risk of NODM increases.
On the other hand, data regarding the possible relationship between NODM risk and statin type or dose are inconsistent. Some studies reported little difference in diabetes rates between patients with medium or high statin doses10,11 while other publications reported a greater risk in subjects treated with rosuvastatin, atorvastatin and simvastatin, and no excess risk with other statins such as pravastatin, fluvastatin, lovastatin and pitavastatin.12–14 In this sense, it is worth highlighting that the possible increased risk of NODM seems to be related to the potency of each statin. A recent meta-analysis showed an association between LDL cholesterol reductions ≥30% with statin therapy and the risk of new-onset diabetes.15
Statin treatment and familial hypercholesterolemiaIn addition, a hypothetical protection from NODM has been described in patients with heterozygous familial hypercholesterolemia (HeFH), although most of these patients receive statins as the main lipid-lowering treatment. This can be partially explained by the cholesterol-lowering effect of statin therapy being mainly due to inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the primary altered mechanism in HeFH.16 In this context, Besseling et al.17 found the prevalence of type 2 diabetes mellitus to be lower in patients with familial hypercholesterolemia compared with unaffected relatives (1.75% versus 2.93%). Moreover, a previous paper published by our group18 also found the prevalence of diabetes in patients with HeFH to be 40% lower than that observed in the general population matched for age and sex. In our study,18 risk factors independently associated with the presence of diabetes in these subjects were age, male sex, body mass index, baseline triglycerides, hypertension and years of statin treatment.
Previous clinical evidencePopulation-based studiesPopulation-based studies reported to date focused on the incidence of NODM in patients treated with statins,11,13,19–23 and observed an increased risk of NODM related to the use of this lipid-lowering therapy. However, in some of those studies the increased risk was only observed for certain types of statin. For instance, Carter et al.13 conducted a cohort study including 471,250 subjects. After 3 years follow-up, the adjusted hazard ratio (HR) for NODM was 1.22 (95% CI 1.15–1.9) for atorvastatin, 1.18 (95% CI 1.10–1.26) for rosuvastatin and 1.10 (95% CI 1.04–1.17) for simvastatin. However, no significant risk of NODM was observed with fluvastatin HR 0.95 (95% CI 0.81–1.11) and lovastatin HR 0.99 (95% CI 0.86–1.14). Similarly, in the meta-analysis of Vallejo-Vaz et al.24 pitavastatin did not adversely affect glucose metabolism or diabetes development compared with placebo or other statins. By contrast, in the Women's Health Initiative Study,25 153,840 postmenopausal women without diabetes were recruited and followed from 1993 to 2005. There were 10,242 incident cases of diabetes, and statins were associated with an increased risk of NODM, with an HR of 1.48 (95% CI 1.38–1.59), with this increased risk being present for all types of statins, and therefore showing a class effect. Finally, the United Kingdom Clinical Practice Cohort study21 included 2,016,094, 430,890 of whom were receiving statins and were matched to 1,585,204 individuals who were not. During follow-up, 130,395 subjects developed NODM, with statin use being associated with an increased risk of NODM with an HR of 1.57 (95% CI 1.54–1.59). Furthermore, this risk increased with longer duration of statin use and a higher baseline body mass index.
Meta-analysesMoving on from population-based studies, previous published trials, systematic reviews and meta-analyses also focused on NODM and statin treatment,5,6,26–30 and confirmed the previously-reported findings.
In 2010, Sattar et al.6 realized the first meta-analysis that demonstrated the increased risk of NODM secondary to statin treatment. Thirteen studies with 91,140 individuals (2226 in the statin group and 2052 in the control group) were included. Statin treatment was associated with a 9% increase in diabetes (OR 1.09, 95% CI 1.02–1.17). Regarding the different statin subtypes, rosuvastatin was found to increase the risk by 18% (OR 1.18, 95% CI, 1.04–1.33) while simvastatin increased the risk by 11%, although the latter result was not significant. After this first meta-analysis, Preiss et al.27 confirmed the present results, demonstrating a 12% increased risk of diabetes (OR 1.12, 95% CI, 1.04–1.22) in patients with statin treatment.
As mentioned above in population-based studies, some previous works found a different effect on the risk of NODM depending on the type or dose of statin. In this respect, the meta-analysis of Navarese et al.28 that included 17 trials, found that pravastatin presented the lowest risk of incident diabetes. However, rosuvastatin 20mg daily was associated with a 25% increased risk compared to placebo (OR 1.25, 95% CI 0.82–1.90) and atorvastatin 80mg daily with a 15% increased risk (OR 1.15, 95% CI 0.90–1.50). In this same line, the meta-analysis published by Swerdlow et al.30 also yielded different results depending on the type of statin. Fifteen studies were included with 96,418 subjects treated with a standard dose of statins compared to placebo and 4 studies including 32,752 subjects comparing high-dose to medium-dose statin treatment. The results obtained reflected a higher incidence of NODM in the statin group versus the placebo group (5.3% versus 4.7%) as well as a higher incidence in the high statin dose compared to the medium statin dose (6.4% versus 5.7%).
