Inhibitors of the protein PCSK9, available since 2015, are capable of reducing the concentration of low density lipoprotein cholesterol by 40–70%, thus reducing the cardiovascular risk. The present case reports an adverse cardiovascular event that appeared when spacing out the administration of lipid-lowering treatment. A discussion will be presented on the importance of maintaining low cholesterol levels in order to achieve a greater benefit, according to the latest published clinical studies.
Los inhibidores de la PCSK9, disponibles desde el año 2015, son capaces de reducir la concentración de colesterol transportado en las lipoproteínas de baja densidad entre un 40-70% y, por consiguiente, disminuir el riesgo cardiovascular. Presentamos un caso en el que un episodio cardiovascular grave apareció al espaciar la administración del tratamiento hipolipidemiante; discutiremos la importancia de mantener una concentración baja de colesterol, para conseguir un mayor beneficio clínico según los últimos estudios publicados.
Hypercholesterolaemia is one of the most common cardiovascular risk factors in our population; however, it is not always easy to control. Until a few years ago, statins were available at varying doses for its treatment, and ezetimibe could be added at a later stage, despite which some patients failed to achieve the objectives of lipid control. A new therapeutic group, PCSK9 inhibitors (PCSK9i), have made it possible to reduce the levels of cholesterol transported in low-density lipoproteins (LDL-C) very significantly (up to 40–70% on what was achieved with statins and ezetimibe), making it possible to maintain concentrations of LDL-C as recommended by clinical practice guidelines.
Recently, there has been some controversy regarding the safety of achieving such low concentrations of LDL-C, mainly at the cognitive level, although these doubts have dissipated with the latest published works.1–4 Regarding the presentation of this case report, the importance of maintaining LDL-C at the lowest possible concentrations is reiterated.
