This study evaluated the performance of lungs that were preserved with different solutions (Celsior, Perfadex or saline) in an ex vivo rat lung perfusion system.
METHODS:Sixty Wistar rats were anesthetized, anticoagulated and randomized into three groups (n = 20). The rats were subjected to antegrade perfusion via the pulmonary artery with Perfadex, Celsior, or saline, followed by 6 or 12 hours of ischemia (4°C, n = 10 in each group). Respiratory mechanics, gas exchange and hemodynamics were measured at 10-minute intervals during the reperfusion of heart-lung blocks in an ex vivo system (IL2-Isolated Perfused Rat or Guinea Pig Lung System, Harvard Apparatus, Holliston, Massachusetts, USA; Hugo Sachs Elektronik, Germany) for 60 minutes. The lungs were prepared for histopathology and evaluated for edema following reperfusion. Group comparisons were performed using ANOVA and the Kruskal-Wallis test with a 5% level of significance.
RESULTS:Gas exchange was not significantly different between lungs perfused with either Perfadex or Celsior at the same ischemic times, but it was very low in lungs that were preserved with saline. Airway resistance was greater in the lungs that were preserved for 12 hours. Celsior lungs that were preserved for 6 and 12 hours exhibited lower airway resistance (p =0.01) compared to Perfadex lungs. Pulmonary artery pressure was not different between the groups, and no significant differences in histopathology and apoptosis were observed between the groups.
CONCLUSIONS:Lungs that were preserved with Celsior or Perfadex exhibited similar gas exchange and histopathological findings. Airway resistance was slightly lower in the Celsior-preserved lungs compared with the Perfadex-preserved lungs.
Lung transplantation is a well-established treatment for end-stage lung disease. However, lung transplant-related mortality remains significant despite the increasing number of transplants that are performed (over 3,200 in 2010) (1). Ischemia-reperfusion injury is the primary cause of lung transplant-related mortality. The severity of injury depends on several factors, including the quality of lung preservation, which is important in the early stages after transplantation (2).
Lung preservation for transplantation enables distant organ procurement, objectively improves organ quality after reperfusion and decreases post-ischemic reperfusion injury. The most widely used preservation method is hypothermia with the administration of preservation solutions with or without pulmonary vasodilators (3),(4).
Low-potassium preservation solutions are classified as extracellular-type solutions and include Perfadex and Celsior, which are most commonly used for lung preservation. The Celsior solution was originally developed for heart preservation (2), and Perfadex was developed for lung preservation (5).
Perfadex was gradually introduced after its original development two decades ago, despite the superiority of the experimental results obtained from this solution compared with other solutions (2). The use of preservation solutions in the lungs reduces the incidence of acute graft failure after transplantation from 30% to less than 15% (2). Controversy exists over the benefits of Perfadex based on its late clinical performance and associated 1-year post-transplant mortality rate (2). The recent introduction of ex vivo lung reconditioning and donation after cardiocirculatory arrest has rekindled this controversy and reinforced the need for the reassessment of the currently used preservation solutions (6).
Ex vivo lung perfusion systems are useful for experimental physiological evaluations because of its reproducibility and relatively low cost for assessments of lung preservation methods (7),(8).
This study compared the functional performance of rat lungs that were subjected to different ischemic times; preserved with Perfadex, Celsior or normal saline; and reperfused in an ex vivo lung perfusion system.
MATERIALS AND METHODSAnimals were handled in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Research and published by the National Academies Press, 8th Edition, 2011.
Male Wistar rats (250 to 300 g) were anesthetized with sodium thiopental (50 mg/kg, intraperitoneally) and subjected to sternolaparotomy, tracheostomy, and mechanical ventilation (room air; tidal volume (Vt) = 10 mL/kg body weight; respiratory rate (RR) = 70 cycles/min; and positive end expiratory pressure (PEEP)=1 cmH2O). The diaphragm was opened radially to expose the supradiaphragmatic vena cava after anticoagulation (heparin 1,500 IU via the inferior vena cava). The right ventricle outflow tract was incised adjacent to the pulmonary artery, the inferior vena cava and the left ventricle. The pulmonary artery was cannulated through right ventriculotomy, and the lungs were perfused antegradely with 20 mL of a hypothermic solution (4°C) at a pressure of 10 cm H2O, which was obtained by the elevation of the reservoir. The effluent was drained via the left ventriculotomy. Animals were randomized into three groups (n = 20 each) based on the perfused preservation solution: Perfadex® (Vitrolife, Kungsbacka, Sweden), Celsior® (Genzyme, Catalent Limoges S.A.S., France) or a 0.9% saline solution (Baxter, São Paulo, Brazil). The heart-lung block was removed partially inflated at the end of perfusion, immersed in the perfusion solution and stored at 4-7°C for 6 or 12 hours according to group assignment. The animals were divided into two groups (n = 30) according to ischemic time (6 or 12 hours), and each group was subdivided into three groups (n = 10) according to perfusion solution.
