To analyze the safety profile of a nifedipine oral solution in the treatment of preterm labor (PTL).
MethodsA multi-center, open-label, prospective, single-arm, observational study was conducted in 500 women with PTL to whom a nifedipine oral solution was prescribed according to its Summary of Product Characteristics. Safety profile and tolerability of oral administration of nifedipine solution during routine clinical practice was assessed as the primary objective of the study and treatment efficacy as secondary objective.
ResultsNo severe adverse events were reported among these women, including severe hypotension. Eight patients (2.3%) reported adverse reactions of moderate intensity, and in 0.9% of the patients (3 cases), these adverse reactions caused the discontinuation of the treatment.
ConclusionsThe results of this study show that nifedipine oral solution exhibits an excellent safety profile used as a tocolytic treatment in women with PTL.
Analizar el perfil de seguridad de una solución oral de nifedipino en el tratamiento del parto prematuro (PP).
MétodosSe llevó a cabo un estudio observacional, prospectivo, de diseño abierto, de rama única y multicéntrico en 500 mujeres que presentaban un PP, a las que se les administró una solución oral de nifedipino según la ficha técnica del producto. El perfil de seguridad y la tolerancia de la solución oral de nifedipino, en el contexto de la práctica clínica rutinaria, fueron evaluados como objetivo primario del estudio, y la eficacia del tratamiento, como objetivo secundario.
ResultadosNo se notificaron efectos adversos graves, incluyendo hipotensión severa. Ocho pacientes (2,3%) presentaron reacciones adversas de intensidad moderada, y en el 0,9% de las pacientes (3 casos) estos efectos adversos provocaron la discontinuación del tratamiento.
ConclusionesLos resultados de este estudio muestran que la solución oral de nifedipino dispone de un excelente perfil de seguridad para su uso como tocolítico en el tratamiento de mujeres con PP.
Prematurity is the main risk factor for mortality and morbidity of newborns in western countries,1–3 with an increased probability of impaired motor and cognitive development during childhood when compared to infants born at term. The most prevalent pathology affecting preterm newborns is related with pulmonary function, mainly due to immaturity.4
Even if prenatal interventions, such as corticosteroids administration or delivering at a center with adequate facilities to handle extreme prematurity, improve outcomes, gestational age at birth is still the main prognostic factor of subsequent morbimortality.5 Consequently, a tocolytic treatment might be used to delay birth at least 48h to allow corticosteroids to provide the maximum benefit for the newborn, and in utero transfer to a high-complexity facility if needed.2,6–8
Nifedipine, a widely known calcium-channel blocker which acts as a smooth muscle relaxant, can attenuate uterine contractions.9 Its efficacy and safety have been demonstrated in several randomized trials and validated in subsequent reviews and meta-analyses.2,6,10 However, nifedipine capsules are used off-label with heterogenous administration patterns according to the available preparation forms and variable medical protocols.
For this reason, a nifedipine oral solution (NifOS) was developed to face the specific problem of tocolysis. This novel formulation has a quicker absorption rate, which increases bioavailability and lowers pharmacokinetic variability.11 A comparative study between nifedipine capsules and nifedipine oral solution showed that although both drugs had similar efficacy, the NifOS had less adverse events like hypotension or tachycardia.12
Subsequently, the Spanish Medicines Agency (Agencia Española de Medicamentos y Productos Sanitarios – AEMPS) approved a NifOS (Nife-par®, Laboratorio Reig Jofre S.A., Spain) in 2013 for its use as tocolysis in the management of preterm labor.13 In 2014 Nife-par® was included as an alternative tocolytic treatment in the Protocol of the Spanish Society of Gynecology and Obstetrics (SEGO).14
Following the generalization of the use of Nife-par®, and as a requirement of the AEMPS, we conducted this study with the aim to prospectively collect further safety information on the administration of this NifOS in pregnant women with preterm labor (PTL) in a clinical setting under current practice conditions.
MethodsDesign and patientsThis was a multi-center, open-label, prospective, single-arm, observational study conducted in women with PTL to whom a NifOS (Nife-par®, Laboratorio Reig Jofre S.A., Spain) was prescribed according to its Summary of Product Characteristics (SmPC).13
Participants were recruited from 30 public and private Spanish hospitals from 2016 to 2020 and ethics approval was obtained from an Ethics Committee at each participant hospital. The use of tocolysis and its particular regimen occurred before the inclusion of the patient in the study. Therefore, this was a non-interventional study.
PTL was defined as the presence of contractions and/or cervical length up to 5th centile by transvaginal ultrasound or positive vaginal fibronectin test, according to the procedures of each center, before 37 weeks of pregnancy.
To be included in the study, patients had to fulfill the following criteria: singleton or twin pregnancies with PTL below 37 weeks under NifOS treatment, aged over 18 years and having signed an informed consent. Patients having already received treatment with nifedipine capsules in the previous hours before starting treatment with NifOS were excluded.