Case reportThis case is with regard to a 68-year-old male former smoker with a history of hypertension, heterozygous familial hypercholesterolaemia on treatment with atorvastatin 80 mg and ezetimibe 10 mg, chronic coronary heart disease since 2007 with three-vessel disease (requiring implantation of up to four stents) on treatment with clopidogrel 75 mg/day, subclinical carotid atherosclerosis, chronic obstructive pulmonary disease-asthma, chronic allergic rhinitis and stage 2 chronic kidney failure. Given the high cardiovascular risk of the patient, and given the history of heterozygous familial hypercholesterolaemia in secondary prevention, with poor control despite optimal treatment (LDL-C >77 mg/dl), he participated in a randomised double-blind, placebo-controlled, clinical trial on the safety and efficacy of alirocumab in patients with heterozygous familial hypercholesterolaemia, being assigned to the treatment arm with alirocumab 75 mg/2 weeks injected subcutaneously.
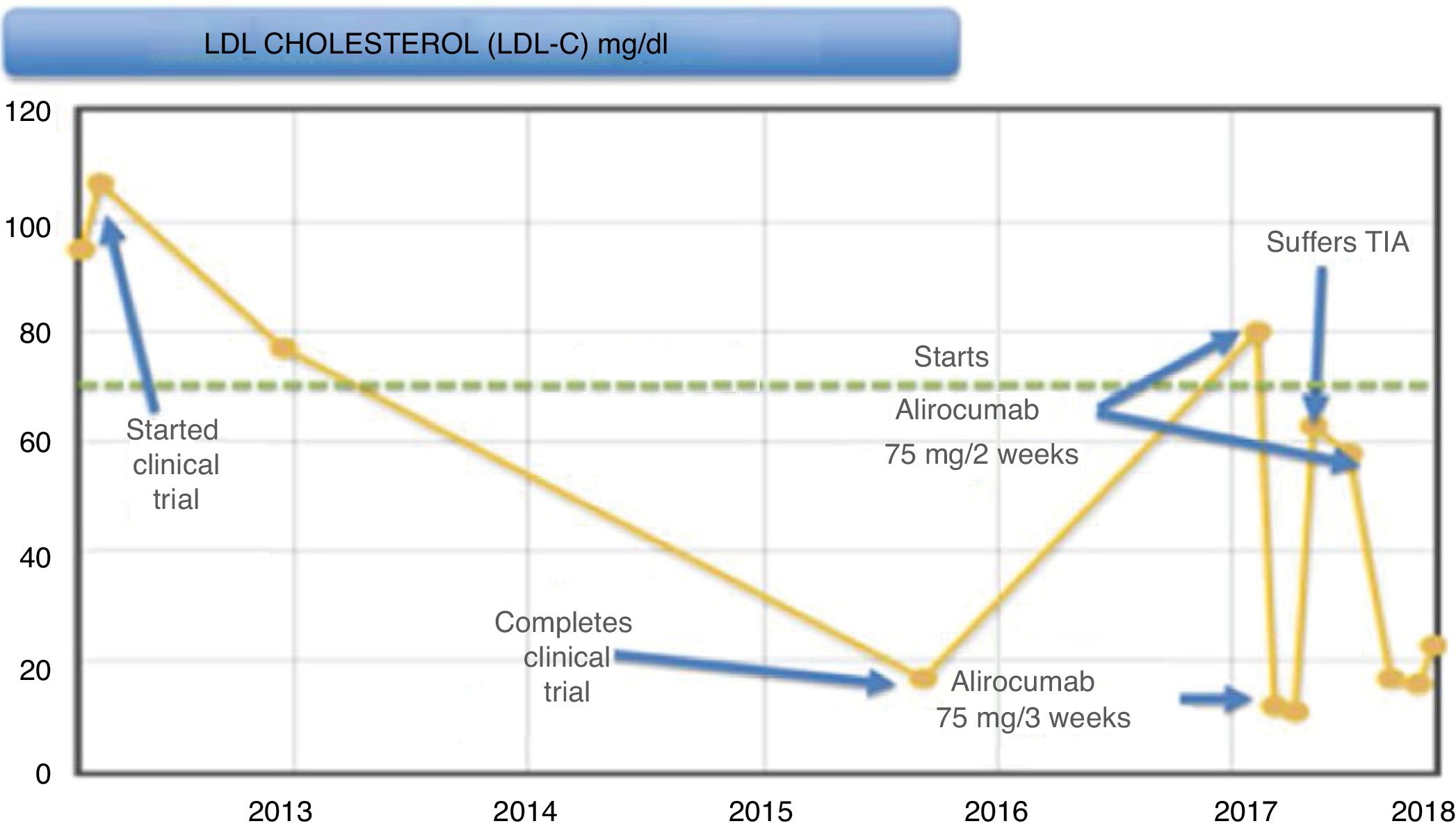
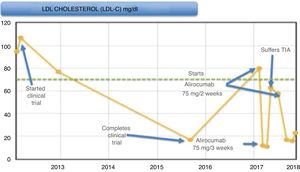
During the trial (Fig. 1), the patient presented a good control of LDL-C levels, with it being <20 mg/dl at the end of it. Subsequently, levels began to rise, remaining around 75−85 mg/dl, and, since it met criteria for the start of this new drug, it was decided to start alirocumab 75 mg/2 weeks after being accepted in the PCSK9i protocol of our hospital, after participating in a clinical trial. In subsequent months, LDL-C levels decreased down to 10 mg/dl, worsening his allergic rhinitis symptoms (considered as a possible adverse effect of this medicine), and, in light of good control, it was decided to space out the 75-mg regimen to one injection every three weeks.
After this adjustment, the LDL-C level rose again up to 63 mg/dl. The patient had a transient ischaemic attack (TIA), which he suffered as motor aphasia. He was admitted to the Stroke Unit and a conservative management was performed, resolving the aphasia at 15 h from the beginning of the condition, starting another antiplatelet therapy (acetylsalicylic acid 100 mg).
Given this event, it was decided to increase the dose of alirocumab again to 75 mg every two weeks, with the patient currently being asymptomatic with LDL-C levels less than 15 mg/dl; he has not had any more cardiovascular episodes.
