To study the effect of short-chain fatty-acids on atrophy and inflammation of excluded colonic segments before and after the development of diversion colitis.
INTRODUCTION:Diversion colitis is a chronic inflammatory process affecting the dysfunctional colon, possibly evolving with mucous and blood discharge. The most favored hypotheses to explain its development is short-chain fatty-acid deficiency in the colon lumen.
METHODS:Wistar rats were submitted to colostomy with distal colon exclusion. Two control groups (A1 and B1) received rectally administered physiological saline, whereas two experimental groups (A2 and B2) received rectally administered short-chain fatty-acids. The A groups were prophylactically treated (5th to 40th days postoperatively), whereas the B groups were therapeutically treated (after post-operative day 40). The mucosal thickness of the excluded colon was measured histologically. The inflammatory reaction of the mucosal lamina propria and the lymphoid tissue response were quantified through established scores.
RESULTS:There was a significant thickness recovery of the colonic mucosa in group B2 animals (p = 0.0001), which also exhibited a significant reduction in the number of eosinophilic polymorphonuclear cells in the lamina propria (p = 0.0126) and in the intestinal lumen (p = 0.0256). Group A2 showed no mucosal thickness recovery and significant increases in the numbers of lymphocytes (p = 0.0006) and eosinophilic polymorphonuclear cells in the lamina propria of the mucosa (p = 0.0022).
CONCLUSION:Therapeutic use of short-chain fatty-acids significantly reduced eosinophilic polymorphonuclear cell numbers in the intestinal wall and in the colonic lumen; it also reversed the atrophy of the colonic mucosa. Prophylactic use did not impede the development of mucosal atrophy.
After colostomy, the nonfunctioning intestinal segment presents inflammatory alterations comprising a nosological entity known as diversion colitis (DC).1 The preferred treatment for DC is intestinal transit reconstruction which, in most cases, resolves the inflammatory process.2
Ma et al.3 analyzed 21 cases of DC and concluded that moderate chronic inflammation with lymphoplasmocytary infiltrate in the lamina propria, vascular congestion, minimal alterations in crypt architecture and a slight decline in their number were the main histopathologic alterations found in the disease. Additionally, these changes were accompanied by the presence of prominent lymphoid nodules with or without hyperplasia of the germinal centers. Haque et al.4 observed the presence of eosinophilic polymorphonuclear cells (EPNs) in the lamina propria and colonic lumen of children with DC. Pinto et al.5 reported that the onset of significant atrophy in the colonic mucosa of Wistar rats coincides with the 40th day after surgical exclusion of the colon.
Grove et al.6 demonstrated the relationship between diet and the presence of short chain fatty acids (SCFAs) in the colonic lumen. Roediger showed that around 70% of colonocyte energy requirements originate in the SCFAs and, further, that 90% of these SCFAs are formed by acetic, n-propionic and n-butyric acid, the latter of which serves as the main energy source for colonocytes.7
The SCFAs exert trophic effects on the large bowel through direct contact of the acids with the colonic mucosa.8 Colonic trophism mechanisms occur as a result of increased energetic oxygenation, stimulated flow of blood microcirculation caused by dilated resistance arteries, enterotrophic hormone production and stimulation of the enteric nervous system. This trophism occurs transmurally and is not restricted to the mucosa.8,9
In addition to stimulating collagen maturation, these aforementioned functions are fundamental in colonic physiology and contribute to decreased bacterial translocation, intestinal adaptation in short bowel syndrome, stimulation of healing and increased anastomosis resistance.8-10
Some authors have postulated that the use of SCFAs in the nonfunctioning segment reverses the alteration of DC.2,11 Other studies were not as successful, however, and their authors question this form of treatment.12,13
The present study aimed to assess the use of SCFAs in the nonfunctioning colonic stump of Wistar rats in order to demonstrate microscopically the existence (or lack thereof) of a prophylactic or therapeutic role.