Thus, the results of previous published papers, both population-based studies and meta-analyses, confirm the close relationship between NODM and statin treatment.
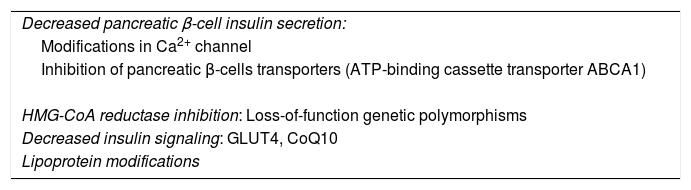
Possible pathophysiologic mechanismsThe possible molecular mechanisms associated with the increased risk of NODM in subjects receiving statins are still under debate. Several mechanisms seem to be involved, all resulting in a decline in insulin secretion by pancreatic β-cells or an increase in insulin resistance.
One of the possible mechanisms is based on the inhibition caused by statins of the Ca2+ channel in pancreatic β-cells, leading to a direct drop in insulin secretion.31 This was confirmed in a previous in vitro study, where rosuvastatin inhibited the Ca2+ channel in pancreatic β-cells and led to reduced exocytosis of insulin granules.31
A further possible mechanism is related to HMG-CoA reductase inhibition, which also leads to modifications in insulin sensitivity. Possible mechanisms associated with this include the production of loss-of-function genetic polymorphisms. In this sense, a study including 5327 non-diabetic subjects found that those who presented certain single nucleotide polymorphisms such as TCF7L2, SLC30A8, HHEX and others had an impaired conversion of proinsulin to insulin together with a limited insulin secretion.32 Similarly, Swerdlow et al.30 also described single nucleotide polymorphisms related to HMG-CoA reductase in 22,463 patients. In this study, each allele was associated to lower LDL colesterol levels together with increased blood glucose and insulin concentrations.
Moreover, a decreased expression of glucose protein transporter 4 (GLUT4) and ubiquinone (CoQ10) levels together with modifications in adiponectin concentrations have also been described.33–35 In this respect, previous human studies have obtained inconsistent results, showing increase, decrease or no change of adiponectine levels in patients treated with statins.33
Other possible pathophysiologic mechanisms that have been proposed are related to the effect of statins on lipoprotein particle size, thus increasing the size of very low-density lipoprotein (VLDL) particles and reducing the size of LDL and HDL particles, this leading to an increased risk of NODM.36,37 In contrast, large LDL and HDL and small VLDL particles appear to have an inverse effect on NODM. This has been demonstrated in experimental models with both rosuvastatin38 and atorvastatin,39 but must be confirmed in further studies.
Finally, other possible mechanisms that have been described are related to alterations on glucose metabolism due to the inhibition of pancreatic β-cell transporters. In this line, ATP-binding cassette transporter ABCA1 defficiency was related to impared insulin secretion in pancreatic β-cells.40,41 All of the possible mechanisms described above are summarized in Table 1.
Possible pathophysiologic mechanisms associated with the diabetes risk of statin treatment.
| Decreased pancreatic β-cell insulin secretion: |
| Modifications in Ca2+ channel |
| Inhibition of pancreatic β-cells transporters (ATP-binding cassette transporter ABCA1) |
| HMG-CoA reductase inhibition: Loss-of-function genetic polymorphisms |
| Decreased insulin signaling: GLUT4, CoQ10 |
| Lipoprotein modifications |
GLUT4: glucose protein transporter 4; HMG-CoA: 3-hydroxy-3-methylglutaryl-coenzyme A; CoQ10: ubiquinone.
It seems clear that statins are associated with an increased risk of NODM and that there could be a potency-dependent effect together with associated factors, such as metabolic syndrome traits, that increase this risk.
On the other hand, statins play an essential role in cardiovascular risk reduction due to their effect on lowering LDL cholesterol levels. Hence, there appears to be agreement that the benefits of statin treatment regarding the cardiovascular profile outweigh to the increased risk associated with the presence of diabetes.42,43 However, questions remain to be answered in this field.
It is also noteworthy that most of the studies describing the possible pathophysiologic mechanisms were conducted in experimental in vitro or animal models, and thus these data must be further confirmed in human studies in the near future to draw more solid conclusions.
ConclusionsStatins present an increased risk of NODM, as described in previous observational studies and confirmed in recent meta-analyses and systematic reviews. However, this risk seems to be present only for certain types of statin. Moreover, individuals may have several risk factors possibly linked to this increased risk: a high body mass index, hypertension and previous glucose metabolism disorders.
The different pathophysiologic mechanisms involved are still under study, but seem to be related to decreased insulin secretion together with increased insulin resistance. However, further studies are mandatory in this field to reach more solid conclusions and hence achieve clinical applicability in daily practice.
FundingThis work has not received any funds.
Conflict of interestThe authors declare no conflict of interest.
We thank Miss Christine O’Hara for review of the English version of the manuscript.