Lysyl oxidase (LOX) participates in the assembly of collagen and elastin fibres. The impact of vascular LOX over-expression on extracellular matrix (ECM) structure and its contribution to oxidative stress has been analysed.
MethodsStudies were conducted on mice over-expressing LOX (Tg), specifically in smooth muscle cells (VSMC). Gene expression was assessed by real-time PCR analysis. Sirius Red staining, H2O2 production and NADPH oxidase activity were analysed in different vascular beds. The size and number of fenestra of the internal elastic lamina were determined by confocal microscopy.
ResultsLOX activity was up-regulated in VSMC of transgenic mice compared with cells from control animals. At the same time, transgenic cells deposited more organised elastin fibres and their supernatants induced a stronger collagen assembly in in vitro assays. Vascular collagen cross-linking was also higher in Tg mice, which showed a decrease in the size of fenestrae and an enhanced expression of fibulin-5. Interestingly, higher H2O2 production and NADPH oxidase activity was detected in the vascular wall from transgenic mice. The H2O2 scavenger catalase attenuated the stronger deposition of mature elastin fibres induced by LOX transgenesis.
ConclusionsLOX over-expression in VSMC was associated with a change in the structure of collagen and elastin fibres. LOX could constitute a novel source of oxidative stress that might participate in elastin changes and contribute to vascular remodelling.
La lisil oxidasa (LOX) contribuye al ensamblaje de las fibras de colágeno y elastina de la matriz extracelular (MEC). Hemos determinado las consecuencias de la sobreexpresión vascular de LOX sobre la estructura de la MEC y su contribución al estrés oxidativo.
MétodosLos estudios se desarrollaron en ratones que sobreexpresan la LOX (Tg) específicamente en células musculares lisas vasculares (CMLV). Se realizaron análisis por PCR a tiempo real, tinción de rojo sirio, producción de H2O2 y actividad NADPH oxidasa. Se caracterizaron las fenestras de la lámina elástica interna mediante microscopia confocal.
ResultadosLas CMLV de ratones transgénicos presentaron niveles de actividad LOX superiores a los de animales control. En consonancia, las células transgénicas depositaron más fibras de elastina organizada y sus sobrenadantes indujeron un mayor ensamblaje de colágeno en ensayos in vitro. El nivel de colágeno maduro fue superior en la pared vascular de ratones Tg, que presentaban una menor área de las fenestras y un aumento de la expresión de la fibulina-5. La producción vascular de H2O2 y la actividad NADPH oxidasa fueron superiores en los ratones transgénicos. La incubación de CMLV con catalasa atenuó el incremento en la deposición de fibras de elastina madura inducido por la transgénesis de LOX.
ConclusionesLa sobreexpresión de la LOX en CMLV se asocia a una alteración de la estructura vascular del colágeno y la elastina. La LOX podría constituir una nueva fuente de estrés oxidativo que participaría en la alteración estructural de la MEC y podría contribuir al remodelado vascular.
Lysyl oxidase (LOX) is an enzyme that catalyses one of the key steps in the synthesis and stabilisation of the extracellular matrix (ECM).1,2 LOX is a copper-dependent amino oxidase that participates in the covalent cross-linking of collagen and elastin fibres in the ECM. Specifically, LOX catalyses the oxidative deamination of lysine and hydroxylysine residues, resulting in the formation of highly reactive peptidyl semialdehydes that condense together to form both intramolecular and intermolecular bonds; a reaction that generates H2O2 as a by-product.1
LOX activity determines the mechanical and structural properties of connective tissues and changes in that activity have been linked to many different pathological processes, including cancer and cardiovascular disease.2,3 Recent studies suggest that LOX activity and increased cross-linking in associated collagen fibres may play a key role in vascular stiffness.4 Alteration in the structure of elastin affects vascular mechanics in both large and small arteries5–7 and at least partly contributes to increased vascular stiffness in hypertension,8,9 although there is less evidence of the role of LOX in this process.
Surprisingly, in addition to its merely structural function, LOX has been implicated in the control of multiple cellular processes, including cell differentiation, migration, transformation and regulation of gene expression.1,2 It is interesting to note that some of these biological functions, such as control of cell migration and vascular smooth muscle cell (VSMC) chemotaxis, have been linked to increased production of H2O2 as a result of LOX activity.10,11 However, whether LOX may contribute to vascular oxidative stress and its possible impact on vascular structure have not been established. Our results obtained in a transgenic mouse model that overexpressed LOX specifically in VSMC show that LOX overexpression is associated with greater oxidative stress in the vascular wall, and that this then contributes to the alteration of elastin structure, which may have significant pathophysiological repercussions.
Material and methodsAnimal modelThe studies were performed on a transgenic mouse model that overexpressed LOX in VSMC (Tg) on a C57BL/6J genetic background.12 C57BL/6J mice of the same litter and age (3 months) were used as controls. The animals were kept in a pathogen-free environment under standard lighting (12h cycles of light/darkness) and temperature (21±1°C) conditions in the animal experimentation unit (CSIC-ICCC, Barcelona, Spain) with free access to food and water throughout the procedure. The research was conducted in accordance with the principles and guidelines established by the American Physiological Society – Animal Research, and all procedures were approved by the Ethics Committee of the Cardiovascular Research Centre of Barcelona, in accordance with the Spanish Animal Protection Policy RD53/2013, which complies with the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes.
