To identify Chlamydia trachomatis via polymerase chain reaction and a direct fluorescent antibody assay in patients with vernal keratoconjunctivitis while comparing the efficacies of both tests for detecting Chlamydia trachomatis in these conditions.
METHODS:Conjunctival scraping samples were obtained from 177 patients who were divided into two groups: a vernal keratoconjunctivitis group (group A) and a control group (group B). The polymerase chain reaction and a direct fluorescent antibody assay were performed. Sensitivity, specificity, receiver operating characteristic curves, and areas under the curve were calculated for both tests in groups A and B. Receiver operating characteristic curves were plotted using a categorical variable with only two possible outcomes (positive and negative).
RESULTS:Statistical analysis revealed a significant association between vernal keratoconjunctivitis and Chlamydia trachomatis infection detected by a direct fluorescent antibody assay with high sensitivity and specificity. All patients in group A with positive polymerase chain reactions also presented with positive direct fluorescent antibody assays.
CONCLUSION:The association between vernal keratoconjunctivitis and Chlamydia trachomatis infection was confirmed by positive direct fluorescent antibody assays in 49.4% of vernal keratoconjunctivitis patients and by positive polymerase chain reactions in 20% of these patients. The direct fluorescent antibody assay detected Chlamydia trachomatis in a higher number of patients than did the polymerase chain reaction. Although the diagnosis of trachoma is essentially clinical, the disease may not be detected in vernal keratoconjunctivitis patients. Due to the high frequency of chlamydial infection detected in patients with vernal keratoconjunctivitis, we suggest considering routine laboratory tests to detect Chlamydia trachomatis in patients with severe and refractory allergic disease.
Vernal keratoconjunctivitis (VKC) is a chronic ocular allergy characterized by itching and conjunctival hyperemia with papillary hypertrophy of the upper palpebral or tarsal conjunctiva and/or limbus. It is frequently associated with corneal involvement.1–3
Trachoma is a chronic follicular keratoconjunctivitis characterized by the presence of follicles in the upper palpebral conjunctiva and limbus. Mucous discharge may be present, along with itching, which is less intense than that experienced in VKC. Follicular necrosis and subconjunctival inflammation may lead to conjunctival scars, Arlt's lines and Herbert's pits, which are pathognomonic features of trachoma. Palpebral and secondary corneal involvement may also be present.
Transmission occurs by direct eye-to-eye and hand-to-eye contact, usually by contaminated ocular discharge, clothes, bed sheets, towels and the proliferation of flies in homes and schools.4,5
The diagnosis of both VKC and trachoma is essentially clinical. With trachoma, especially in endemic areas and in chronic and cicatricial trachoma, clinical diagnosis is straightforward. However, in the early stages of the infection, due to its relatively benign and asymptomatic course, it may not be easily differentiated from viral, bacterial, or allergic conjunctivitis. In these cases, laboratory tests become essential, especially direct fluorescent antibody assays (DFAs).6,7
DFAs are easily performed but require adequate transportation and storage of the samples in a refrigerator, a fluorescent microscope, and well-trained personnel.
Polymerase chain reaction (PCR) has been suggested to test for Chlamydia trachomatis that has both the specificity of cell culture and a level of sensitivity similar to that of DFA. PCR is an in vitro method for detecting DNA sequences by enzymatic amplification of a specific fragment that can synthesize more than one million copies of one DNA sequence in a short period of time.
VKC and trachoma share many features. They both affect school-age children and young adults in hot, dry climate areas. They are characterized by chronic keratoconjunctivitis, usually bilateral, that waxes and wanes throughout the year.8,9 One key difference is that whereas VKC stimulates a papillary reaction of the conjunctiva, trachoma stimulates a follicular response. However, following the early follicular hypertrophy of trachoma (phase TF), a papillary reaction (phase TI) may cover the follicles. Also, follicles and papillae may coexist. In these cases, giant papillae would be dominant and would obscure the follicles. Limbal follicles may also be obscured by the characteristic papillae and edema of limbal VKC. In this phase, we believe that many cases of trachoma may not be getting clinically diagnosed, especially in the presence of a common comorbid papillary disease such as VKC.
Vérin et al. first described a possible association between VKC and trachoma in 1980.8 Later, in 1988, Friedlaender & Cameron presented four cases of possible association.3 One year later, Vérin et al. (1989) described 8 (23.5%) cases of Chlamydia trachomatis infection confirmed the Wang and Grayston technique in 34 patients with VKC.9
The possible association between VKC and trachoma was not addressed again until 2000, when Melo et al. studied 72 patients with allergic conjunctivitis, 38 (52.8%) of whom had a positive DFA for Chlamydia trachomatis.10 In that study, the control group (60 patients) did not have any positive DFAs. Statistical analysis identified a significant association between the diseases.