MATERIALS AND METHODSAnimalsForty male Wistar rats weighing between 220 g and 230 g were housed in a room under standard conditions of temperature, light, humidity, water and diet (Labina-Purina, São Paulo, Brazil) according to specifications described by Reeves et al.14 The animals were supplied by the vivarium of the Center for Experimental Surgery, Department of Surgery of the CCS (Centro de Ciências da Saúde) (Health Science Center) of UFRN (Universidade Federal do Rio Grande do Norte) (Federal University of Rio Grande do Norte). The animals were treated according to the use of Nonhuman Animals in Research: a Guide for Scientists.15 The surgery was performed in the Laboratory of the Operatory Technique discipline (UFRN) with the collaboration of the Department of Pathology (UFRN) and the Postgraduate Program in Health Sciences (PPGCSA (Programa de Pós-graduação do Centro de Ciências da Saúde), UFRN). This was a prospective, analytical, experimental, intervention study.
Surgical StudyAll of the animals were submitted to a 12-hour fast with the exclusive use of water. Immediately before surgery, a retrograde intestinal wash was performed with physiological saline to remove all fecal matter.5 All surgical procedures were conducted under aseptic conditions. Anesthesia was obtained using pentobarbital (20 mg/Kg intraperitoneally) and ketamine (50 mg/Kg intramuscularly). The animals were allowed to breathe spontaneously throughout the experiment. They were fixed in dorsal decubitus, and trichotomy and asepsis using povidone-iodine were performed.16
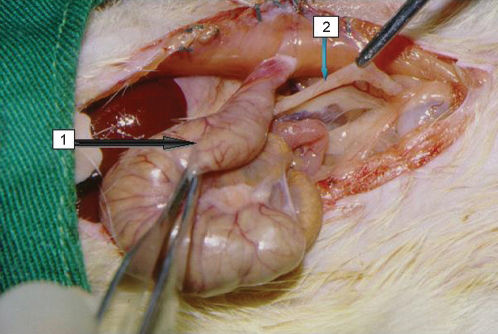
The abdominal cavity was opened by a median laparotomy of around 5 cm to identify the cecum and proximal colon, which was divided 5 cm from the ileocecal valve. The distal colon was submitted to terminal suture. After its sectioning, the distal colonic segment was kept inside the abdominal cavity. The proximal segment was colostomized and exteriorized through the abdominal wall left of the median incision. A single-barreled end colostomy was performed along with fixation of the stoma to the abdominal wall (primary maturation) with a 6-0 polypropylene thread. The abdominal wall was then closed with separate 3-0 cotton sutures (Fig. 1).5
The macroscopic aspect of the colon of a group B1 animal submitted to intestinal derivation. Arrow 1 indicates the functional intestinal segment, after derivation in intestinal wall colostomy. Arrow 2 shows the defunctionalized segment, with whitish coloration and visibly atrophic.
Four groups of ten adult Wistar rats submitted to colostomy underwent additional interventions. The protocol distributed the animals into groups A (A1 and A2) and B (B1 and B2). Two control groups (A1 and B1) received infusions of physiological saline administered rectally, whereas the two experimental groups (A2 and B2) received short-chain fatty-acids rectally. The A groups were prophylactically treated (5th to 40th post-operative days, twice a week), whereas the B groups were therapeutically treated (after post-operative day 40, for seven days). The intervention posologies are described in table 1.
Experimental design. Distribution of animal groups according to type of intervention, treatment period, and posologies.
| Group | η | Infused substance | Infusion period | Posology |
|---|---|---|---|---|
| A1 (control) | 10 | SS 0.9% | Between the 5th and 40th POD | Twice a week |
| A2 (prophylaxis) | 10 | SCFA | Between the 5th and 40th POD | Twice a week |
| B1 (control) | 10 | SS 0.9% | After the 40th POD | Twice a day for 7 days |
| B2 (treatment) | 10 | SCFA | After the 40th POD | Twice a day for 7 days |
SS = saline solution. SCAF = short-chain fatty acids. POD = post-operatory day.