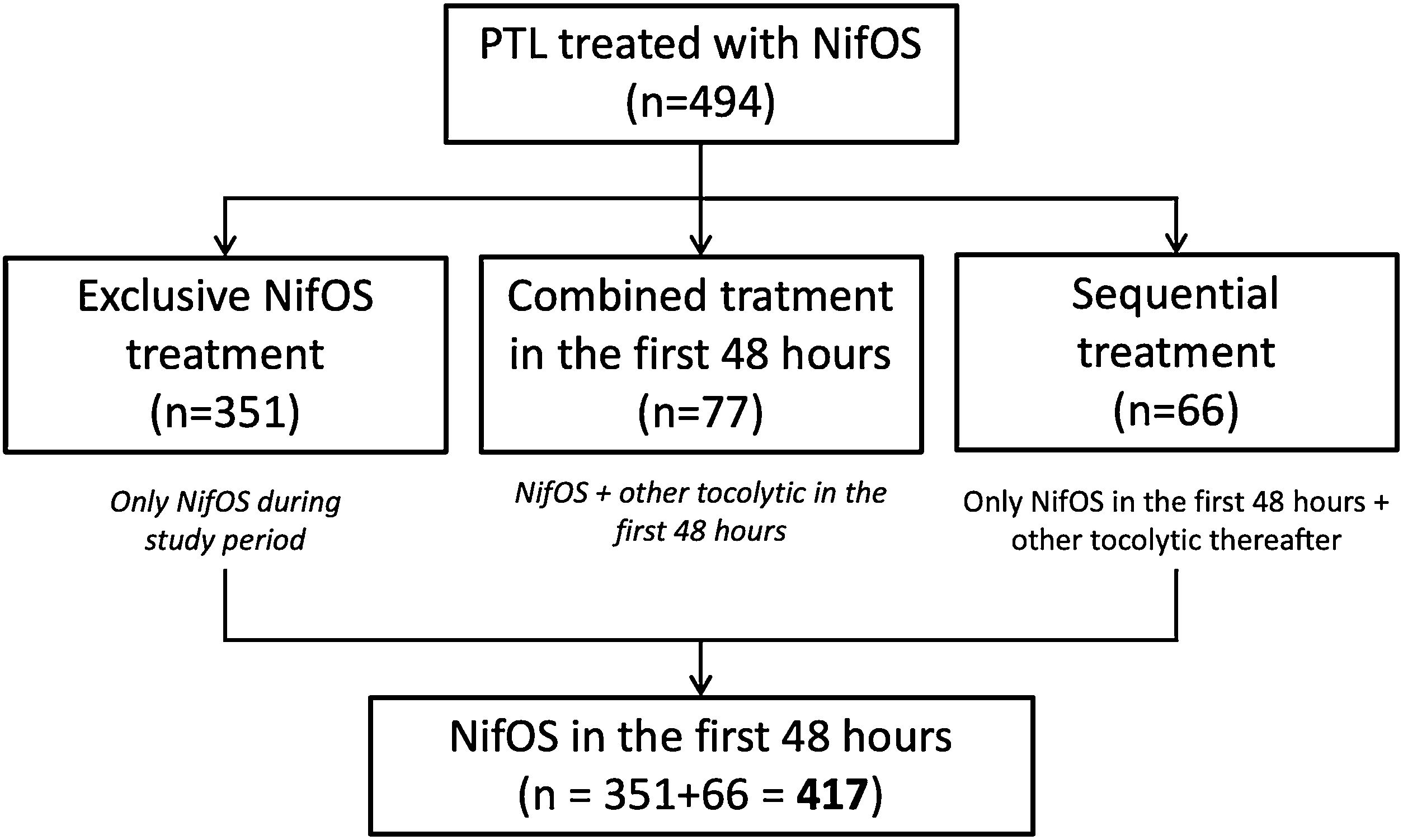
Two analysis sub-populations were defined according to the use of NifOS with or without other tocolytic treatment: (1) NifOS in the first 48h: no other tocolytic was administered during the first 48h from admission, regardless the need of other tocolytic thereafter. (2) Combined treatment, including NifOS, in the first 48h: women who started NifOS and required rescue tocolytic treatment in the first 48h.
A subanalysis have been performed in the subgroup with exclusive treatment with NifOS during all the study period.
Briefly, clinical management of pregnant women diagnosed of PTL consisted in admission, record of vital signs, and blood tests to rule out signs of chorioamnionitis; administration of corticosteroids between 24+0 and 34+6 weeks of gestation and administration of magnesium sulfate between 24+0 and 32+0 weeks. Antibiotic therapy was only administrated when an infection was suspected. Tocolysis was discontinued if delivery was required because of maternal or fetal indication (chorioamnionitis, placental abruption, loss of fetal well-being or other).
The initial dose of NifOS was 2.0mL (10.0mg nifedipine). If contractions were not suppressed, a second dose of 1.5mL (7.5mg nifedipine) after 15min was administrated and repeated every 15min until contractions were suppressed (maximum dose during the first hour is 8mL (40mg)). The maintenance dose is 3mL (15mg nifedipine) every 6–8h (maximum daily dose: 32mL/day (160mg)) for 48h.
The primary objective was to assess the safety profile and tolerability of oral administration of NifOS in pregnant women with PTL during routine clinical practice. As secondary objective, we aimed to assess the efficacy of NifOS in women with PTL.
All variables were described for all patients and separately within each subgroup and collected from the start of treatment until delivery or hospital discharge, whichever occurred first. Baseline, perinatal and admission characteristics were recorded, including each tocolytic regimen administered.