The purpose of this study was to use PCR and DFA to detect the presence of Chlamydia trachomatis in patients with VKC compared with a control group and also to compare the efficacies of both tests for detecting Chlamydia trachomatis in patients with VKC.
MATERIALS AND METHODSOne hundred seventy-seven patients were divided into two groups.
Group A consisted of 87 patients with VKC from the Ocular Allergy Service of the Department of Ophthalmology. Patients using topical or systemic antibiotics were excluded. Patients were diagnosed with VKC using the following criteria: a clinical history of chronic bilateral conjunctivitis (at least one year) with seasonal exacerbations (i.e., itching, photophobia, and foreign body sensation); hypertrophic papillae at the superior palpebral conjunctiva and/or limbus; and, eventually, Horner-Trantas dots, superficial punctate keratitis and shield ulcers or corneal scars from shield ulcers.
Group B (the control group) consisted of 90 patients who presented for regular eye examinations (refractometry) and were neither complaining of allergic conjunctivitis nor taking topical or systemic antibiotics.
All patients in groups A and B were informed of the purpose of the study, and all patients signed an informed consent. The institutional review ethical committee approved this study.
Patients were asked about their disease length, symptoms, and familial and personal histories of atopy and other ocular diseases.
The symptoms assessed included itching, tearing, photophobia, discharge, and reduced visual acuity.
The following components were included in the examination: a measurement of visual acuity; slit lamp biomicroscopy to evaluate conjunctival hyperemia; a test for the presence of papillae at the conjunctiva and/or limbus and other conjunctival, limbal, and corneal alterations (follicles and scars); tonometry; and a fundus examination. All patients were examined by the same doctor.
All patients underwent tissue sampling for the detection of Chlamydia trachomatis by DFA. The superior palpebral conjunctiva of the right eye was scraped five times with a Kimura spatula. The sample was then placed in a demarcated circle on the appropriate slide, dried for 5 minutes, fixed with absolute methanol and stained with the fluorescent monoclonal antibody (Microtrak-SyvaTM). After 30 minutes of incubation in a moist chamber at room temperature, the slides were washed with distilled water and were left to dry again. The samples were examined by an experienced technician under immersion fluorescent microscopy with epi-illumination at 1000X magnification. The material was considered adequate when it included at least 100 epithelial cells per field. The criterion for a positive diagnosis was the presence of five or more elementary bodies per sample.
The PCR was calibrated by obtaining a positive control from a patient who had no allergic conjunctivitis and a clinical diagnosis of trachoma, which was confirmed by a positive DFA for Chlamydia trachomatis. The negative control was obtained from a patient who presented with no signs or symptoms of either trachoma or allergic conjunctivitis and had a negative DFA for Chlamydia trachomatis. The positive and negative controls were obtained to accurately calibrate the PCR for the detection of Chlamydia trachomatis.
All patients in groups A and B had the superior palpebral conjunctiva of their right eye scraped with a Dacron swab. The part of the swab containing the collected material was placed in a plastic tube with the transportation medium. Samples were taken immediately to a Molecular Biology laboratory within 4 hours after collection. The material was processed according to the technique introduced by Bobo.11
For each sample, an initial PCR was performed to verify the presence of β-human globin to avoid false negative results. The primers had the following sequences: Primer HGH1: 5′ – TGCCTTCCCAACCATTCCCTTA – 3′ and Primer HGH2: 5′ – CCACTCACGGATTTCTGTTGTGTTTC – 3′. Detection of the fragment occurred at 420-bp long.
In the samples in which β-human globin was detected, a second PCR was performed to detect and amplify a highly conserved region of the specific major outer membrane protein (MOMP) for Chlamydia trachomatis. The sequences of the primers used to detect Chlamydia trachomatis follow: Primer 1: 5′ - GAT AGC GAG CAC AAA GAG AGC TAA – 3′; Primer 2: 5′ – TTC ACA TCT GTT TGC AAA ACA CGG TCG AAA ACA AAG – 3′; Primer 3: 5′ - TCT GCT TCC TCC TTG CAA GCA AGT CTG CC – 3′; and Primer 4: 5′ - CCA TAG TAA CCC ATA CGC ATG CTG – 3′.
The amplified fragment was then examined by electrophoresis in agarose gel that was stained with ethidium bromide. Detection of the fragment occurred at 151-bp long.