The animals were sacrificed by CO2 inhalation and the different vascular beds were isolated, cleaned to remove excess fat and connective tissue and processed for the different analyses.
Organisation of the internal elastic laminaPressurised mesenteric resistance artery segments were fixed with 4% paraformaldehyde and assessed by pressure myography (45mmHg). The organisation of the elastin in the internal elastic lamina was studied by fluorescence confocal microscopy based on the elastin autofluorescence properties (excitation wavelength: 488nm; emission wavelength: 500–560nm) as described previously.5 The experiments were carried out with a Leica TCS SP2 confocal microscope (Leica Microsistemas S.L.U., Barcelona, Spain). Serial optical sections were captured from the adventitia to the vascular lumen (distance between sections on the z-axis=0.5μm) with a 40× oil immersion zoom lens 4. A minimum of two sets of images of different regions were captured in each arterial segment. Quantitative analysis of the inner elastic lamina was performed with the Metamorph image analysis software (Molecular Devices, Sunnyvale, CA, USA) as described above.5 From each series of images, the individual maximum projections of the inner elastic lamina were reconstructed and the area and number of fenestrae were determined.
ImmunohistochemistryThe carotid arteries of the mice were perfused with PBS, fixed in 4% paraformaldehyde for 24h and embedded in paraffin. Sections 5μm thick were prepared with a microtome (Jung RM2055, Leica Microsistemas S.L.U., Barcelona, Spain), which were deparaffinised and rehydrated in a gradient of ethanol solutions. The slides were stained with picrosirius red and the birefringence was visualised by polarised light microscopy.13
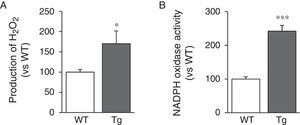
Measurement of NADPH oxidase activityNAD(P)H oxidase activity was determined in vascular homogenates by a lucigenin chemiluminescence method. The tissues were homogenised in a lysis buffer (50mM KH2PO4, 1mM ethylene glycol tetra-acetic acid and 150mM sucrose, pH 7.4). The reaction was started with the addition of NAD(P)H (0.1mM) to the suspension containing the sample, lucigenin (5μM) and phosphate buffer. Luminescence was measured with a plate luminometer (Auto Lumat LB 953, Berthold Technologies GmbH, Bad Wildbad, Germany). The reading corresponding to the buffer blank was subtracted from the value of each reading. NADPH oxidase activity was normalised for the protein content and expressed relative to the control animals (WT).14
Measurement of H2O2 productionH2O2 production was analysed in aortic segments using the compound Amplex Red (Molecular Probes, Eugene, OR, USA) as a fluorogenic horseradish peroxidase (HRP) substrate. Amplex Red (100μM) and HRP type II (0.2U/ml) were added to the aortic homogenate. The fluorescence readings were performed in triplicate in 96-well plates at an Ex/Em of 530/580nm using 50μl of sample. The H2O2 level was extrapolated from a standard curve, normalised by protein content and expressed as a percentage of that of the control animals (WT).
Cell cultureMurine aorta VSMC were obtained by a modification of the explant technique as previously described.15 Briefly, the aorta was opened longitudinally, adventitia and endothelium were removed, and the middle layer was divided into 1–2mm fragments which were transferred to 25cm2 culture flasks containing 5ml DMEM (Dulbecco's Modified Eagle's Medium, Gibco, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (Biological Industries, Kibbutz Beit-Haemek, Israel) and antibiotics (Gibco, Carlsbad, CA, USA). The VSMC migrated from the explants after 2 to 3 weeks, at which time the explants were removed and the cells were trypsinised and subcultured routinely. Cells were used between steps 3 to 6.
Real-time PCRTotal RNA was isolated by Ultraspec™ (Biotecx, Houston, TX, USA) and reverse transcription was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Thermo Fisher Scientific Inc., Waltham, MA, USA) in the presence of random hexamers. Quantification of mRNA levels was performed using the ABI PRISM 7900HT sequence detection system and specific probes and oligonucleotides provided by the TaqMan™ gene expression assays-on-demand system (Applied Biosystems, Thermo Fisher Scientific Inc., Waltham, MA, USA) for the amplification of murine FBLN5 (Mm00488601_m1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1) was used as an endogenous control. Each sample was amplified in duplicate and relative levels of mRNA were determined by the 2−ΔΔCT method.