Regarding primary safety and tolerability objectives, adverse events (AEs) suspected to be related to NifOS were collected, as well as the need of NifOS discontinuation due to each of these reactions. The events collected were: (1) moderate adverse reactions (headache, palpitations, hypotension, dizziness, rash, dyspepsia, malaise, tachycardia, urticarial, pharyngeal mucositis, and morning sickness when they limited daily activities and required minimal, local, or non-invasive intervention) and/or (2) severe adverse reactions (severe hypotension).
Hypotension was described as a patient presenting a change over 20% in her basal pressure and if the systolic blood pressure decreased under 100mmHg. It was considered moderate when the patient remained asymptomatic or did not require any intervention, while severe when symptoms required treatment.
These results are presented for those women receiving the exclusive treatment with NifOS during the study period, to rule out any adverse events related to other tocolytic treatment. The complete detail of adverse events in the overall population is presented as supplementary material.
Regarding secondary efficacy outcomes, we recorded delivery and neonatal variables, including: (1) gestational age at delivery (before 32 weeks, between 32+0 and 36+6 weeks and after 36+6 weeks); (2) latency from admission to delivery (women with latency more than 48h and more than 7 days); and (3) neonatal outcomes during a month after delivery, or until discharge if the neonate was admitted for a longer period (birth weight, APGAR score <7 at 5min of life, NICU admission, neonatal death, culture-proven sepsis, necrotizing enterocolitis, respiratory distress syndrome).
Sample sizeSample size was determined based on the accuracy of estimated incidence of AEs. According to available literature of nifedipine as a tocolytic, the incidence of moderate or severe AEs was around 12%.12 Thus, to identify the same proportion with 3% accuracy, a sample size of 500 participants is needed.
Data collection, management and quality controlA specific on-line electronic case report form (e-CRF) was created. Variables were obtained from each hospital medical records. Regular monitoring was performed to guarantee quality information on the e-CRF.
Statistical methodsAnalyses were performed using SAS v9.3 (Cary, North Carolina). All study data were analyzed for the whole study population and for each of the subgroups described above. Descriptive summary statistics for categorical/qualitative variables included frequency count and percentage. The denominator for the percentage calculations was the total number of patients with non-missing observations in each treatment subgroup. Descriptive summary statistics for continuous variables included the number of patients with non-missing observations, median and interquartile range (IQR).
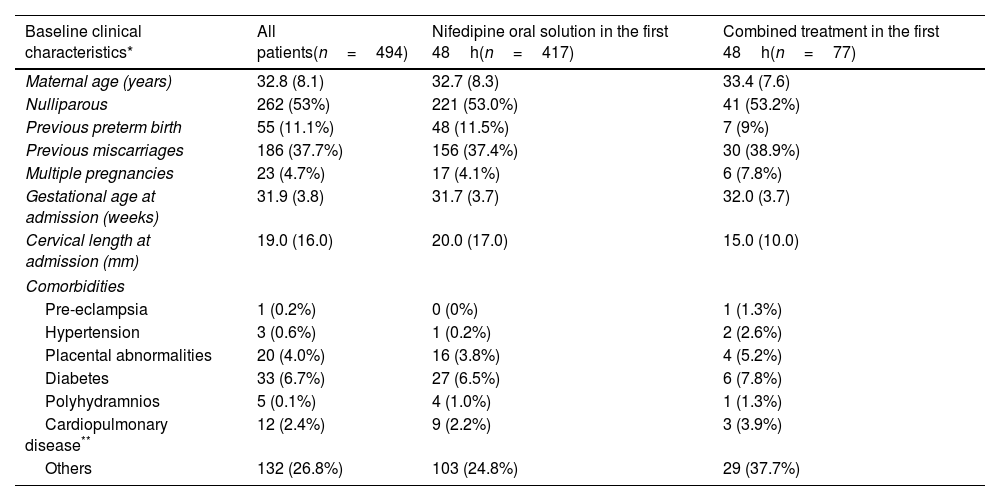
ResultsA total of 494 women with PTL between 22+3 and 35+4 weeks of gestation to whom NifOS was prescribed according to its SmPC were enrolled from September 2016 to March 2020 inclusive and analyzed for this study. The median (IQR) maternal age was 32.8 (8.1) years and the median (IQR) gestational age at admission was 31.9 (3.8) weeks. Six patients (1.21%) were at 23+6 weeks or less gestation, 248 patients (50.2%) were between 24+0 and 31+6 weeks, and 240 patients (48.5%) were at or over 32+0 weeks. Baseline, perinatal and admission characteristics are shown in Tables 1a and 1b.
Baseline characteristics of the population at admission.