The heart-lung block was connected to the ex vivo perfusion system at the end of the cold ischemia period (IL-2 Isolated Perfused Rat or Guinea Pig Lung System; Harvard Apparatus, Holliston, MA, USA; Hugo Sachs Elektronic, Hugstetten, Germany) and reperfused for 60 minutes using homologous blood from donor rats diluted in saline to a hematocrit of 15-20%. The pulmonary venous blood was deoxygenated using a membrane oxygenator (D-150 Hemofilter, Medsulfone, Italy) that contained a continuously administered (100 mL/min flow rate) gas mixture (90% N2 and 10% CO2).
Ventilation was initiated with the heart-lung block positioned in the ex vivo perfusion system (RR = 60 bpm; inspiratory/expiratory ratio = 60%; one breath/minute = 50% increase in tidal volume; and PEEP =1 cmH2O), beginning at 50% of the tidal volume and increasing until a ventilation of 10 mL/kg body weight was reached. Perfusion of the block began at a low flow rate (2 mL/min) and was increased over 5-10 minutes until the desired flow rate (5-7 mL/min) was reached. Ventilation and perfusion were stabilized, and data (hemodynamics, ventilatory mechanics, and pulmonary arterial and venous gases) were collected every 10 minutes for 60 minutes.
Blood samples were taken from the pulmonary arterial and left atrial cannulae for blood gas measurements (ABL 800, Radiometer, Denmark). The lung’s relative oxygenation capacity (ROC) was calculated using the following formula: ROC = [(PvO2-PaO2) ×100]/PaO2 (7). The PaO2 corresponds to the deoxygenated blood that was taken from the pulmonary arterial cannula, and the PvO2 is the oxygenated blood from the left atrial cannula. The pH of the blood in the reservoir was corrected with sodium bicarbonate (0.3 mEq/L/dose) to maintain the pH between 7.1-7.4 (9),(10). The ventilatory mechanics parameters were provided by the IL2 system and included tidal volume, lung compliance and maximum inspiratory and expiratory flows. The hemodynamic parameters included pulmonary artery pressure and pulmonary resistance.
The left lung was dissected out at the end of perfusion. The lung was weighed, stored at 70°C for 72 hours, and re-weighed for the calculation of the wet-to-dry (W/D) weight ratio.
The right lung was immersed in 10% buffered neutral formalin solution for 24 hours and then longitudinally sectioned. The most volumetrically significant half was selected for embedding and processing. The specimen was dehydrated, diaphanized and embedded in paraffin. Histological sections (5 μm thickness) were stained with hematoxylin and eosin (HE) for light microscopy analysis. Histopathological analyses of the fragments was performed to assess the presence or absence of congestion, alveolar edema, bleeding (alveolar and/or interstitial), acute thrombosis, interstitial inflammatory infiltrates (mononuclear and/or granulocytic) and pneumonic foci.
Fragments were obtained from the right lung immediately after reperfusion for the detection and quantification of apoptotic cells using the In situ Cell Death Detection Kit (Roche, Mannheim, Germany). The fragments were immersed in 10% buffered formalin for fixation for 24 hours. Paraffin blocks of the specimens were prepared and sectioned into slices of 5 μm thick sections. The sections were deparaffinized in three xylene baths (five minutes each) and rehydrated in an ethanol gradient (100, 95, 90, 80, and 70%). Proteinase K was applied to the sections for 30 minutes at room temperature. The sections were washed in two (three minutes each) PBS (phosphate-buffered saline) baths. The sections were incubated in a 0.3% hydrogen peroxide (H2O2) and methanol solution for 30 minutes at room temperature and immediately washed twice in PBS. A TUNEL mixture (50 μl:5 μl of the TdT enzyme solution and 45 μl of the labeled nucleotide solution) was applied to each specimen. The specimens remained in the wet chamber at 37°C for 60 minutes. The sections were washed three times in PBS and coverslipped with glycerin for fluorescence microscopy analysis.
