To study the role of angiogenesis and cyclooxygenase-2 expression in cartilaginous tumors and correlate these factors with prognosis.
INTRODUCTION:For chondrosarcoma, the histological grade is the current standard for predicting tumor outcome. However, a low-grade chondrosarcoma can follow an aggressive course—as monitored by sequential imaging techniques—even when it is histologically indistinguishable from an enchondroma. Therefore, additional tools are needed to help identify the biological potential of these tumors. The degree of angiogenesis that is induced by the tumor could assist in this task. Angiogenesis can be quantified by measuring the expression of vascular endothelial growth factor and CD34, and cyclooxygenase-2 can induce angiogenesis by stimulating the production of pro-angiogenic factors.
METHODS:In total, 21 enchondromas and 58 conventional chondrosarcomas were studied by examining the clinical and histopathological findings in conjunction with the immunostaining markers of angiogenesis and cyclooxygenase-2 expression.
RESULTS:The significant variables that were associated with poor outcome were 1) higher-grade chondrosarcomas, 2) tumors that developed in flat bones, and 3) over-expression of CD34 (with a median count that was higher than 5.9 vessels in 5 high power fields). Moreover, CD34 expression (measured using the Chalkley method) revealed significantly higher microvessel density in flat bone chondrosarcomas.
DISCUSSION:Previous studies have shown a positive correlation between Chalkley microvessel density and histological grade; however, in our sample, we found that the former is predictive of the outcome. Chondrosarcomas in flat bones have been shown to correlate with a poor prognosis. We also found that CD34 microvessel density values were significantly higher in flat-bone chondrosarcomas. This could explain—at least in part—the more aggressive biological course that is taken by these tumors.
CONCLUSIONS:These results provide evidence that CD34 microvessel density in chondrosarcomas can be helpful in predicting patient outcome and may add to our understanding of chondrosarcoma pathogenesis.
Among bone tumors, cartilage-producing tumors rank as the third most frequent type, representing 30% of benign tumors and 10–20% of malignant tumors.1,2 The World Health Organization (WHO) Classification of Bone Tumors (2002), which is based on the histological grade of the tumor, has been accepted as the standard for predicting tumor outcome.3,4 The histological grading system for chondrosarcomas (CSs) categorizes these tumors into three grades that are based on cellularity, nuclear atypia, and pleomorphism.4 Low-grade CSs tend to grow slowly and are associated with a 90% five-year survival rate but can recur and metastasize. In contrast, high-grade tumors have a higher incidence of metastasis and a 45% five-year survival rate.3–6 In some borderline tumors (i.e., a grade 1 CS), distinguishing a CS from an enchondroma (EC) may be difficult when using only a routine histopathological examination, as the current diagnostic criteria are not definitive. Furthermore, these tumors have a broad array of clinical, radiographic, and histological presentations that cause difficulties in both diagnosis and treatment.3–7 Thus, it is important to establish additional tools that could help predict a tumor's biological potential. The degree of angiogenesis that the tumor elicits might assist in making this distinction.
Angiogenesis is a fundamental step in both neoplastic transformation and the regulation of tumor growth, as demonstrated by Folkman8 and Dvorak et al.9 The interaction of vessels with cartilaginous tissue is not a physiological step, except during skeletal development. For example, in enchondral ossification (the period in which the growth plate is active), vessels can be seen penetrating the zones of hypertrophied cartilage during matrix ossification.10,11 However, normal adult cartilage is avascular. Thus, the presence of vessels within cartilaginous tissue is associated with pathological conditions, such as osteoarthritis and tumors.10,11 Angiogenesis (or neoangiogenesis) can be quantified by measuring the expression of certain molecules.