| Baseline clinical characteristics* | All patients(n=494) | Nifedipine oral solution in the first 48h(n=417) | Combined treatment in the first 48h(n=77) |
|---|---|---|---|
| Maternal age (years) | 32.8 (8.1) | 32.7 (8.3) | 33.4 (7.6) |
| Nulliparous | 262 (53%) | 221 (53.0%) | 41 (53.2%) |
| Previous preterm birth | 55 (11.1%) | 48 (11.5%) | 7 (9%) |
| Previous miscarriages | 186 (37.7%) | 156 (37.4%) | 30 (38.9%) |
| Multiple pregnancies | 23 (4.7%) | 17 (4.1%) | 6 (7.8%) |
| Gestational age at admission (weeks) | 31.9 (3.8) | 31.7 (3.7) | 32.0 (3.7) |
| Cervical length at admission (mm) | 19.0 (16.0) | 20.0 (17.0) | 15.0 (10.0) |
| Comorbidities | |||
| Pre-eclampsia | 1 (0.2%) | 0 (0%) | 1 (1.3%) |
| Hypertension | 3 (0.6%) | 1 (0.2%) | 2 (2.6%) |
| Placental abnormalities | 20 (4.0%) | 16 (3.8%) | 4 (5.2%) |
| Diabetes | 33 (6.7%) | 27 (6.5%) | 6 (7.8%) |
| Polyhydramnios | 5 (0.1%) | 4 (1.0%) | 1 (1.3%) |
| Cardiopulmonary disease** | 12 (2.4%) | 9 (2.2%) | 3 (3.9%) |
| Others | 132 (26.8%) | 103 (24.8%) | 29 (37.7%) |
*Categorical variables are expressed as number (percentage) and continuous variables as medians (interquartile ranges).
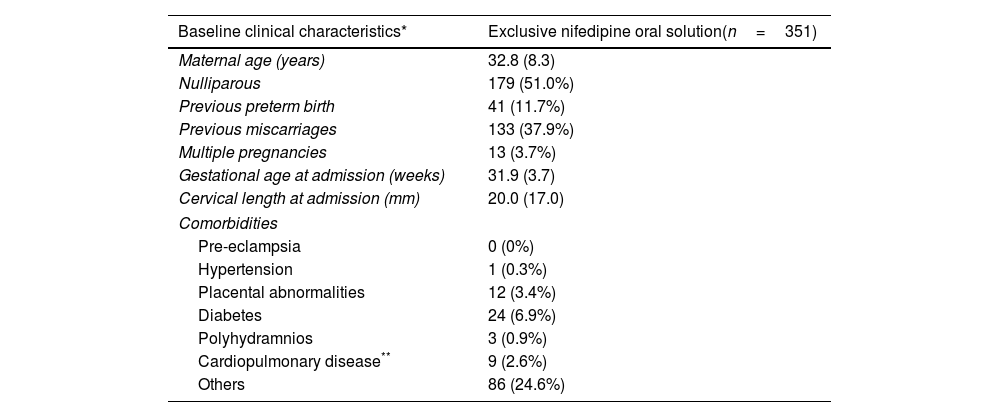
Baseline characteristics of the population with exclusive treatment with Nifedipine oral solution tratment at admission.
| Baseline clinical characteristics* | Exclusive nifedipine oral solution(n=351) |
|---|---|
| Maternal age (years) | 32.8 (8.3) |
| Nulliparous | 179 (51.0%) |
| Previous preterm birth | 41 (11.7%) |
| Previous miscarriages | 133 (37.9%) |
| Multiple pregnancies | 13 (3.7%) |
| Gestational age at admission (weeks) | 31.9 (3.7) |
| Cervical length at admission (mm) | 20.0 (17.0) |
| Comorbidities | |
| Pre-eclampsia | 0 (0%) |
| Hypertension | 1 (0.3%) |
| Placental abnormalities | 12 (3.4%) |
| Diabetes | 24 (6.9%) |
| Polyhydramnios | 3 (0.9%) |
| Cardiopulmonary disease** | 9 (2.6%) |
| Others | 86 (24.6%) |
*Categorical variables are expressed as number (percentage) and continuous variables as medians (interquartile ranges).
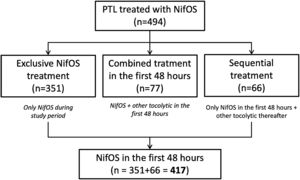
Regarding the administered treatment, 84.4% (417/494) women received only NifOS in the first 48h and 15.6% (77/494) of the participants started tocolytic treatment with NifOS and subsequently required alternative combined tocolytic therapy during the first 48h. Only 15.8% (66/417) of the patients who received only NifOS in the first 48h required other tocolytic thereafter. Thus, 71.1% of all the patients (351/494) received exclusive NifOS treatment during all the study period. Flowchart of the grouping process is presented as Fig. 1.
Regarding the population who received exclusive treatment with NifOS, 8 patients (8/351, 2.3%) presented moderate AEs related with study medication. No severe adverse events were reported among these women. The most frequent AEs were headache (2/351, 0.6%), palpitations (2/351, 0.6%), dyspepsia (2/351, 0.6%) and malaise (2/351, 0.6%).
Only 0.9% of the patients (3/351) discontinued the study due to at least one moderate or severe AE that was possibly related to the treatment: malaise (2/351, 0.6%) and rash (1/351, 0.3%). None of these participants discontinued treatment with NifOS because of severe hypotension. The complete description of moderate and severe AEs is presented in Table 2, while Table S1 shows the moderate or severe events among the overall population. No maternal or fetal deaths were observed.