The SCFA solution was developed in the biochemistry laboratory of the Bioscience Center at UFRN and was composed of: 75 mmol/L of sodium acetate; 35 mmol/L of sodium propionate; 20 mmol/L of butyric-N acid; 2.5 mmol/L of calcium chloride; 7.5 mmol/L of magnesium chloride; 10 mmol of potassium chloride.17 The solutions were elaborated in an iso-osmolar (280 mosm/L), and the pH was adjusted to seven by using appropriate amounts of NaOH or HCI.
HistomorphometryThe mean of four microscopic measures of mucosal thickness in the rats of each subgroup was measured using a Nikon Lobophot 10 × microscope (Nikon, Tokyo, Japan). This equipment expresses the mean thickness in millimeters by multiplying the value obtained by the standard correction factor indicated for the lens (0.0078) as a function of its amplification and diameter. The measures were obtained for each of the transversal and longitudinal histological sections of the colonic wall using the final mean for each animal from the values found.5
Qualitative Histological AnalysisDifferent cell type counts were performed to assess neutrophilic polymorphonuclear cells (NPNs), lymphocytes, and EPNs. The lymphoid follicle size of the mucous associated lymphoid tissue (MALT) was assessed in the lamina propria of the mucosa as well as in the colonic wall.
The intensity of alterations was graduated on a 6-point scale ranging from 0 to 5. These values were later converted into whole numbers using our modified version of Myers' index,18 which is based on variables appropriate to the study of DC. This procedure gave rise to a histologic score. A variable was stipulated for each type of variable analyzed, depending on whether it was favorable for DC diagnosis. This value was multiplied by the intensity of the alterations observed in the histologic sections.
StatisticsIn quantitative assessment, statistical analysis compared the measures obtained in the two paired groups (A1 and A2) and (B1 and B2) using analysis of variance procedures (Student's t-test). For qualitative assessment, exploratory analysis was performed by building tables and then comparing the mean intensity of the cells observed in each group according to cell type. To determine the existence of significant differences in cellularity between groups (A1 and A2) and (B1 and B2), the non-parametric Mann-Whitney test was applied. A significance level of 5% (p-value ≤ 0.05) was set for all of the results assessed.
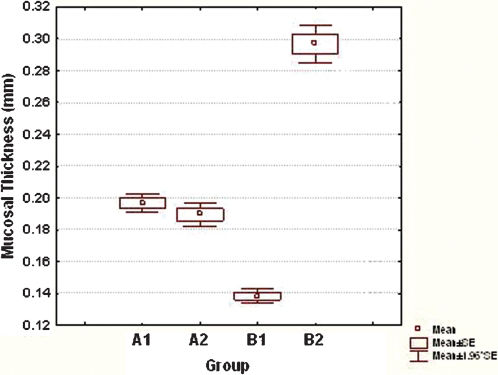
RESULTSHistomorphometryValues for colonic mucosal thickness are shown in Figure 2. No significance difference was found between the control (A1) and prophylactic (A2) groups in the prevention of DC in terms of mucosal atrophy (p = 0.1680). However, there was a significant difference between the control group (B1) and treatment group (B2), p = 0.0001.
Comparative box plots of the mean of mucosal thickness measures in control rats (A1), rats treated prophylactically using SCFAs (A2), control rats (B1), and rats treated with SCFAs (B2). No statistically significant difference between A1 and A2 was noted (p = 0.1680). A statistically significant difference between B1 and B2 was observed (p = 0.0001). SCFAs, short chain fatty acids.
Table 2 shows the different cell types, including the presence and size of MALT lymphoid follicles (LFs), with values expressed in accordance with Myers' modified score. The highest indices observed were the presence of LFs (×5), mainly large size (×3), and number of EPNs (×3) in the colonic lumen; these findings favored the diagnosis of DC.