In vitro collagen polymerisation assayThe VSMC of WT and Tg mice were plated in 6-well plates (150,000cells/well). After 3 days the cell supernatants were collected and centrifuged at 10,000×g for 5min at room temperature. The supernatants were then concentrated 4 times by centrifugation with Amicon Ultra 10K filters (Merck Millipore, Darmstadt, Germany). The rat tail type I collagen (150μl) was neutralised, added onto glass bottom plates (Willco Wells B.V., Amsterdam, The Netherlands) and incubated for 40min at 37°C until gelled. Next, 400μl of concentrated supernatant was added to the surface of the gel and incubated for 24h at 37°C. The collagen matrix was visualised by confocal reflection microscopy (Leica DMIRE2) as previously described.16
Lysyl oxidase activityLOX activity was determined by a fluorescent method as previously described.17 The VSMC were seeded at a density of 150,000cells/well in 6-well plates, and after 48h the medium was replaced by RPMI medium without phenol red (Gibco, Carlsbad, CA, USA), serum, antibiotics or glutamine. After 24h, the LOX activity was quantified in the cell supernatant. To do this, an aliquot of medium (50μl) was incubated in the presence and absence of β-aminopropionitrile (BAPN; 500μM) at 37°C for 30min with 1U/ml HRP, 10μM of Amplex Red (Molecular Probes, Eugene, OR, USA) and 10mM of 1,5-diaminopentane in 1.2M of urea and 0.05M of sodium borate at pH8.2. The reaction was stopped on ice and the difference in fluorescence intensity (excitation λ: 563nm; emission λ: 587nm) between samples with and without BAPN was determined.17
ImmunocytochemistryVSMC of WT and Tg mice were plated onto μ-Dishes 35mm in diameter (Ibidi, Planegg/Martinsried, Germany) where they were kept growing in complete medium for 10 days. The medium was changed every 2 days. In some experiments, catalase (500U/ml) was added. The cells were then fixed with 4% paraformaldehyde and, without prior permeabilisation, incubated overnight with a rabbit polyclonal anti-elastin antibody (Abcam, Cambridge, UK; ab21607). After several thorough washes, the plates were incubated for 1h with a fluorescence-conjugated secondary antibody (goat anti-rabbit Alexa Fluor 488; Thermo Fisher Scientific Inc., Waltham, MA, USA). The nuclei were stained with Hoechst 33342 (Thermo Fisher Scientific Inc., Waltham, MA, USA).18 Fluorescence images were captured with a Leica DMIRE2 confocal microscope (Leica Microsistemas S.L.U., Barcelona, Spain). Quantitative analysis was performed using Image J software.
Statistical analysisThe results are shown as mean±SEM. Statistically significant differences were established by t-test or ANOVA followed by the Bonferroni test using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA). Differences were considered significant when p<0.05.
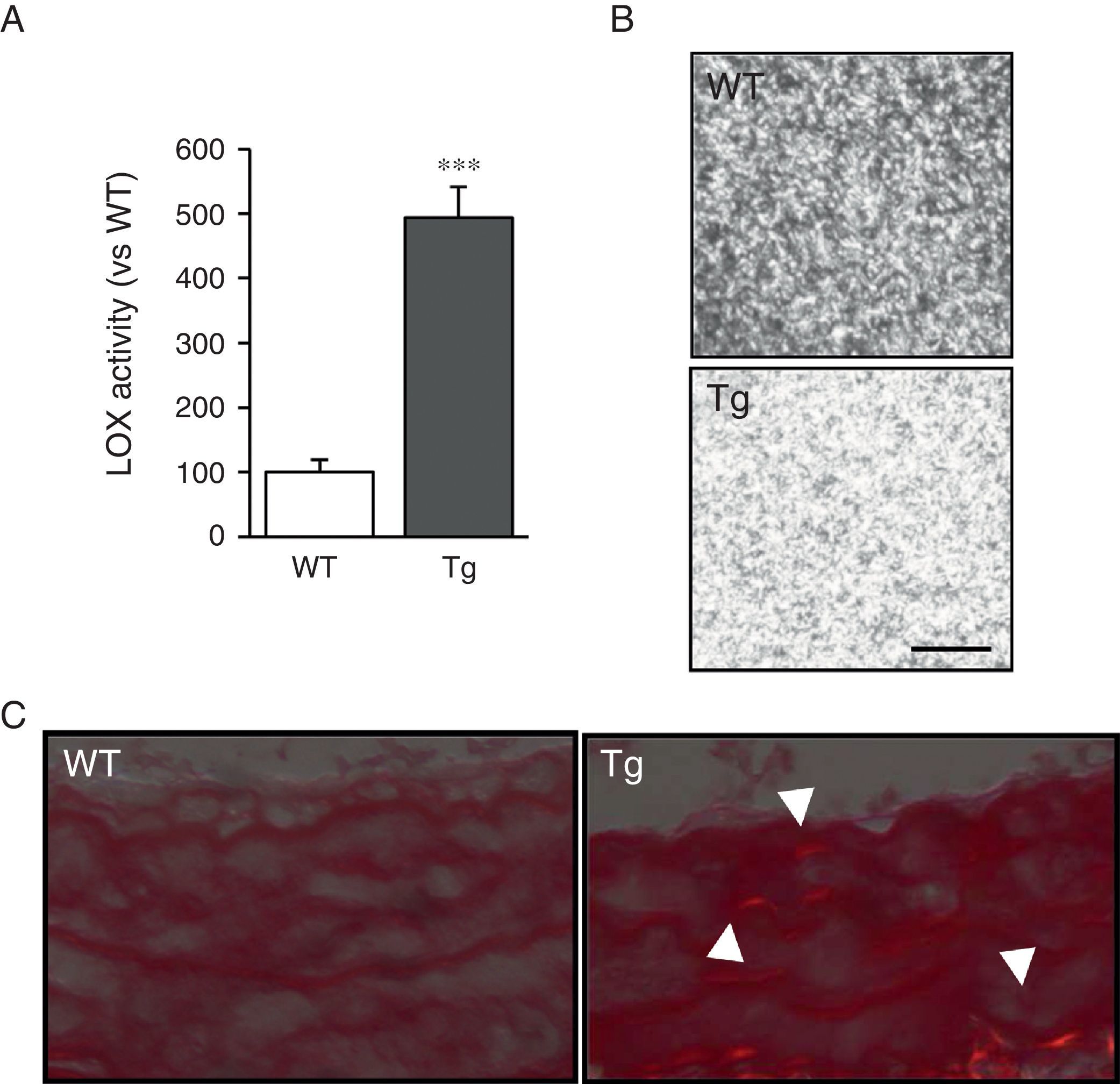
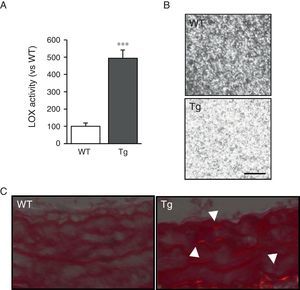
ResultsMice transgenic for LOX have a higher level of LOX activity and alterations in vascular collagen assemblyAortic VSMC from transgenic mice showed levels of LOX activity approximately 4-fold higher than the cells isolated from control mice (WT; Fig. 1A). Accordingly, supernatants from the VSMC of transgenic animals induced closer packing of the collagen fibres when incubated with soluble type I collagen (Fig. 1B). Also, after staining with Sirius red and visualisation with polarised light, an increase in mature collagen was observed in the middle layer of the carotids of these animals (Fig. 1C). These results indicate that the high LOX activity of the VSMC of transgenic animals would promote closer packing of the collagen in the vascular wall.