Moderate and severe adverse events possibly or probably related to nifedipine oral solution in exclusive nifepar oral solution group (n=351). It must be highlighted that a patient may present more than one adverse event.
| Patients with at least one treatment-related moderate/severe AE, n (%) | 8 (2.3%) |
| Moderate | 8 (2.3%) |
| Severe | 0 |
| Adverse events | Patients with AEs | Patients with AEs leading to treatment discontinuation |
|---|---|---|
| Headache | 2 (0.6%) | 0 |
| Palpitations | 2 (0.6%) | 0 |
| Hypotension | 0 | 0 |
| Dizziness | 1 (0.3%) | 0 |
| Rash | 1 (0.3%) | 1 (0.3%) |
| Dyspepsia | 2 (0.6%) | 0 |
| Malaise | 2 (0.6%) | 2 (0.6%) |
| Tachycardia | 0 | 0 |
| Urticaria | 0 | 0 |
| Pharyngeal mucositis | 0 | 0 |
| Morning sickness | 0 | 0 |
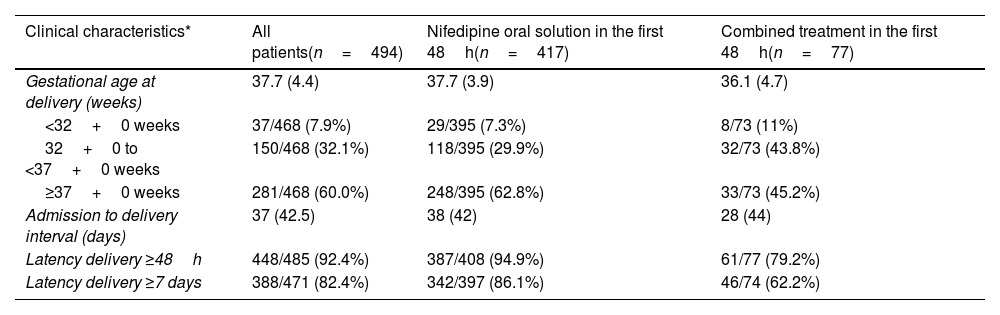
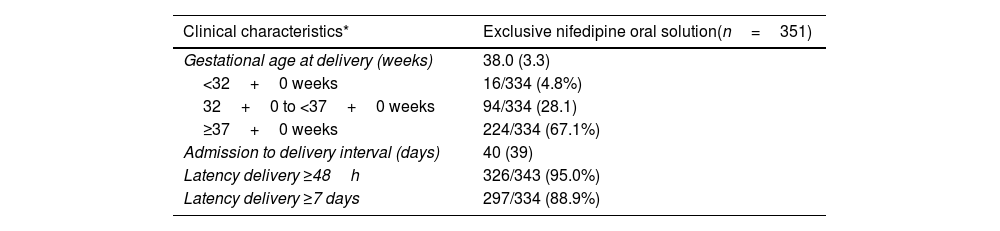
The proportion of women who remained undelivered for 48h or more and 7 days or more after initiation of tocolytic treatment was 92.4% (448/485) and 82.4% (388/471), respectively (Table 3a).
Delivery outcomes.
| Clinical characteristics* | All patients(n=494) | Nifedipine oral solution in the first 48h(n=417) | Combined treatment in the first 48h(n=77) |
|---|---|---|---|
| Gestational age at delivery (weeks) | 37.7 (4.4) | 37.7 (3.9) | 36.1 (4.7) |
| <32+0 weeks | 37/468 (7.9%) | 29/395 (7.3%) | 8/73 (11%) |
| 32+0 to <37+0 weeks | 150/468 (32.1%) | 118/395 (29.9%) | 32/73 (43.8%) |
| ≥37+0 weeks | 281/468 (60.0%) | 248/395 (62.8%) | 33/73 (45.2%) |
| Admission to delivery interval (days) | 37 (42.5) | 38 (42) | 28 (44) |
| Latency delivery ≥48h | 448/485 (92.4%) | 387/408 (94.9%) | 61/77 (79.2%) |
| Latency delivery ≥7 days | 388/471 (82.4%) | 342/397 (86.1%) | 46/74 (62.2%) |
*Categorical variables are expressed as number (percentage) and continuous variables as medians (interquartile ranges).
The median (IQR) gestational age at time of delivery was 37.7 (31) weeks for the whole study population. More than half of women (281/468; 60%) delivered after 37 weeks of gestation. This percentage was lower in patients that required tocolytics apart from NifOS in the first 48h. Alternative tocolytics were used as rescue therapy in the first 48h in 77 patients (15.6%). Conversely, the rate of preterm delivery at <32 weeks of gestation was 7.9% (37/468). This percentage was lower in women with exclusive treatment with NifOS treatment who never required other tocolytic treatment (Table 3b).
Delivery outcomes in the population with exclusive treatment with nifedipine oral solution tratment at admission.
| Clinical characteristics* | Exclusive nifedipine oral solution(n=351) |
|---|---|
| Gestational age at delivery (weeks) | 38.0 (3.3) |
| <32+0 weeks | 16/334 (4.8%) |
| 32+0 to <37+0 weeks | 94/334 (28.1) |
| ≥37+0 weeks | 224/334 (67.1%) |
| Admission to delivery interval (days) | 40 (39) |
| Latency delivery ≥48h | 326/343 (95.0%) |
| Latency delivery ≥7 days | 297/334 (88.9%) |
*Categorical variables are expressed as number (percentage) and continuous variables as medians (interquartile ranges).