Vascular endothelial growth factor (VEGF) is one of the most important angiogenic factors that have been described. Among its many functions are the formation, organization, and migration of blood vessels. VEGF also promotes the degradation of soft tissues around the sprouting endothelium and increases the permeability of venules. An increase in VEGF staining has been described in many epithelial, germ cell, lymphoid, melanocytic, and mesenchymal tumors.12–16
CD34 is a surface glycophosphoprotein that is expressed in small-vessel endothelial cells and is associated with angiogenesis.16–20
Cyclooxygenases (COXs) are enzymes that catalyze the synthesis of prostaglandins from arachidonic acid. Cyclooxygenase-2 (COX-2) is associated with inflammatory and mitogenic stimuli, resulting in increased prostaglandin synthesis in both inflamed and neoplastic tissues.21–23 COX-2 also plays an important role in carcinogenesis, as it can induce angiogenesis by stimulating the production of pro-angiogenic factors, such as VEGF;21–23 in addition, COX-2 can inhibit tumor cell apoptosis and immune surveillance, thus increasing the tumor's invasive and metastatic potential.21–23,25 Indeed, recent studies of various human tumors showed that COX-2 over-expression is associated with poor prognosis.21 In musculoskeletal tumors (e.g., osteosarcomas, rhabdomyosarcomas, and CSs), studies of COX-2 over-expression have yielded conflicting results.21–29
In fact, neovascularization is only one of the many elements involved in neoplastic transformation and the progression of a tumor to a higher histological grade. However, because inhibiting angiogenesis is so important in the preservation of intact cartilage (as seen in the osteoarthritis therapeutic approach),10,11,15,16 it is possible that the lack of this inhibition is a significant component in the pathogenesis of cartilage tumors.
The present report is a retrospective study of 21 ECs and 58 conventional CSs, in which we addressed the clinical and follow-up data and histopathological findings with regard to immunostaining for markers of angiogenesis and COX-2 expression. The aim of this study was to evaluate the value of these findings as a prognostic tool in CSs and as a possible aid in the differential diagnosis between an ECs and a low-grade CSs.
MATERIALS AND METHODSPatients and tumor samplesFrom 1988 through 2007, 141 patients with a primary EC or CS were admitted to the University Hospital – Unicamp (Campinas-Brazil) and Centro Médico de Campinas (Brazil). The tumor specimens were routinely fixed in 10% formalin and later decalcified with hydrochloric acid and ethylenediaminetetraacetic acid. The embedded tumor tissue from each patient was obtained from the Department of Pathology. The clinical, radiographic, and follow-up information were obtained from the patients' medical records. The routine staining of the tumor sections was reviewed to confirm the diagnosis. The histological grade (on a scale of 1 to 3) of each CS was determined based on nuclear size and staining (hyperchromasia) and cellularity according to the WHO Classification of Bone Tumors (2002).1
Pathological material with insufficient tissue samples for new cuts or in poor fixative condition (n = 54) and chondrosarcoma variants (mesenchymal: n = 4; de-differentiated: n = 2; clear cell: n = 2), were excluded from the analysis. Therefore, a total of 79 patients (30 men and 49 women, ranging from 5 to 87 years of age, with a median age of 42 years) entered the study. There was no loss of follow-up data. The patient data are summarized in Table 1.
Correlation between variables in groups I, II, and III.
| Group I (n = 21 ECs) | Group II (n = 31 Low-Grade CSs) | Group III (n = 27 Moderate/High-Grade CSs) | |
|---|---|---|---|
| Gender | |||
| Male | 7 (33.33%) | 13 (41.93%) | 10 (37.03%) |
| Female | 14 (66.67%) | 18 (58.07%) | 17 (62.97%) |
| Mean age at Dx (years) | 29.6 | 43 | 50 |
| Follow-up (months) | 98 | 66 | 71 |
| Site | |||
| Small bone | 16 (76.2%) | 0 | 1 (3.7%) |
| Long bone | 5 (23.8%) | 23 (74.20%) | 20 (74.1%) |
| Flat bone | 0 | 8 (25.8%) | 6 (22.22) |
| COX-2 | |||
| No expression | 2 (9.5%) | 20 (64.5%) | 15 (55.56%) |
| <10% | 9 (42.9%) | 7 (22.6%) | 6 (22.22) |
| 10-50% | 8 (38.1%) | 4 (12.9%) | 3 (11.11%) |
| >50% | 2 (9.5%) | 0 | 3 (11.11%) |
| VEGF | |||
| ≤10.5% | 11 (52.38%) | 17 (54.84%) | 14 (51.85%) |
| >10.5% | 10 (47.69%) | 14 (45.16%) | 13 (48.15%) |
| CD34 | |||
| ≤5.9 | 21 (100%) | 27 (87%) | 20 (74.1%) |
| >5.9 | 0 | 4 (13%) | 7 (25.9%) |
| Outcome | 100% cured | 3 (9.6%) poor | 11 (40.7%) poor |
Dx: diagnosis; ECs: enchondromas; CSs: chondrosarcomas; Mod: moderate.