LOX transgenesis increases lysyl oxidase activity and induces the organisation of collagen fibres. (A) LOX activity measured in the supernatant of VSMC from mice transgenic for LOX (Tg) and control animals (WT). The results are expressed as mean±SEM (***: p<0.001 vs WT). (B) VSMC supernatants from Tg and WT mice were added on type I collagen gels and incubated at 37°C for 24h. A representative image is shown of the visualisation of those gels by confocal reflection microscopy. The images correspond to the maximum projection of a series in z (18 sections). Bar: 20μm. (C) Representative image of Sirius red staining of carotid arteries from the above animals visualised under polarised light. The arrows indicate red birefringent regions corresponding to coarse and compact collagen fibres (i.e. mature collagen).
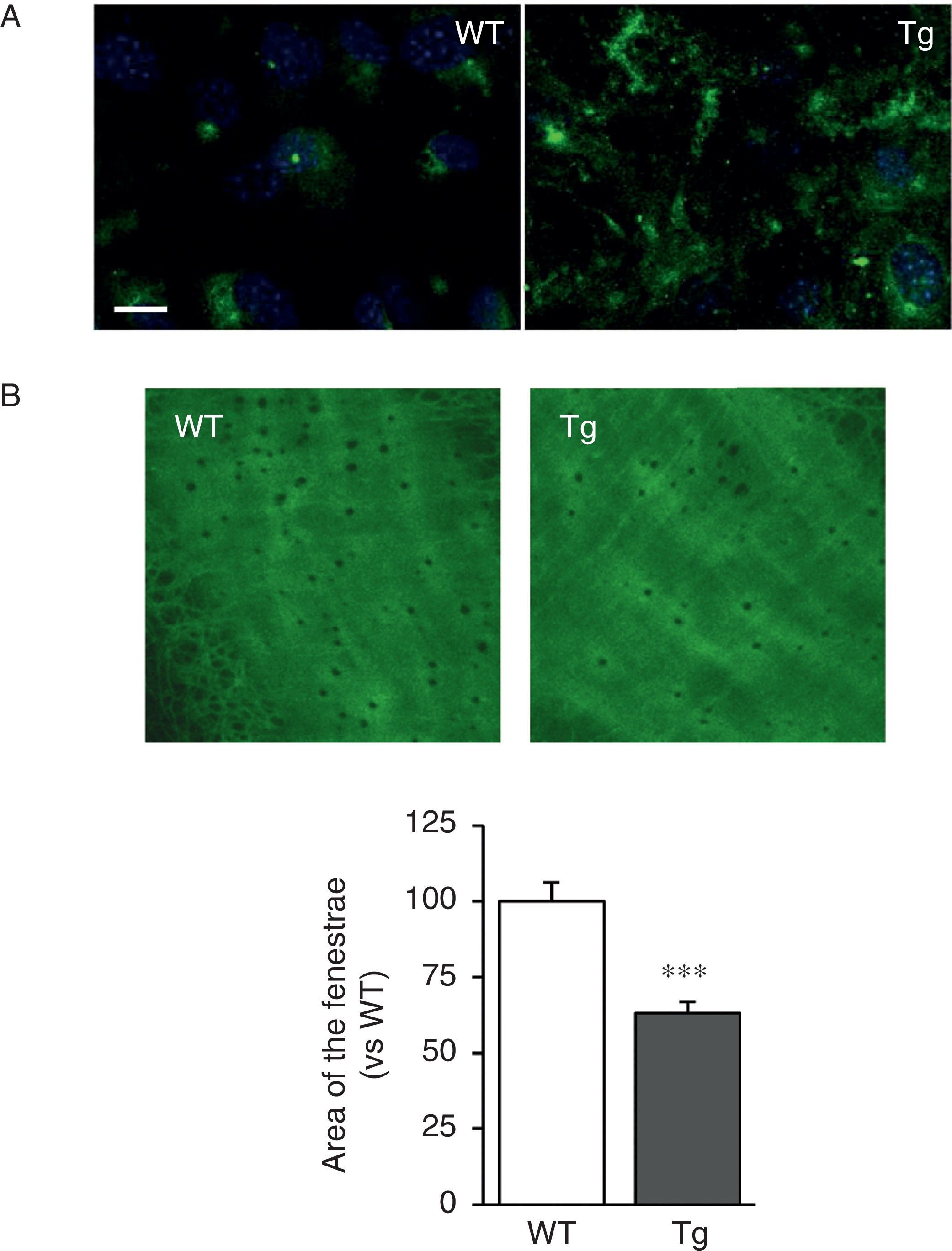
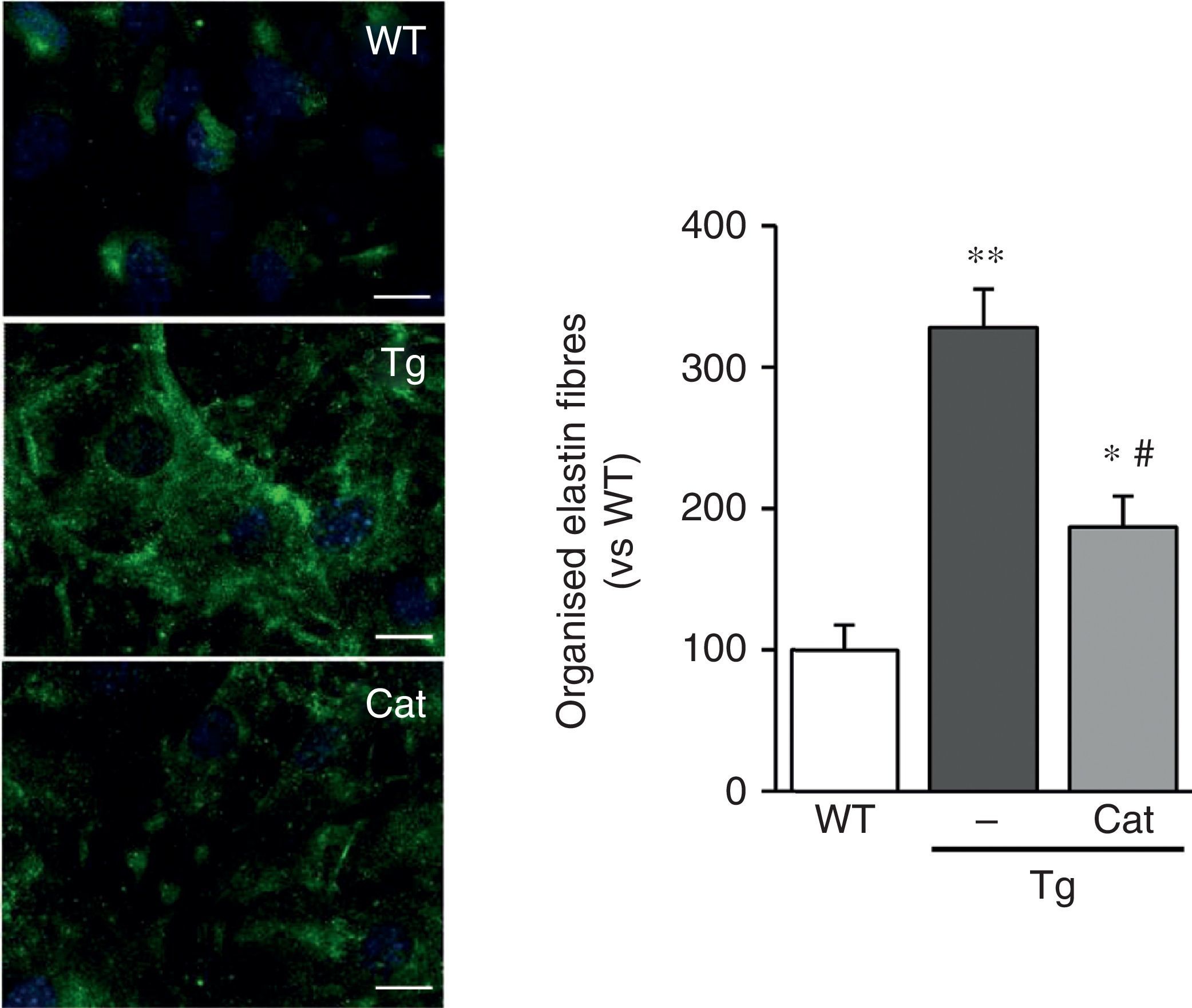
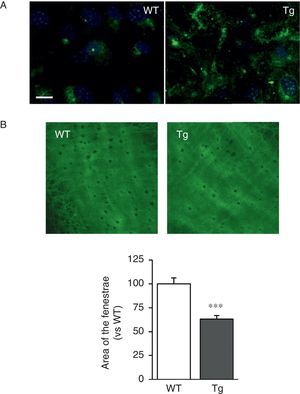
The VSMC of mice transgenic for LOX deposited thicker and more structurally organised elastin fibres, indicative of a higher degree of assembly than the cells isolated from WT mice (Fig. 2A). LOX transgenesis was also associated with a reduction in the size (Fig. 2B) and number of fenestrae of the internal elastic lamina (WT: 3804±258; Tg: 2550±170, n=6; p<0.01 vs WT).