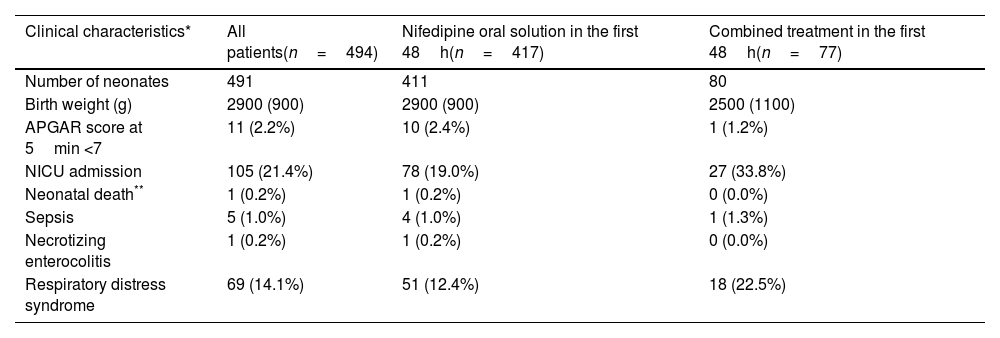
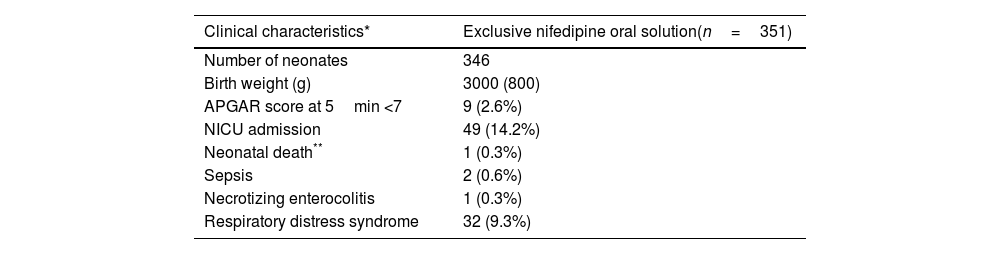
Most of the women had a spontaneous onset of labor (339/447; 75.8%) and a vaginal delivery (368/491; 74.9%). Neonatal median (IQR) weight was 2.9 (0.9)kg. In 11 cases (2.2%), the Apgar score at 5min was below 7. About a fifth of babies (105/490; 21.4%) required neonatal intensive care unit (NICU) admission and 14.2% (69/486) presented respiratory distress syndrome. One neonatal death at three days of life was accounted, associated to congenital malformations diagnosed before NifOS treatment (1/489; 0.2%). Other perinatal morbidities are summarized in Tables 4a and 4b.
Neonatal outcomes.
| Clinical characteristics* | All patients(n=494) | Nifedipine oral solution in the first 48h(n=417) | Combined treatment in the first 48h(n=77) |
|---|---|---|---|
| Number of neonates | 491 | 411 | 80 |
| Birth weight (g) | 2900 (900) | 2900 (900) | 2500 (1100) |
| APGAR score at 5min <7 | 11 (2.2%) | 10 (2.4%) | 1 (1.2%) |
| NICU admission | 105 (21.4%) | 78 (19.0%) | 27 (33.8%) |
| Neonatal death** | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) |
| Sepsis | 5 (1.0%) | 4 (1.0%) | 1 (1.3%) |
| Necrotizing enterocolitis | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) |
| Respiratory distress syndrome | 69 (14.1%) | 51 (12.4%) | 18 (22.5%) |
*Categorical variables are expressed as number (percentage) (over total neonates with available data) and continuous variables as medians (interquartile ranges).
Neonatal outcomes in the population with exclusive treatment with nifedipine oral solution tratment at admission.
| Clinical characteristics* | Exclusive nifedipine oral solution(n=351) |
|---|---|
| Number of neonates | 346 |
| Birth weight (g) | 3000 (800) |
| APGAR score at 5min <7 | 9 (2.6%) |
| NICU admission | 49 (14.2%) |
| Neonatal death** | 1 (0.3%) |
| Sepsis | 2 (0.6%) |
| Necrotizing enterocolitis | 1 (0.3%) |
| Respiratory distress syndrome | 32 (9.3%) |
*Categorical variables are expressed as number (percentage) (over total neonates with available data) and continuous variables as medians (interquartile ranges).
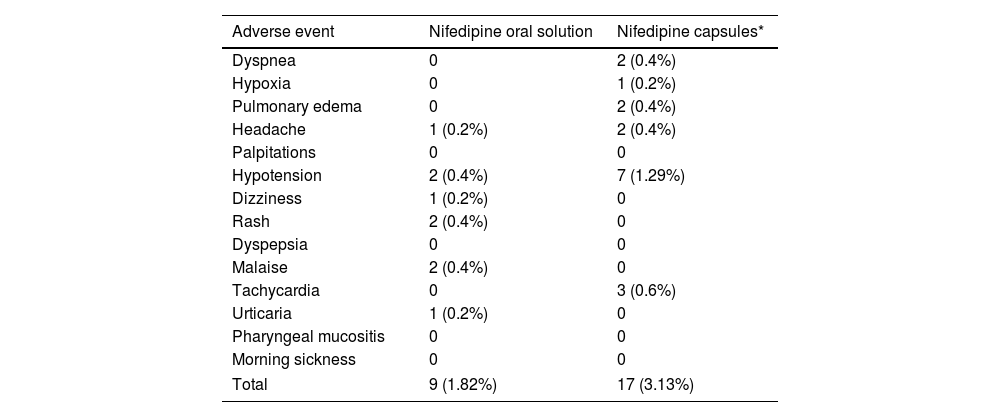
The results of our study show that NifOS presents a remarkable security profile. Only three women (0.9%) under exclusive treatment with NifOS needed to discontinue the study medication due to adverse events. Of them, none of them had to do it due to severe hypotension. Previous studies conducted with nifedipine capsules1 reported a greater rate of treatment interruptions due to AEs (which, for illustrative purposes, the prevalence of AEs in our cohort together with the rates reported in previous studies1 is shown in Table 5), and, additionally, the proportion of women presenting headache or mild hypotension was lower than in other studies using nifedipine capsules. The metanalysis on calcium-channel blockers for tocolysis reported a proportion of side-effects reaching almost 20% of women, and during APOSTEL-III, 6% of women stopped nifedipine capsules because of side-effects, which was not significantly different from those under atosiban.15
Moderate and severe adverse events leading to treatment discontinuation.