Twenty-one ECs that developed in the long (n = 5) and short bones (n = 16) were studied. Among the CSs were 31 grade 1 CSs that developed in the long (n = 23) and flat bones (n = 8). Twenty-four grade 2 CSs developed in the long (n = 18) and flat bones (n = 6), and 3 grade 3 CSs developed in the long (n = 1) and flat bones (n = 2). With the goal of correlating the clinical and histological results, the patients were divided into the following three groups: group I (n = 21) comprised the patients with an EC; group II (n = 31) comprised the patients with a grade 1 CS; and group III (n = 27) comprised the patients with a grade 2 or 3 CS.
Because flat-bone CSs are associated with poor prognosis,1 the benign and malignant tumor anatomical sites were also divided into three groups. Seventeen tumors (16 ECs and 1 high-grade CS) developed in small bones (metacarpal bone, n = 11; hand phalanx, n = 4; metatarsal bone, n = 1; calcaneus, n = 1). Forty-eight tumors (5 ECs and 43 CSs) developed in long bones (femur, n = 18; fibula, n = 3; tibia, n = 10; humerus, n = 13; radius, n = 3; lumbar vertebra, n = 1). Fourteen tumors (all CSs) developed in flat bones (ribs, n = 6; scapula, n = 2; pelvis, n = 6).
All of the ECs (n = 21) were treated with curettage. The CSs were removed by either wide resection or amputation, with a wide margin in 12 cases, a marginal resection in 26 cases, and an intralesional resection or curettage in 18 cases (all 18 of which were grade 1 CS cases).30 Instead of surgery, two extremely large pelvic tumors (in the sacrum and iliac bone) were treated using chemotherapy and/or radiotherapy after establishing the diagnosis by biopsy. Four of the patients underwent chemo/radiotherapy for local recurrence and metastasis after undergoing a marginal resection. Chondrosarcoma size and the adequacy of the surgical margins were excluded from analysis, as 18 of the 58 CS patients were treated with curettage.
The follow-up interval was recorded from the time of the surgery until January 2009. The minimum follow-up period was 24 months (or shorter in cases of death), and the median follow-up was 77 months (with a range of 4-250 months).
Immunohistochemical techniqueThe primary antibodies that were used included anti-CD34 (Mo a Hu CD34 Class II, Clone QBEnd 10, Dako Cytomation, Carpenteria, CA, USA) at a dilution of 1:50, anti-VEGF (A-20 rabbit polyclonal IgG, 200 μg/ml, Santa Cruz Biotech., Inc, Santa Cruz, CA, USA) at a dilution of 1:200, and anti-COX-2 (Cyclooxygenase-2 antibody clone-4h12, Diagnostic Byosystem, Pleasanton, CA, USA) at a dilution of 1:50. Epitope retrieval was achieved by steaming with citrate buffer (at 95°C). The EnVision + Dual Link System HRP polymer (Dako) was used as a reaction amplifier. The antibody complex was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining according to the manufacturer's instructions. The sections were counterstained with Mayer hematoxylin. The appropriate negative and positive controls were included in each assay.
Immunohistochemical analysisAll of the immunostained sections were evaluated simultaneously (using a double-headed microscope) by two investigators (EMIA and FFC) who were unaware of the clinical status of the patient being studied.