Small fragments of the right lung (approximately 2 mm ×2 mm) were used for transmission electron microscopy analysis. The material was placed in universal fixative (1% glutaraldehyde, 1% paraformaldehyde, pH = 7.4) immediately after biopsy, postfixed in osmium tetroxide (2%), dehydrated, and embedded in epon-araldite. Slices (1 µm thick) were analyzed using a transmission electron microscope for the qualitative evaluation of apoptotic changes in type II pneumocytes.
Statistical analysisStatistical analyses included the parametric ANOVA and nonparametric Kruskal-Wallis tests. The chi-square test was employed for qualitative variables. The data are presented as the means and standard errors of the mean, and the significance level in this study was 5% (p<0.05). Descriptive and inferential statistical analyses were performed with SPSS software version 13 (SPSS 13.0 for Windows).
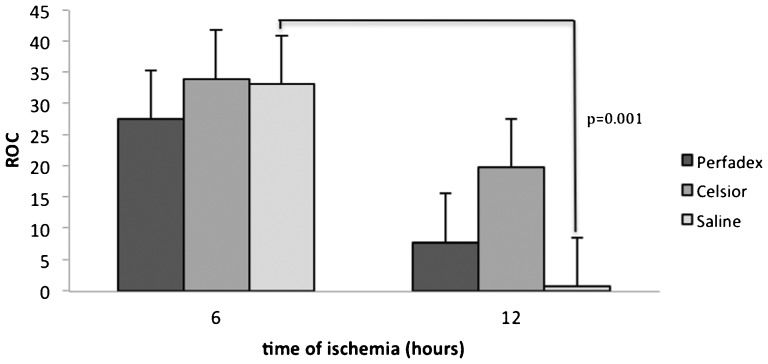
RESULTSThe ROC of Celsior lungs was superior to that of Perfadex or saline lungs, regardless of the ischemic time. However, this difference did not reach statistical significance (6 hours, p =0.75; 12 hours, p =0.18). Oxygenation was lowest in the lungs that were preserved with saline for 12 hours (p =0.001). However, no significant differences between Celsior and Perfadex lungs were observed at either ischemic time (Perfadex, p =0.06; Celsior, p =0.17) (Figure 1).
Mean relative oxygenation capacity (ROC) of rat lungs subjected to ischemia and reperfusion for 60 minutes, illustrating no significant differences between Celsior- and Perfadex-perfused lungs at either ischemic time. The lungs subjected to 6 hours of cold ischemia exhibited higher ROCs than the 12-hour ischemic lungs, but this difference was significant only for the lungs preserved with saline (p =0.001).
The compliance of the lungs that were preserved with Celsior was superior to that of the saline lungs at 6 hours (p =0.03) but not at 12 hours (p =0.07). However, lung compliance was not different between the Celsior and Perfadex lungs at either ischemic time (6 hours, p =0.16; 12 hours, p =0.31). Overall, the compliance of the 6-hour lungs was greater than that of the 12-hour lungs (Perfadex, p =0.02; Celsior, p =0.01; saline, p =0.01) (Figure 2).
Mean lung compliance of rat lungs submitted to ischemia and reperfusion for 60 minutes, illustrating no significant differences between lungs preserved with Celsior and Perfadex. Lungs subjected to ischemia for 6 hours performed better than those subjected to 12 hours of ischemia.
The Celsior lungs exhibited the lowest airway resistance for both ischemic times (p =0.01). The Perfadex-preserved lungs exhibited lower pulmonary resistance than the saline-preserved lungs at 6 hours (p =0.03), but this difference was not observed at 12 hours (p =0.16). Airway resistance was higher in the 12-hour ischemic lungs regardless of the preservation solution (Perfadex, p =0.001; Celsior, p =0.003; saline, p =0.006) (Figure 3).
Airway resistance of rat lungs subjected to ischemia and reperfusion for 60 minutes. The Celsior lungs exhibited the lowest airway resistance for both ischemic times. The airway resistance was lower in lungs submitted to 6 hours of ischemia compared with those submitted to 12 hours of ischemia.