| Adverse event | Nifedipine oral solution | Nifedipine capsules* |
|---|---|---|
| Dyspnea | 0 | 2 (0.4%) |
| Hypoxia | 0 | 1 (0.2%) |
| Pulmonary edema | 0 | 2 (0.4%) |
| Headache | 1 (0.2%) | 2 (0.4%) |
| Palpitations | 0 | 0 |
| Hypotension | 2 (0.4%) | 7 (1.29%) |
| Dizziness | 1 (0.2%) | 0 |
| Rash | 2 (0.4%) | 0 |
| Dyspepsia | 0 | 0 |
| Malaise | 2 (0.4%) | 0 |
| Tachycardia | 0 | 3 (0.6%) |
| Urticaria | 1 (0.2%) | 0 |
| Pharyngeal mucositis | 0 | 0 |
| Morning sickness | 0 | 0 |
| Total | 9 (1.82%) | 17 (3.13%) |
Uncertainty remains over which tocolytic is more effective.16 The only tocolytic drugs approved in Spain are betamimetics (ritodrine) and oxytocin receptor antagonist (atosiban). Although extensively used, calcium-channel blockers (nifedipine) had never been approved for this indication until recently, when the nifedipine oral solution Nife-par® received the authorization to be used for this purpose. However, given its recent commercialization, we intended to collect data regarding safety of this formulation in a clinical setting under current practice circumstances.
The differences that we found may be explained by the different pharmacokinetic profile of NifOS and nifedipine capsules. NifOS has a quicker absorption rate, which increases bioavailability and lowers pharmacokinetic variability.11 This leads to less fluctuant and more predictable serum concentrations which, in turn, minimize the potential risk of acute hypotension. On the contrary, with nifedipine capsules, the maximum plasma levels of a dose may not have been reached at the time of deciding whether to administer the next one.8 This may lead to an accumulative effect of several doses that can result in excessive hypotension after administration, as occurred in DeHeus1 study, where most serious hypotensive events appeared 2–4h after the last dose.
No deleterious effects were reported on the newborns. The only registered demise was a baby carrying multiple malformations, already diagnosed before the enrollment, so any possible association with the study medication can be ruled out. Therefore, it is safe to stablish that NifOS, used at the recommended doses and following clinical protocols, is safe to be used in the population of pregnant women (and their offspring) admitted for PTL.
Even if the efficacy of nifedipine as a tocolytic agent has already been demonstrated,6 there is always a legitim concern regarding the effectivity of a novel preparation, particularly if the galenic and the posology differs from what is usually administered. When administered following clinical protocols, more than 90% and 80% of women did not give birth within 2 and 7 days after admission for PTL, respectively. The authors of the APOSTEL-III trial15 found rates of deliveries before 48h of 68% if the participant was treated with nifedipine capsules, while almost half of women were delivered during the 7 days following admission. Furthermore, the most recent metanalysis on randomized controlled trials on this subject (which did not include the results of the APOSTEL-III) concluded that nifedipine was effective to avoid birth within 48h in almost 75% of women. Of course, the conditions of a randomized trial are not the same of those of an observational study with no aleatory allocation of patients, where selection bias may hinder the findings. However, our results show that nifedipine, administered as an oral solution, is at least as good as capsules when evaluating its ability to delay delivery.
As abovementioned, to be able to inhibit labor during at least two days is a matter of extreme importance when facing the problem of preterm labor. The beneficial effects of corticosteroids begin 6h after injection and achieve their maximum 48h after the first dose.17,18 Hence, these additional two days represent a major boost in neonatal odds of survival without sequels, which are also improved by the solely increase in gestational age, as it is still the main prognostic factor when discussing preterm delivery. The neonatal outcomes analyzed in our study are in line with those described in the literature.
It must be highlighted that slightly more than 15% of patients required an alternative rescue tocolytic in the first 48h. Of these, 20.8% and 37.8% of them delivered in the first 2 and 7 days, respectively, meaning that most probably, these were the patients at a higher risk of delivery at admission. To go further into these differences, a multivariate analysis overcoming the risk of selection bias would be necessary, and even so, the obtained conclusions would have to be carefully interpreted. Yet, it was beyond the objectives of this study to evaluate what are the factors leading either to the absence of response to tocolysis (nifedipine or other treatments).