With regard to COX-2, a consensus judgment was adopted as the proper tumor immunohistochemical score according to the method of Endo et al.,22 with adaptations characterized by the mean of the proportion of stained tumor cells in 5 “hot-spot” high-power fields (at 400x magnification) as follows: 0 = no stained tumor cells; 1+ = less than 10% of all tumor cells stained; 2+ = 10–50% of all tumor cells stained; and 3+ = more than 50% of all tumor cells stained.
For VEGF, cellularity was quantified by counting the relative number of immunostained cells per 5 “hot-spot” high-power fields (HPFs) (at 400x magnification) and was expressed as a percentage of the total number of neoplastic cells according to the method of Ayala et al.12
For the CD34 analysis, the sections were scanned using a low-power view (at 50x magnification). Five areas displaying the highest number of immunostained microvessels (i.e., hot-spots) were identified.17,18 Next, one 400x microscopic field (corresponding to an area of 0.1449 mm2) was chosen within each hot-spot. A 25-point Chalkley eyepiece graticule (Leitz Orthoplan, Leica) was applied to each selected hot-spot field (corresponding to a Chalkley grid area of 0.041 mm2). The graticule was positioned such that the immunostained vessels hit the maximum number of points. The final Chalkley count for an individual tumor was taken as the mean value of the five graticule counts. The generally accepted criteria for determining a vessel's profile17,18 were followed and included any stained endothelial cells or endothelial cell clusters that were separated from the adjacent microvessels. A visible lumen was not a requirement for a structure to be counted as a microvessel. Necrotic or sclerotic areas within the tumor and non-tumor areas that were adjacent to the tumor were excluded from the vessel counts.31–33
Statistical analysisChi-squared or Fisher's tests were used to compare outcome, gender, site, follow-up span, and COX-2 expression. For the VEGF and CD34 results, Mann-Whitney test, Kruskal-Wallis test or analysis of variance (ANOVA) with transformation by ranks was used, followed by Tukey's test to identify differences when necessary. To test the linear association between two immunomarkers, the Spearman correlation coefficient was determined. A multiple logistic regression analysis (the generalized log model) was used to identify factors that differentiate the histological grade of the tumor. The process of selecting the variables that were used was stepwise. The level of significance for the statistical tests was set at p<0.05. The data analysis was performed using the SAS System for Windows version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTSThe results are summarized in Tables 1 and 2.
Correlation between variables in groups II (grade I CSs) and III (high-grade CSs).
| Outcome | Good (n = 44) | Poor (n = 14) | p-value |
|---|---|---|---|
| Gender | 0.7293∗ | ||
| Male | 18 (40.1%) | 5 (35.7%) | |
| Female | 26 (39.9%) | 9 (64.3%) | |
| Age (years) | 0.1966∗ | ||
| ≤51 | 28 (63.6%) | 7 (50%) | |
| >51 | 16 (36.4%) | 7(50%) | |
| Histological Grade | <0.0001∗ | ||
| Grade 1 | 28 (63.6%) | 3 (21.4%) | |
| Grade 2 or 3 | 16 (36.4%) | 11 (78.6%) | |
| Site | <0.0001∗ | ||
| small bone | 1 (2.3%) | 0 | |
| long bone | 36 (81.8%) | 7 (50%) | |
| flat bone | 7 (15.9%) | 7 (50%) | |
| CD34 MVD | 0.0104¥ | ||
| ≤5.9 | 37 (84%) | 7 (50%) | |
| >5.9 | 7 (16%) | 7 (50%) | |
| COX-2 | 0.3364∗ | ||
| no expression | 28 (63.6%) | 7 (50%) | |
| <10% | 10 (22.7%) | 3 (21.4%) | |
| 10-50% | 5 (11.3%) | 2 (14.3%) | |
| >50% | 1 (2.4%) | 2 (14.3%) | |
| VEGF expression | 0.2094¥ | ||
| ≤10.5% | 22 (50%) | 9 (64.3%) | |
| >10.5% | 22 (50%) | 5 (35.7%) |
The following 14 patients had poor outcomes: three in group II (metastasis: n = 1; local recurrence: n = 2) and 11 in group III (local recurrence: n = 4; death: n = 7). None of the ECs recurred. None of the patients whose CS was treated with curettage presented a tumor recurrence or poor outcome.