Calculations of sensitivity and specificity were conducted, and a receiver operating characteristic (ROC) curve was plotted to evaluate and compare the diagnostic capabilities of both laboratory tests. Sensitivity, specificity, ROC curves, and areas under the curve were calculated with the assumption that DFA is the gold standard for Chlamydia spp. diagnosis. ROC curves were plotted using a categorical variable with only two possible outcomes (positive and negative), which can lead to underestimation of the area under the curve. MedCalc software version 9.3.7.0 was used for all calculations.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
RESULTSClinical evaluationGroup A consisted of 87 patients with VKC. Patient ages in this group ranged from 2 to 23 years (mean age = 9.6±4.8 years); 74 patients (73.6%) were white, and 23 (26.4%) were black; 27 (31.0%) were female, and 60 (69.0%) were male (Table 1).
Distribution by sex and race of patients with vernal keratoconjunctivitis (Group A) and controls (Group B).
| Group | Sex | Race | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | White | Black | |||||
| N | % | N | % | N | % | N | % | |
| A | 27 | 31 | 60 | 69 | 64 | 73.6 | 23 | 26.4 |
| B | 45 | 50 | 45 | 50 | 68 | 75.6 | 22 | 24.4 |
Legend:
N: Number.
χ2 = 5.831 (for sex); 0 (for race).
p = 0.016 (for sex); 1 (for race).
Group B (control group) consisted of 90 patients. Patient ages ranged from 2 to 23 years (medium age = 9.0±5.0 years); 45 patients (50.0%) were male, and 45 (50.0%) female; 68 patients (75.6%) were white, and 22 (24.4%) were black (Table 1).
No statistical differences were identified between groups A and B based on either race (χ2 = 0, p = 1.0) or age (t = -0.862; p = 0.39). The majority of VKC patients (86.2%) were less than 15 years old. In group B, 88.9% of the patients were under the age of 15. Caucasians were predominant in both groups (73.6% in group A and 75.6% in group B).
In group A, 69.0% of the patients were male, and in group B, 50.0% of the patients were male. This difference between the groups was statistically significant (χ2 = 5.831, p = 0.016).
All patients in group A presented with itching and red eyes. Symptoms of corneal involvement (i.e., punctate keratitis, superficial corneal opacities, shield ulcer, and peripheral neovascularization) were identified in 15 patients (17.2%). Seventeen patients (19.5%) in group A (i.e., patients with VKC) had conjunctival and limbal scars (i.e., scars in the upper tarsal conjunctiva and pits in the superior limbus), both of which were suggestive of trachoma (TS). Follicles (more than six), suggesting follicular trachoma (TF), were identified in the upper tarsal conjunctiva in two patients (2.3%) from group A. No patients presented with typically trachomatous corneal opacities or palpebral alterations.
Direct fluorescent antibody testAll samples had more than 100 epithelial cells per field and were considered adequate for laboratory analysis.
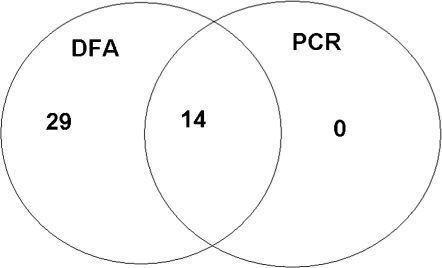
In group A, 44 samples (50.6%) were negative (less than five fluorescent elementary bodies), and 43 samples (49.4%) were positive (more than five fluorescent elementary bodies). Positive DFA was identified in all patients with suggestive signs of trachoma (two patients with more than six follicles in the upper tarsal conjunctiva and 13 of 17 patients with conjunctival and limbal scars). In group B, all samples were negative.
Polymerase Chain ReactionIn group A, human β-globin was detected in 70 samples (80.4%); 14 (20.0%) were positive for Chlamydia trachomatis. In group B, human β globin was identified in 60 samples (66.7%), one (1.7%) of which was PCR-positive for Chlamydia trachomatis.
Correlating the clinical findings with the results of the PCR, we observed that two patients from group A with suggestive signs of TF had positive PCR results. The PCR results were also positive in eight of the 17 patients with suggestive TS.
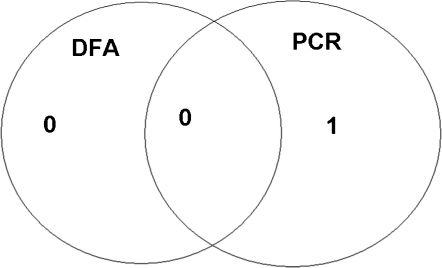
Direct Fluorescent Antibody Assay vs. Polymerase Chain ReactionAll patients in group A with positive PCR results also had positive DFAs. Only one patient from group B with a negative DFA had a positive PCR result (Figures 1 and 2)
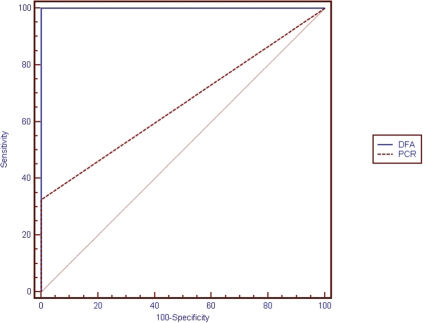
Figure 3 displays ROC curves for DFA and PCR.