The W/D ratio was not different between the 6- and 12-hour lungs regardless of the preservation solution (p =0.29 and p =0.26, respectively). The Perfadex-preserved lungs exhibited a higher W/D ratio at 12 hours of ischemia compared with 6 hours (Perfadex, p =0.001; Celsior, p =0.27; saline, p =0.13).
Pulmonary artery pressure was not different between the 6- and 12-hour lungs regardless of the preservation solution (p =0.88 and p =0.98, respectively), and no significant difference between lungs in the two ischemic time groups was observed (Perfadex, p =0.17; Celsior, p =0.34; saline, p =0.19).
The saline-preserved lungs exhibited the highest levels of alveolar edema on light microscopy regardless of the ischemic time (6 hours, p =0.006; 12 hours, p =0.001). The Celsior and Perfadex lungs exhibited some level of alveolar edema at both ischemic times (6 hours, p =0.131; 12 hours, p =1.00), but no significant differences in edema formation across groups and between 6- and 12-hour ischemic lungs were observed (Perfadex, p =0.37; Celsior, p =0.3; saline, p =0.47) (Figure 4).
No differences in type II pneumocyte changes were observed on electron microscopy between the different groups and between the 6- and 12-hour ischemic lungs. Very few epithelial cells exhibited gross chromatin aggregates peripheral to the nucleus, which is indicative of the initial stages of apoptosis. A slight thickening of the basement membrane due to edema was observed in all groups (Figure 5).
The TUNEL assay for the assessment of apoptosis did not demonstrate any statistically significant differences between groups, regardless of the preservation solution and ischemic time (Figure 6).
DISCUSSIONThis study demonstrated a similar reperfusion performance of gas exchange in lungs that were preserved with Perfadex or Celsior. Both Perfadex and Celsior are extracellular solutions, but the potassium content of the Perfadex solution is lower than that of the Celsior solution (4 vs. 15 mEq/L, respectively). Therefore, Perfadex is potentially less harmful to the structural and functional integrity of endothelial cells and may, as a result, decrease the production of oxidants and vasoconstrictors. The addition of Dextran-40 increases the oncotic pressure, which improves the deforming capacity of red blood cells, prevents erythrocyte aggregation and induces disaggregation of the aggregated red blood cells. Therefore, Dextran-40 elicits an antithrombotic effect due to its action on the surfaces of endothelial cells and platelets. These effects improve lung microcirculation and preserve the endothelial-epithelial interface, which can reduce water and protein extravasation during reperfusion (2).
Previous studies on the use of Celsior or Perfadex for lung preservation reported comparable results, but these studies used shorter ischemic times (7). The Celsior solution contains antioxidant substances, such as histidine, mannitol, lactobionate, and glutathione (11).
All lungs that were subjected to 6 or 12 hours of ischemia in the present study completed the 60-minute reperfusion period and provided consistent data for analysis. Other authors have used ischemic times of 2 and 4 hours using the same model used in the present study (7),(12).
The ex vivo lung perfusion model is suitable for initial evaluations of lung preservation because of its simplicity, reproducibility, reliability, and low cost compared with larger animal models (13).
Similar lung compliance results were found in this study between the Celsior and Perfadex groups for both ischemic times. The Celsior solution exhibited a tendency for improved lung compliance during reperfusion, but this difference was not significant. Sommer et al. observed comparable lung compliances in a pig model in both experimental groups after 24 hours of cold ischemia and 7 hours of reperfusion (14). Wittwer et al. also described that dynamic lung compliance in a pig model remained stable over 6 hours of reperfusion even after 27 hours of hypothermic ischemia. These authors did not demonstrate a significant difference between solutions regardless of the perfusion route (antegrade or retrograde). However, preservation with Celsior produced the lowest lung compliance values across the investigated groups (15). Both preservation solutions in our study were associated with significantly lower values of lung compliance after 12 hours of ischemia, which suggested that this parameter was negatively affected by longer ischemic times regardless of the preservation solution.
Celsior performed better than Perfadex with regard to airway resistance, as demonstrated by the lower resistances measured during the reperfusion of the Celsior lungs. Increased resistance may be secondary to the increases in permeability and alveolar-capillary injury that result from edema. However, airway resistance may vary due to the denervation of the heart-lung block and to the airway reactivity, which is less likely to play a role in this model. However, the microscopic observations of alveolar edema were similar in the Perfadex and Celsior lungs despite the significant differences in airway resistance caused by these two solutions.