LOX transgenesis alters the structure of elastin. (A) Elastin staining (in green) in non-permeabilised VSMC isolated from control mice (WT) and mice transgenic for LOX (Tg). The nuclei were stained with Hoechst 33342 (blue). Bar: 20μm. (B) Maximum projections of the internal elastic lamina of mesenteric arteries from the animals indicated in A. The projections were obtained from serial optical sections captured with a fluorescence confocal microscope (oil immersion objective 40× zoom 4). Image size 93.75μm×93.75μm. The results are expressed as mean±SEM (n=4–10; ***: p<0.001 vs WT).
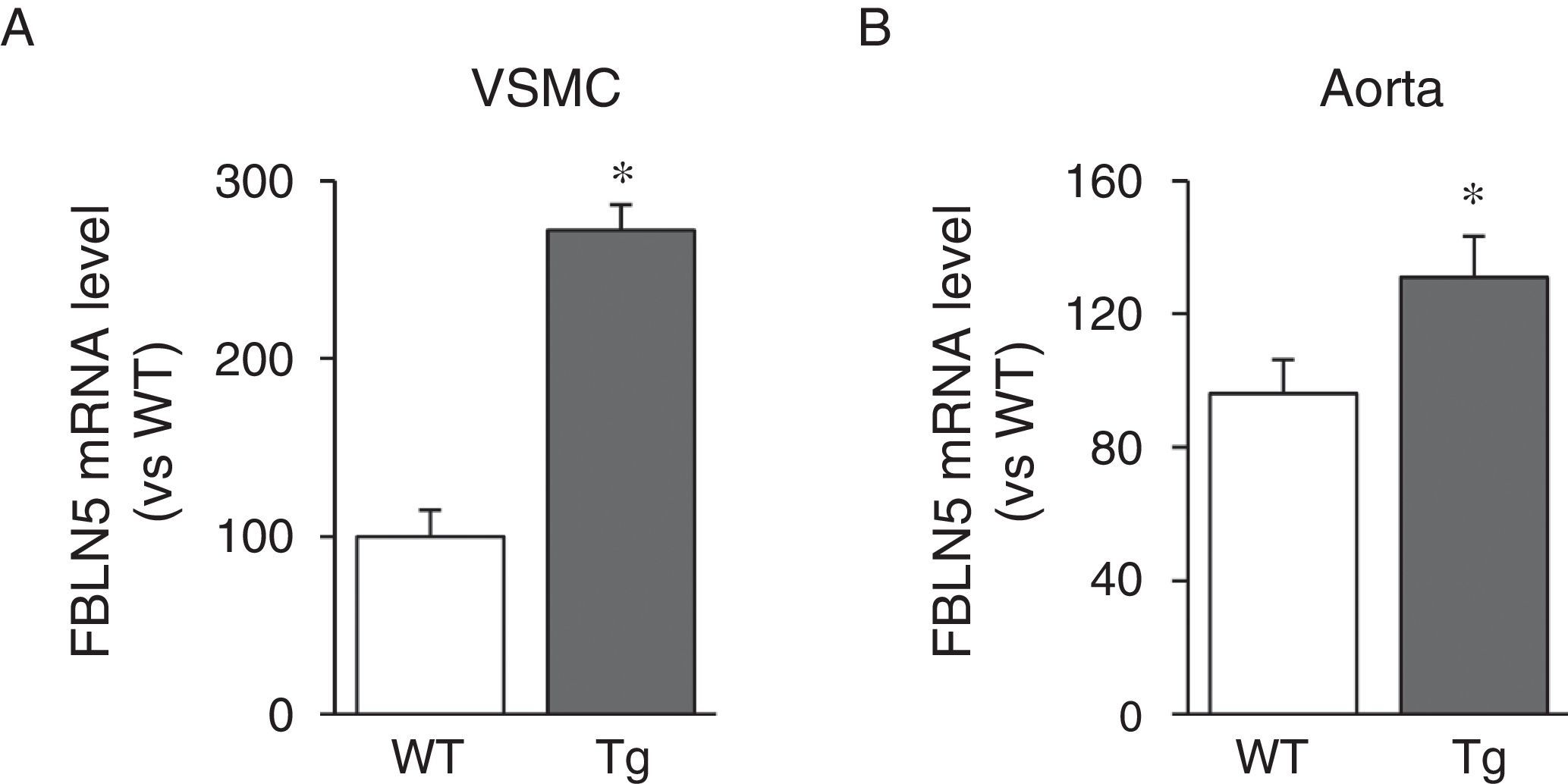
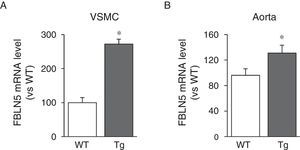
We then analysed whether LOX transgenesis could affect the expression of elastogenic proteins such as FBLN5. As can be seen in Fig. 3A, over-expression of LOX in VSMC increased the expression of FBLN5 by approximately 2.7-fold compared to cells from WT mice. Similarly, a significant increase in the level of FBLN5 mRNA was detected in the aorta of the transgenic mice (Fig. 3B).