Cell types, assessment of lymphoid follicles of mucous associated lymphoid tissue (MALT), and respective values proposed by the Myers index modified for conversion into absolute numbers.
| Histologic parameters | Indices adapted to DC |
|---|---|
| NPN in the lamina propria of the colonic mucosa | × 1 |
| Lymphocytes in the lamina propria of the colonic mucosa | × 2 |
| EPN in the lamina propria of the colonic mucosa | × 2 |
| EPN in the colonic lumen | × 3 |
| Presence of LF of MALT | × 5 |
| LF size | |
| Large | × 3 |
| Medium | × 2 |
| Small | × 1 |
MALT = mucous associated lymphoid tissue. NPN = neutrophilic polymorphonuclear cells. EPN = eosinophilic polymorphonuclear cells. LF = lymphoid follicles.
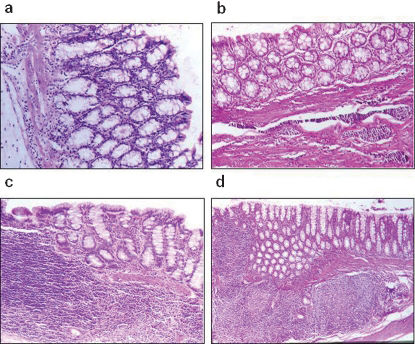
The histologic sections from group A1 (control) exhibiting mucosal atrophy are shown in Figure 3(a). Figure 3(b) shows sections from group A2 (prophylactic) exhibiting mucosal atrophy and slight MALT hyperplasia, and Figure 3(c) shows sections from group B1 (control) exhibiting mucosal atrophy and MALT hyperplasia. Figure 3(d) shows sections from group B2 (treatment) exhibiting normal mucosal thickness and MALT hyperplasia.
(a) Histologic section of the colon of a group A1 animal (control) exhibiting an atrophic mucosa (H&E ×400). (b) Atrophic colonic mucosa and slight MALT hyperplasia in a group 2 animal (prophylaxis) (H&E ×200). (c) Atrophic colonic atrophy with MALT hyperplasia in a group B1 animal (control) (H&E ×100). (d) Normal mucosal thickness with MALT hyperplasia in a group B2 animal (treatment) (H&E ×40). MALT, mucous associated lymphoid tissue.
The assessment of histologic scores by cell type and lymphoid follicles of MALT, which are represented by the sum of the median values in the control (A1) and prophylactic (A2) groups, is shown in Table 3. The results obtained in the qualitative analysis of the samples revealed a significant numerical increase in lymphocytes (p = 0.006) and EPNs in the lamina propria of the colonic mucosa (p = 0.0022) in the prophylactic group (A2) compared to the control group (A1). The prophylactic use of SCFAs (group A2) did not significantly reduce MALT hyperplasia compared to the control group (A1) (p = 0.0670). With respect to total cellularity, there were no significant differences between groups A1 and A2 (p = 0.2233).
Results of the Mann-Whitney U test for the comparison of histologic scores by cell type and lymphoid follicles between the control group (A1) and prophylactic group treated with short chain fatty acids (SCFAs) (A2).
| Type of cell | Median | U-value | p-value | Group size | ||
|---|---|---|---|---|---|---|
| A1 | A2 | A1 | A2 | |||
| NPN in the lamina propria | 1 | 1 | 39.5 | 0.3222 | 10 | 10 |
| Lymphocytes in the lamina propria | 2 | 4 | 9 | 0.0006* | 10 | 10 |
| EPN in the lamina propria | 2 | 4 | 13.5 | 0.0022* | 10 | 10 |
| EPN in the intestinal lumen | 9 | 6 | 36 | 0.2296 | 10 | 10 |
| Lymphoid follicles of MALT | 5 | 0 | 27 | 0.0670 | 10 | 10 |
| Final histologic scores (modified Myers index) | 20.9 | 19.9 | 34 | 0.2233 | 10 | 10 |
Table 4 shows a significant reduction in EPNs in the lamina propria of the treatment group (B2) compared to the control group (B1) (p = 0.0126), and a reduction was also observed in the intestinal lumen (p = 0.0256). We also found that the therapeutic use of SCFAs had little effect on the inhibition of MALT hyperplasia when comparing groups B2 and B1 (p = 0.5514). Total cellularity showed no significant difference between groups B1 and B2 (p = 0.0781).