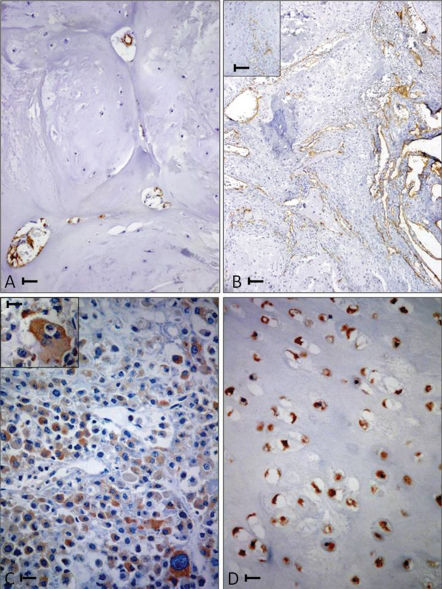
There were no significant differences among the three groups with regard to the duration of follow-up (p = 0.7431). A poor outcome was significantly associated with the following: 1) moderate/high-grade CS (p<0.0001); 2) tumors that developed in a flat bone (p<0.0001); and 3) over-expression (p<0.02) of CD34 (when the number of immunostained vessels was higher than the median of 5.9 vessels in 5 high-power fields) (Figures 1A and B). No significant correlations were found between outcome and age (p = 0.1966), gender (p = 0.7293), COX-2 expression (p = 0.3364), or VEGF expression (p = 0.2094). An over-expression of VEGF (when the percentage of immunostained cells was higher than the median of 10.5% in five high-power fields) was found in 48% of low-grade CSs (group II) and in 59% of moderate/high-grade CSs (group III).
(A) A grade I femur chondrosarcoma (CS) with low CD34 microvascular density (CD34 positive MVD). Bar = 0.02 mm. (B) A grade II iliac bone CS with high CD34 positive MVD. Bar = 0.04 mm. A magnified view is shown in the inset (bar = 0.02 mm). (C) A grade 3 humerus CS. Bar = 0.02 mm. Bizarre cells are shown in the inset (bar = 0.01 mm) with diffuse strong immunoreactivity to COX-2 antibody. (D) A hand phalanx enchondroma with uniform and diffuse immunoreactivity to COX-2 antibody. Bar = 0.02 mm.
None of the variables proved useful for differentiating between low-grade CSs and ECS (p = 0.7152).
A multivariate analysis of CD34 immunostaining (using Mann-Whitney, ANOVA, and Tukey's tests) revealed a positive association between higher microvessel density (MVD) and flat bone sites (p<0.01). Indeed, flat bone CSs (Figure 1B) had a mean of 6.1 CD34-positive vessels in the Chalkley count compared with 4.7 and 4.6 CD34-positive vessels in the long and small bones, respectively.
Although COX-2 expression was not correlated with patient outcome, its over-expression (Figure 1C) (more than 10% positive COX-2 cells in five high-power fields) was positively correlated (p<0.02) with increased CD34-positive MVD (using an ANOVA test followed by the Kruskal-Wallis test). Among the EC cases, we found that 19/21 (90%) of the tumors stained positively for COX-2 (Fig. 1D). In 10 of the 21 ECs (47%), an over-expression (i.e., >10% of positive cells) was found; VEGF over-expression was found in 13 (61%) of the ECs. Intra-tumoral CD34-positive vessels were found in 100% of the ECs but displayed weak CD34 expression (none had a Chalkley count above 5.5).