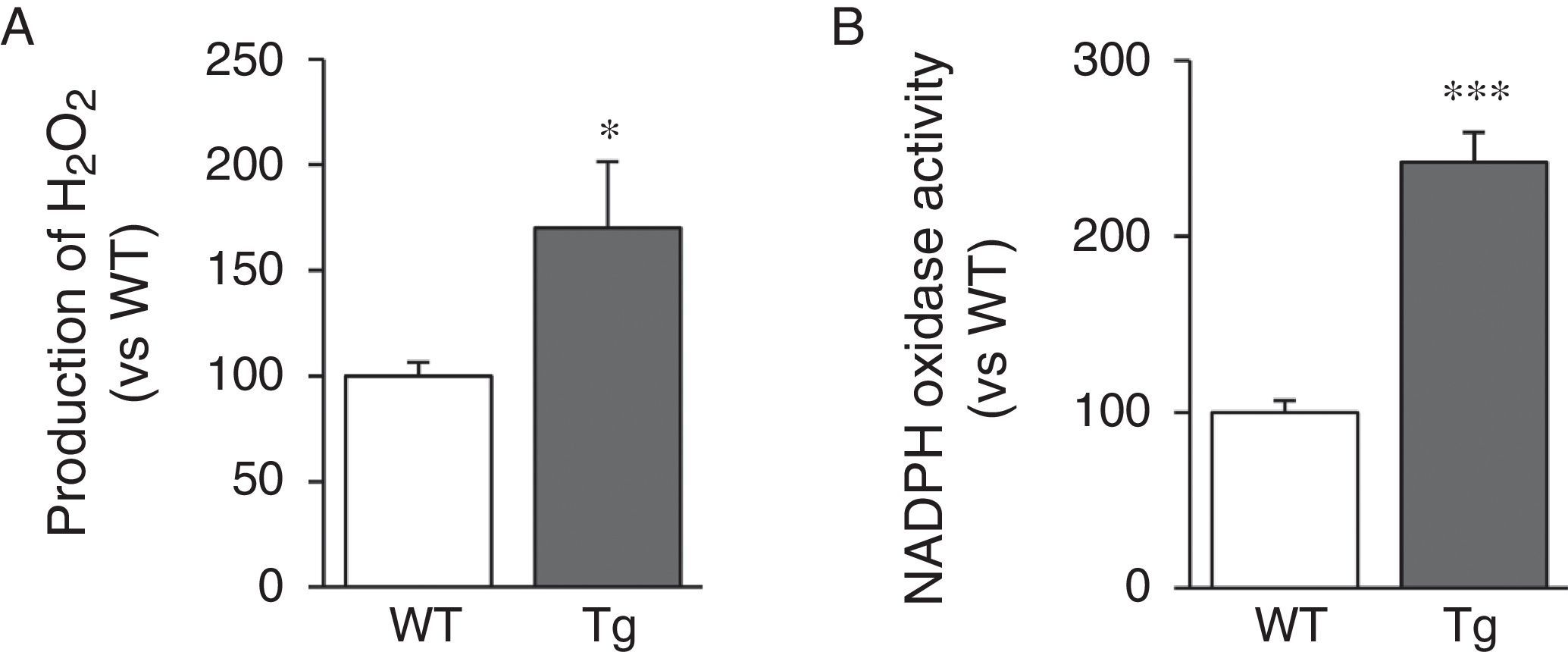
LOX transgenesis induces oxidative stress in the vascular wallSince LOX activity results in the production of H2O2 as a by-product, we analysed H2O2 levels in the aorta of transgenic mice. We can see that aortic production of H2O2 increased in animals transgenic for LOX (Fig. 4A). LOX over-expression also induced a significant increase in activity of NADPH oxidase, a major superoxide anion-producing enzyme (Fig. 4B).
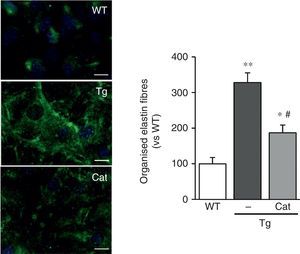
Catalase prevents changes in elastin structureIn view of the results described above, we wanted to evaluate whether the oxidative stress induced by LOX transgenesis might play a part in the changes in elastin structure observed in this model. To do this, we analysed the impact of incubation with catalase, an H2O2-detoxifying enzyme, on the deposition of elastin in VSMC. As shown in Fig. 5, incubation with catalase partially attenuated the enhanced organisation of the elastin fibres deposited by the VSMC from transgenic animals.
The production of H2O2 is involved in the alteration of elastin structure induced by LOX over-expression. VSMC from control mice (WT) and mice transgenic for LOX (Tg) were incubated in the presence or absence of catalase (Cat) for 10 days. Elastin staining (in green) is shown in non-permeabilised VSMC. The nuclei were stained with Hoechst 33342 (blue). Bar: 20μm. The results are shown as mean±SEM (n=6–10; *: p<0.05 vs WT; #: p<0.05 vs Tg).