Results of Mann-Whitney U test for comparisons between types of inflammatory cells along with the role of lymphoid follicles in the control group (B1) and group treated with short chain fatty acids (SCFAs) (B2).
| Type of cell | Median | U-value | p-value | Group size | ||
|---|---|---|---|---|---|---|
| B1 | B2 | B1 | B2 | |||
| NPN in the lamina propria | 1 | 1 | 44.5 | 0.9389 | 9 | 10 |
| Lymphocytes in the lamina propria | 4 | 4 | 33 | 0.2796 | 9 | 10 |
| EPN in the lamina propria | 4 | 2 | 18 | 0.0126* | 9 | 10 |
| EPN in the intestinal lumen | 9 | 6 | 20 | 0.0256* | 9 | 10 |
| Lymphoid follicles of MALT | 0 | 0 | 39 | 0.5514 | 9 | 10 |
| Final histologic scores (modified Myers index) | 19.6 | 13.8 | 23.5 | 0.0781 | 9 | 10 |
In clinical practice, the objective assessment of DC is made according to three main criteria: (1) analysis of mucosal thickness; (2) study of the role of inflammatory cells of the lamina propria and MALT and; (3) investigation of surface colonocyte alterations. The colonic mucosa exhibited reduced thickness in all of the cases studied. The latter parameter is therefore more reliable than the others, as it can be easily measured.19
The relevant results obtained in the present study show that SCFAs are effective for the treatment of DC as they reverse colonic mucosal atrophy. Therapeutic use of SCFAs has also been shown to reduce the number of EPNs in the lamina propria of the mucosa and colonic lumen, thereby decreasing the inflammatory process. In contrast, therapeutic SCFA use did not benefit the nonfunctioning colonic tissue by reversing lymphoid follicular hyperplasia in MALT.20 SCFAs were ineffective when used as DC prophylaxis, as shown by the fact that they did not impede mucosal atrophy on the 40th day postoperatively.
These findings corroborate data described in the literature. Harig et al.,2 for example, observed clinical, endoscopic, and histopathologic reversal in four patients with DC who underwent SCFA treatment in the excluded segment. Worsening resulted with treatment interruption or when the infusion solution was replaced by physiological saline.
With the exclusive use of topically infused SCFAs, Kiely et al.11 observed symptom remission and significant improvement in the endoscopic and histopathologic DC findings in three out of five patients studied.
Sengupta et al.21 submitted rats to a fiber-free diet to establish DC-like intestinal atrophy. These authors observed that the topical use of butyrate favored increased cellularity in the colonic crypt, elevated mitoses, and consequent cell proliferation and atrophy reversal. The authors also observed that the effect of butyrate was dose-dependent and that its action was of short duration.
Oliveira-Neto and Aguilar-Nascimento22 assessed the infusion effect of a solution containing fibers on the nonfunctioning colonic stump of 11 patients. These authors observed a significant decrease in the degree of DC, as well as a significant increase in crypt depth after infusion. Given that SCFAs are a result of fiber degradation caused by anaerobic bacteria in the colonic lumen, these findings suggested that SCFAs were directly responsible for DC remission.
Some researchers, however, found no relevance in the use of SCFAs as an active agent in DC remission. Guillemot et al. published a double-blind study with thirteen patients.12 These authors observed no significant endoscopic or histopathologic differences in DC reversal between the SCFAs and physiological saline infusion groups.