Our experimental design included a saline solution group to assess the stability and reliability of the model. The absence of significant differences in oxygenation and pulmonary resistance parameters between Perfadex and saline lungs may be partly attributed to blood dilution. Puskas et al described these effects in a similar ex vivo perfusion model in which blood dilution with a crystalloid solution significantly improved post-ischemic reperfusion injury (2). The blood dilution may have been higher in the saline group in the present model because the vascular bed of the lungs was filled with saline prior to reperfusion. This act may explain the lower pulmonary resistance values in the saline-preserved lungs compared with the Perfadex lungs for the 12-hour ischemic period. This act may also underlie the biases that are inherent in the absence of the body of the animal and its replacement with a deoxygenator. The use of Krebs solution instead of saline may be an option for preventing the aforementioned effects of blood dilution.
Wittwer et al. (12) also observed no significant differences in pulmonary artery pressure between Perfadex- and Celsior-preserved lungs using a similar model. One limitation of this model is that it does not enable the calculation of pulmonary vascular resistance, which yields a better assessment of pulmonary hemodynamics. These authors used a swine model and pulmonary vascular resistance calculations to conclude that preservation with Celsior produced the highest resistance values, but Sommer et al. (14) reported that Perfadex was inferior to Celsior.
The ROC is the parameter of choice for the assessment of gas exchange in this ex vivo model and likely represents the most important physiological parameter (7). We did not observe a significant difference in the gas exchange performance of Celsior lungs compared with Perfadex lungs at either ischemic timepoint examined. A previous study using a similar model with 4 hours of ischemia demonstrated differences between the two solutions that favored Celsior (7), but the ischemic time in the present study is three times longer. However, the saline solution group exhibited a decline in ROC for the longest ischemic time.
The W/D ratios demonstrated that the increased edema during reperfusion was closely related to the ischemic time. Increased edema results from post-ischemic reperfusion injury and the characteristics of the ex vivo model, which uses an extracorporeal circuit, inorganic interfaces and non-pulsatile flow. The Perfadex-preserved lungs in the 6-hour ischemic group exhibited a trend toward a lower weight gain than the Celsior-preserved lungs, but this difference was not statistically significant. The Perfadex solution is associated with less edema regardless of the perfusion route (15). An assessment of lung edema in pigs demonstrated that Perfadex better prevents the formation of intra-alveolar, peribronchovascular and septal edema and injury to the alveolar-capillary barrier during ischemia-reperfusion than Celsior (16). Another study demonstrated that the water content of Perfadex lungs was not different than that of Celsior lungs after 24 hours of cold ischemia and 7 hours of reperfusion in pig lungs (14). Conversely, our study demonstrated that Celsior lungs subjected to 12 hours of ischemia were less edematous at the end of reperfusion than Perfadex lungs. Overall lung performance in the Celsior lungs that were subjected to 12 hours of ischemia was similar to the 6-hour ischemic lungs, thus suggesting that the use of the Celsior solution produced less edema than the Perfadex solution over longer ischemic times. Unfortunately, such differences did not achieve statistical significance, and the results should be confirmed in future studies.
In conclusion, this animal model of ex vivo lung perfusion demonstrated that lungs preserved with Perfadex and Celsior exhibited similar gas exchange, hemodynamics and histopathological findings. The Perfadex-preserved lungs subjected to 12 hours of ischemia were more edematous, but Celsior-preserved lungs exhibited slightly better ventilatory mechanics, as suggested by the lower airway resistance. Future studies are required to confirm these results and clarify the underlying mechanisms.
This study was performed as part of the Thoracic and Cardiovascular Surgery Post-graduate Program, Heart Institute, Hospital das Clínicas, São Paulo University Medical School, São Paulo, SP, Brazil. The study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, SP, Brazil.
No potential conflict of interest was reported.
Menezes AQ executed the experimental protocol, conducted the data analysis and participated in the manuscript writing. Pego-Fernandes P supervised all experimental stages and the manuscript preparation. Cardoso PF executed the experimental protocol and participated in the manuscript preparation. Braga KA and Nepomuceno NA executed the experimental protocol and performed the data analysis. Pazetti R executed the experimental protocol. Correia AT performed the statistical analysis. Canzian M performed the histopathological analysis. Santim JK executed the experimental protocol. Jatene FB supervised all stages of the study.