The importance of LOX in the development of many different diseases and its involvement in the control of key processes in cardiovascular pathophysiology have become apparent in recent years.1,2,4,12,19–21 The multifunctional character of LOX, along with the discovery of the biological activity of the LOX propeptide (released during the maturation of the enzyme) and the existence of active intracellular forms located even in the cellular nucleus have increased the interest in this enzyme.1,2 Studies in this field have benefited from the development of a transgenic animal model that over-expresses LOX specifically in VSMC. This model has allowed us to demonstrate that LOX plays a fundamental role in vascular remodelling, as it limits the proliferation of VSMC.12 It is the only genetically modified animal model currently available to help improve our knowledge of the biology of this enzyme, since animals deficient in LOX are not viable.22
The VSMC isolated from animals transgenic for LOX were found to have a high level of production of bioactive LOX protein12 and, consequently, they had high levels of LOX activity, which leads to an increase in the capacity of the supernatants of these cells to polymerise collagen, one of the main substrates of this enzyme. Accordingly, transgenic animals also show a higher degree of assembly of the collagen fibres in the vascular wall.
Similarly, the elastin fibres deposited by VSMC isolated from the transgenic animals show a higher degree of assembly, which results in thicker fibres. Since elastogenesis is a complex process involving multiple steps, the increased level of organisation of elastin fibres observed in transgenic animals may be associated not only with high LOX activity, but also with other effects deriving from LOX over-expression, including the increase in vascular expression of FBLN5 found in this study. FBLN5 is a glycoprotein that regulates the proteolytic activation of LOX and is essential in the maintenance of elastic fibres and in vascular remodelling.23,24 FBLN5 also regulates integrin-mediated signalling and controls the adhesion, migration, proliferation and survival of vascular cells.18,25,26 Therefore, if FBLN5 is induced in response to LOX over-expression, this may profoundly affect vascular homeostasis. In addition, analysis of the elastin structure of the internal elastic lamina in the Tg mice revealed a reduction in the number and size of the fenestrae. We have shown in previous studies that the decrease in the area of the fenestrae of the internal elastic lamina at least partly explains the increase in vascular stiffness in animal models of hypertension,5,27–29 and it has been suggested that the reduction in the size of the fenestrae observed in hypertensive animals could lead to significant alterations in myoendothelial communication and so critically affect vascular function.30 Therefore, the changes in the number and size of the fenestrae induced by the transgenesis of LOX could result in greater stiffness and abnormal vascular function. It is clear, however, that this hypothesis will have to be confirmed in the future and further studies will be required to clarify the pathophysiological consequences of this effect.
LOX catalyses the transformation of an amino group by an aldehyde group into peptidyl lysine residues of collagen and elastin fibres, and this results in the generation of H2O2 as a by-product.1,2 Animals transgenic for LOX show elevated H2O2 levels in the vascular wall, most likely as a result of the higher LOX activity in the VSMC of these animals. Recent studies in developing rat heart have identified LOX as a significant source of H2O2 in the vascular wall.31 Our results expand on these observations and confirm the contribution of LOX to the production of vascular H2O2. At the same time, LOX transgenesis is associated with an increase in vascular NADPH oxidase activity. The mechanism behind this effect is not yet fully understood. However, it should be noted that H2O2 is a stable and permeable reactive oxygen species (ROS) that carries out multiple functions in the vascular wall and, among other responses, it may affect the production of oxidative stress from other sources of ROS, including NADPH oxidase.32,33 The increase in H2O2 production resulting from LOX activity is implicated in the ability of this enzyme to induce cell migration and VSMC chemotaxis, among other effects.10,11,34 The results obtained in VSMC grown in the presence of catalase suggest precisely that the high levels of H2O2 produced by VSMC that over-express LOX at least partly contribute to the higher deposition of mature elastin fibres detected in these cells. In fact, H2O2 has been reported to induce the deposition of different components of microfibrils, which may also contribute to the alteration in the structure of the ECM observed in our model.35
To sum up, our findings demonstrate that LOX over-expression is associated with an increase in vascular oxidative stress and suggest that this mechanism may at least partly contribute to the change in the elastin structure induced by LOX activation. Since oxidative stress plays a key role in the development of cardiovascular diseases, these results suggest that the control of LOX activity could be a useful strategy for limiting ROS production in disorders characterised by increased activity/expression of LOX.
Ethical disclosuresProtection of human and animal subjectsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and to the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study was funded by the Sociedad Española de Arteriosclerosis [Spanish Arteriosclerosis Society], SEA/FEA 2016 Grant for Basic Research, and the Spanish Ministry of Economy and Competitiveness (MINECO)-Instituto de Salud Carlos III (ISCIII) [Carlos III Health Institute] [projects PI15/01016, PI13/01488, SAF2012-36400; SAF2015-64767-R]. The Biomedical Research Network Centres (CIBER) for Cardiovascular Diseases is an ISCIII initiative. AMB received a grant from the Ramón y Cajal programme (RYC-2010-06473). The study was co-financed by the European Regional Development Fund (ERDF), “A way to make Europe”.
Authors’ contributionsCR and AMB designed the study, interpreted the data and wrote the manuscript. SV and ABGR performed the experiments and analysed the data, and participated in the drafting of the manuscript. JMG and MS participated in the analysis of the results and writing of the manuscript. All the authors approved the final version of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Laura García Redondo and Silvia Aguiló for their technical support.
Please cite this article as: Varona S, García-Redondo AB, Martínez-González J, Salaices M, Briones AM, Rodríguez C. La sobreexpresión vascular de la lisil oxidasa altera la estructura de la matriz extracelular e induce estrés oxidativo. Clin Invest Arterioscler. 2017;29:157–165.