Schauber et al.13 examined 9 patients in a double-blind study and only 7 of these completed the protocol. All of the subjects had intestinal inflammatory disease and colostomy was indicated. The study showed no significant endoscopic or bacteriologic differences between the control and SCFA groups.
The disparity in results between these authors and those who observed data favoring the use of SCFAs may be explained by the differences in clinical indications for colostomy. Recent studies have revealed the inhibitory action of butyrate metabolism in patients with idiopathic ulcerative colitis.23
The trophic action of SCFAs on the large intestine, which occurs through its direct contact with the colonic mucosa, is important in the clinical control of DC.8 This action reduces the signs and symptoms associated with the condition itself (e.g. mucous discharge and transrectal bleeding). Further, SCFAs also prevent the emergence of complications related to mucosal atrophy and colonic epithelium lesion, as well as complications inherent to re-establishing intestinal transit.
Neut et al.20 reported that the colonic mucosal atrophy with loss of integrity seen in DC could predispose patients to bacterial translocation by interfering with local immunity and modifying native bacterial flora, both quantitatively and qualitatively. The present study found that the use of SCFAs for a short period of time not only reversed atrophy but also reduced the risk of losing mucosal integrity. Additionally, it did not interfere with MALT hyperplasia of the nonfunctioning colon. The fact that this latter finding has not been reported in the literature makes it scientifically relevant. These results, therefore, favor the preservation of local immunity while avoiding the modification of native bacterial flora. Furthermore, bacterial translocation was not observed in the results reported by Pinto Jr. et al.,24 corroborating the results obtained in this study.
Lim et al.25 raised the hypothesis that DC is a factor predisposing patients to the emergence of idiopathic ulcerative rectocolitis. This occurs through the sensitization of leukocytes in the nonfunctioning colon and subsequent leukocyte aggression toward the endothelium of the functional colon as a result of the emergence of anticolonic self-antibodies. The therapeutic action of SCFAs observed in this study would reduce the possibility of disease evolution.
Owing to their high morbidity and mortality rates, intestinal anastomosis fistulae remain the greatest threat to gastrointestinal tract surgeons. Pearce et al.26 observed higher re-anastomosis dehiscence indices when the interval for transit reconstruction was more than six months, which is sufficient time for atrophy of the nonfunctioning colon wall to occur. SCFA enemas facilitated the healing process of colonic anastomosis in rats.10 Possible mechanisms that might mediate this effect include an increase in cell proliferation of the colonic mucosa and the acceleration of collagen maturation.27 The first mechanism accelerates re-epithelization and increases blood flow, with a consequent rise in oxygen supply. The topical use of SCFAs twice daily for 7 days (the therapeutic methodology used in this study) reversed colonic mucosal atrophy. This therapy would reduce the potential risk of fistula formation in patients undergoing intestinal transit reconstruction.
The use of SCFAs is important for proper nutrition of the nonfunctioning colonocyte, because SCFAs reverse colonocyte atrophy and therefore minimize symptoms in some patients. This treatment may be particularly valuable for those patients undergoing intestinal transit reconstruction, however, because the aim of atrophy reversal is to prevent complications inherent to the surgery.
CONCLUSIONIn conclusion, the prophylactic action of SCFAs on DC in terms of colonic mucosal trophism was not confirmed. This study demonstrates the therapeutic use of SCFAs in experimental DC by showing the significant effects of SCFAs on atrophy regression and EPN reduction in the intestinal lumen and lamina propria of the colonic mucosa, despite their lack of interference with the intensity of MALT hyperplasia. Thus, the therapeutic application of SCFAs may be of great significance in clinical practice, especially in patients without associated inflammatory disease. SCFAs may shorten hospitalization and favor better postoperative management in colostomized patients by reducing complications.
This study was partially funded by a grant (no. 135226/06-6) from CNPq.