DISCUSSIONHistological grading of CSs is considered to be the most useful predictor of outcome,1,3,4 but this is a subjective procedure, and the current criteria are not definitive.5,6 Therefore, supplemental methods for assessing the prognosis of CS patients have been sought and include the evaluation of DNA synthesis and content; flow cytometry using molecular markers, such as p53 and MIB-1; cytogenetic features; histomorphometry; and radiographic classifications. Nevertheless, none of these methods is considered to be definitive.1,
The present study provides immunohistochemical evidence that MVD—as evaluated by CD34 staining—is significantly associated with poor prognosis. Our results differ from those of Nakagawa et al.,25 who found no correlation between CD34 expression and outcome. The two additional predictive variables that we identified (i.e., histological grade and tumor site in flat bones) are well known in the medical literature.1,4,5 However, in our study, we also found that CD34 positive MVD was significantly higher in flat bone CSs. In animal experiments, flat and long bones had the same density of vessels.34 However, in adults, flat bones have functional bone marrow that contains stem cells and produces many growth factors16 that could elicit tumor angiogenesis.
The role of COX-2 in cartilage tumors is controversial. Endo et al. in 2006 and Schrage et al. in 201022,23 have reported that COX-2 over-expression is associated with poor prognosis in CSs. Our results are in line with those of Sutton et al.,21 who studied 32 cartilaginous tumors by Western blot analysis and found no statistical correlation between the expression of COX-2 and the following variables: age, sex, stage, anatomical site, metastasis development, or survival rate. In addition, Nakagawa et al.25 studied 101 CS specimens and found no correlation between COX-2 expression and prognosis. We previously studied 53 CSs (seven of which were unconventional and included four mesenchymal, two de-differentiated and one clear-cell).35 In the previous sample, we found that a COX-2 expression frequency above 50% was associated with poor prognosis; all of the six patients with either mesenchymal or de-differentiated CSs died within 24 months of their diagnosis. In the present study, we added 12 additional conventional CS patients and excluded the unconventional tumors from the sample and found no significant correlation between COX-2 expression and outcome. Another difference between our findings and those of Endo et al.22 was that they did not find any COX-2 staining in enchondromas, whereas 90% of our enchondroma specimens had positive COX-2 staining.
Contradictory results have also been obtained from studying other bone tumors, such as osteosarcomas. Xu et al.26 found that COX-2 over-expression was associated with a better prognosis. In contrast, Dinckens et al.27 found no correlation between COX-2 over-expression and patient outcome. Finally, Masi et al.28 and Liao et al.29 reported that cases of osteosarcoma had worse prognosis and decreased patient survival rates whether COX-2 was overexpressed. We suggest the following two reasons for these discordant results: 1) the COX-2 antibody clone that we used was different from that used by Endo et al.; and/or 2) any problems with the fixation or decalcification of the samples could have biased the results. Nevertheless, we found a positive correlation between CD34 positive MVD and COX-2 expression, which is in line with the well-known relationship between COX-2 and angiogenesis. Therefore, this enzyme might have at least an indirect role in patient prognosis.
With respect to VEGF expression, our quantitative results are similar to those of Ayala et al.12 and Kalinski et al.,13,14 which were descriptive studies that found a higher rate of VEGF expression in moderate- and high-grade CSs. In our sample, no significant correlation was observed between VEGF expression and outcome.
CONCLUSIONSChalkley MVD—as evaluated through CD34 antibody expression—may be a useful tool to help predict patient outcome in chondrosarcoma (CS) cases. These data can be considered for selective therapeutic inhibitory targeting. The higher MVD in flat bone CSs could explain the poorer outcome of these tumors relative to long/short bone CSs. None of the variables that were examined in this study were found to be useful in distinguishing a low-grade chondrosarcoma from an enchondroma.
The authors are grateful to Ana Cláudia S. Piaza and Luzia A. M. Reis for technical assistance, Cleide Ap. M. Silva and Helymar C. Machado for assistance with the statistical analyses, Adilson A. Piaza for assistance with photographic documentation and Ms. Diane Ellis, for her assistance with English grammar. This study was supported by FAPESP (grant number 09/51473-0).