DiscussionThe new lipid-lowering agents known as PCSK9i, including alirocumab, are approved for the treatment of hypercholesterolaemia in patients with high cardiovascular risk who do not reach therapeutic range,5 with the patient in our case being a clear example. A clear relationship between the LDL-C levels and the onset of cardiovascular diseases (including stroke) has been shown, so that maintaining low LDL-C levels is associated with a lower cardiovascular risk.1–4
Given the good control of the patient with LDL-C levels <15 mg/dl and in the presence of rhinitis as a possible side effect of the drug, it was decided to space out the dose, partly due to ignorance of the possible effects that such low cholesterol levels could cause (at that time the new published clinical trials were not available). Consequently, the LDL-C levels increased, and despite encountering levels in therapeutic range, the patient experienced a TIA.
In the latest published studies (FOURIER, SPIRE, ODYSSEY OUTCOMES), which included patients with similar clinical characteristics as our case, it is observed that these drugs manage to reduce the concentration of LDL-C by up to 60%, for a prolonged period of time,1–4 with the exception of bococizumab, which, as it was partly murine, lost its long-term effectiveness by generating antibodies against it.6
Regarding its clinical efficacy, multicentre studies of morbidity and mortality and long-term safety have been designed. Both the FOURIER study, conducted with evolocumab,1,2 and the recently published ODYSSEY OUTCOMES study, conducted with alirocumab,3 demonstrated a significant reduction in cardiovascular diseases, including ischaemic stroke, even among patients with lower LDL-C, with this benefit being greater when the treatment was prolonged, and no differences in the occurrence of adverse events were found.1–3 Regarding the screening of patients who benefit most from applying these treatments, there are those who at the time of inclusion have LDL-C figures >100 mg/dl, presenting a significant reduction of 24% in terms of cardiovascular diseases, and 29% for all-cause mortality.3
The possible increase in adverse effects at the neurocognitive level has been discussed in recent years7; however, in the EBBINGHAUS study (FOURIER substudy) this aspect has been analysed in more detail, excluding patients with previous cognitive disorders with clinical relevance; no differences were observed when patients were compared, even with LDL-C <25 mg/dl.8
In short, it is a patient with a very high cardiovascular risk and a history of subclinical carotid atherosclerosis, so the appearance of a TIA does not seem remarkable. However, it is striking that the episode occurred when LDL-C levels were close to the therapeutic limit. We must consider whether that elevation of LDL-C had some pathogenic role in the development of the TIA, a fact that has been demonstrated by reducing the dose of other drugs such as statins.9 In the JUPITER study, it was already observed that those patients who reached cholesterol levels below 50 mg/dl presented a reduction in cardiovascular events of 65%, compared to 44% of the overall reduction.10 Furthermore, given these LDL-C levels that these new drugs are able to reach, it should be considered if it would be indicated to reduce the LDL-C levels in the clinical practice guidelines in these patients with extreme cardiovascular risk, in order to reduce the incidence of cardiovascular episodes.
ConclusionThe PCSK9i cause a greater reduction in LDL-C levels, associating a lower cardiovascular risk, including ischaemic strokes. However, the possible adverse effects that could be caused by maintaining a much lower LDL-C level have been the subject of interest in the different studies carried out. The evidence published to date coincides in that LDL-C figures lower even than 25 mg/dl continue to present a clinical benefit in reducing cardiovascular risk without having reported a higher rate of adverse effects, so they seem to reaffirm once again the hypothesis that “the lower the levels of LDL-C, the better”.
Conflicts of interestLuis Álvarez-Sala Walther has received conference fees from Sanofi, Amgen, Pfizer and MSD. Pablo Demelo Rodríguez has received conference fees from Sanofi and AstraZeneca. The other authors have no conflicts of interest to declare.
Please cite this article as: Piqueras Ruiz S, Parra Virto A, Torres do Rego A, Demelo Rodríguez P, Álvarez-Sala Walther L. Accidente isquémico transitorio tras espaciar la dosis de alirocumab: ¿es recomendable reducir las dosis de iPCSK9 ante c-LDL muy bajos? Clin Investig Arterioscler. 2019. https://doi.org/10.1016/j.arteri.2019.04.003