The route of administration of nifedipine represents an additional advantage of this molecule. Either in capsules or in solution, having an oral tocolytic is still necessary in obstetrics emergency departments. Apart from its proven efficacy in true clinical situations of preterm labor at high risk of delivery, it may have a utility in those cases consulting for contractions with long cervices or negative biochemical tests. Even if the risk of preterm birth is low, these women may experience discomfort and pain, and they may not be completely reassured if contractions persist. Thus, in this kind of circumstances, where a full course of tocolysis may not be indicated, a non-intravenous treatment may become useful. If the patient is relieved after the administration of a single dose of nifedipine, she may avoid hospitalization and the consequent set of medical interventions (ranging from venous catheterization to corticosteroids administration) which, if preterm delivery does not occur, are not harmless and may represent a risk for infants in the future.18 However, nifedipine capsules require off-label prescriptions and are being discontinued in many countries, so NifOS is an opportunity to maintain available this molecule of proven efficacy and an excellent security profile.
Our study is the largest series of patients treated with this novel formulation in women at risk of preterm delivery, which represents its main strength. Furthermore, it has been prospectively tested in hospital settings, following current protocols, and providing results that echo clinical reality. Nevertheless, this pragmatic design may inevitably introduce bias, as physicians in charge may influence on the selection of patients who will receive the studied molecule. However, this is only an additional spitting image of reality, where obstetricians may decide, among different options, which treatment they consider best for their patients. This is also reflected in the characteristics of the patients that were included in the study, as our population is heterogeneous regarding the criteria leading to the diagnosis of PTL. While all patients had contractions, the additional criterion that triggered the tocolytic treatment may have been different depending on the protocol followed by each center. In this way, pregnancies at a high-risk of preterm labor (and therefore requiring tocolysis) would be considered as such if cervix is shortened, if fetal fibronectin determination was positive (irrespectively of cervical length), or if the woman presented additional risk factors (such as a twin pregnancy or a previous preterm delivery). Consequently, the efficacy results must be assessed within the framework of a non-experimental clinical setting, but this should not affect the analysis of the safety outcomes. Considering that the latter was the main objective of the study, the design is appropriate to answer this question, even if other results should be carefully interpreted. Finally, as previously mentioned, the scope of the study was out of the research of the factors that may lead to a failure of nifedipine, needing a rescue treatment, or even further, leading to a failure of tocolysis.
Future research should focus on the identification of those situations in which nifedipine may be the adequate treatment for selected patients, as well as for those women presenting with contractions without cervical changes, where the oral treatment may provide relief and reassurance when facing the threat of a preterm delivery.
In conclusion, nifedipine 5mg/mL oral solution shows a good safety and tolerability profile, with a small percentage of treatment discontinuation due to secondary effects directly related to it. Severity of adverse outcomes was also reduced compared with available literature for nifedipine capsules. In addition, data obtained from this analysis may support the efficacy for delaying delivery in pregnant women with preterm labor.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Patient consentWe declare that the protocols of all the participating institutions regarding publication of patient data have been followed, and that their privacy has been respected.
FundingThis study has been founded by Laboratorio Reig Jofre under the requirement of the Spanish Medicines Agency (Agencia Española de Medicamentos y Productos Sanitarios – AEMPS). Neither CM, FM, CR, MP nor any of the NifePar Collaborators Team cited at the end of the manuscript have received any financial amount from Laboratorio Reig Jofre for participating in this study. CN, HM, RB, and AC are employed by Laboratorio Reig Jofre.
Conflicts of interestThe authors have no conflicts of interest to declare.
We must thank all the Nife-par Collaborators Team: Dra. Montse Palacio Riera Hospital Clinic i Provincial. Dr. Stefan Iliev Savchev Grupo Hospitalario Quiron Barcelona. Dra. Nuria Elías Santo-Domingo Hospital Universitari Dexeus. Dr. Ignacio Cristóbal García Hospital La Zarzuela. Dr. Javier Plaza Arranz Hospital Fundacion Jimenez Diaz (IDCSALUD). Dra. Carmen Barbancho López Hospital Universitario Infanta Sofía. Dr. Ernesto González Mesa Hospital Materno Infantil Málaga Carlos Haya. Dr. Antonio Carballo García S.A.S. Complejo Hospitalario De Jaen. Dr. Javier Álvarez-Sala Torreano Hospital Universitario Araba. Dra. Mercedes Fraca Padilla Hospital De Basurto. Dra. Valentina Fernández Ladrón Hospital San Pedro. Dr. Manuel Macia Cortiñas Hospital Clínico Universitario Santiago Compostela. Dra. Emilia Exojo Hospital Universitario Infanta Cristina. Dr. Vicente Diago Almela Hospital Universitario Y Politecnico La Fe. Dra. Marta Pérez Adán Hospital Ourense. Dr. Antonio Payà Panadés Hospital Del Mar. Dr. José Luis Bartha Hospital Universitario La Paz. Dra. Águeda Rodríguez y Dra. Laia Martí Corporació Sanitaria Parc Taulí. Dr. Adrián Carlos Troncoso Saleh Hospital Virgen Del Castillo. Dra. Alicia Rodríguez Zurita Hospital Universitario Nuestra Señoara Candelaria. Dra. Erika Padrón Hospital Universitario De Canarias. Dr. Orlando Rafael Dávila Hospital San Pedro Alcántara. Dr. Federico Heredia Hospital General De L’hospitalet. Dra. Mónica González Hospital Universitario Marques De Valdecilla. Dra. Cristina Montes Hospital Arquitecto Marcide.