Areas under the ROC curves were 1.0 (95% CI = 0.958 to 1.000) for the DFA and 0.663 (95% CI = 0.553 to 0.761) for the PCR. A pairwise comparison of ROC curves revealed a difference of 0.337 (95% CI = 0.223 to 0.451, p<0.001).
The sensitivity and specificity of the DFA were 100 (95% CI = 91.7 to 100) and 100 (95% CI = 91.9 to 100), respectively. The sensitivity of the PCR was 23.56 (95% CI = 19.1 to 48.5), and the specificity of the PCR was 100 (95% CI = 91.9 to 100).
DISCUSSIONIn our study, as in previous publications, VKC occurred more frequently in children less than 15 years old (86.25%) and in males (69.0%).1,2 A clinical diagnosis of VKC was made in all patients from group A, and they all reported itching.
A clinical diagnosis of Chlamydia trachomatis infection could also have been made by the presence of scars. However, in the earlier stages of infection, the relatively benign and asymptomatic course and the preponderance of signs and symptoms of VKC could obfuscate the correct diagnosis. In our study, scars suggestive of trachoma were detected in 17 patients (19.5%) from group A.
All the samples that were prepared for DFA were used because all had the minimum of 100 epithelial cells per field. The presence of five or more fluorescent elementary bodies was considered a positive result in the analysis of the samples.6
In group A, 49.4% of the samples presented positive DFA, and in group B, all 90 samples were negative. Of the 43 patients with positive DFA, 19 had suggestive clinical signs of trachoma, and the majority of the remaining cases could be diagnosed only by laboratory tests.
PCR is considered the most sensitive non-culture test for the detection of Chlamydia trachomatis in non-ocular samples. Problems related to contamination and the presence of DNA polymerase inhibitors, such as blood and lysozyme, could result in false-negative and false-positive results. The routine use of a negative control and an internal control (for the detection of human β-globin) would eliminate the risk of misreading for the methodology applied in this study.
Human β-globin was detected in 70 of the 87 samples from group A and in 60 of the 90 samples from group B. Therefore, 80.4% and 66.7% of the samples, respectively, were used. Although many studies have used the plasmid for DNA amplification, some Chlamydia trachomatis strains with no plasmid have been described.7 Therefore, we opted to amplify a conserved region of nucleotides in the MOMP gene.
We obtained fewer positive PCRs than DFAs: 14 samples in group A (corresponding to 20.0%) and 1 sample in group B (corresponding to 1.7%).
Frequent questions raised by practitioners include whether VCK causes trachoma, whether trachoma causes VKC or whether their co-occurrence is merely coincidental. These questions arise because both diseases affect the same group of patients (i.e., school children in hot and dry areas).
Vérin et al. reported that Chlamydia trachomatis could act as an allergen or as the trigger of an allergic process, suggesting that a chronic chlamydial infection, which alters the immunologic events on the ocular surface, could be associated with the local allergic response.8,9
Friedlaender and Cameron observed that the infectious nature of trachoma was not relevant in the pathogenesis of VKC. Atopic patients, such as those with VKC, present with some local or systemic immunological compromise, which could explain the greater incidence of staphylococcal and herpetic infections and possibly chlamydial infections.3 In addition, the integrity of the epithelium is considered the first line of defense of the ocular surface in many infectious conditions. VKC patients have conjunctival alterations due to chronic inflammation, vascular hyperpermeability, and excoriation, all of which compromise the integrity of the ocular surface, rendering the patient more susceptible to secondary infections, such as trachoma.3
Another factor to be considered is that all VKC patients present with itching as their main symptom, and they excessively manipulate their eyes, thus transferring significant numbers of microorganisms, possibly including Chlamydia trachomatis, from their hands to their eyes. Chlamydia trachomatis can also be carried by contaminated secretions, which are commonly found in the eyes of VKC patients.
SCARPI et al. observed in their study that itching was the main symptom in trachoma patients (11.47%); they likely also included some patients with concomitant trachoma and allergic conjunctivitis.12
Rao et al. investigated the presence of Chlamydia trachomatis in 127 patients with acute, chronic, and recurrent conjunctivitis.5 Of the 22 patients with clinically diagnosed allergic conjunctivitis, seven (31.8%) had a positive culture for Chlamydia trachomatis.
Notably, DFA is still considered the gold standard for Chlamydia trachomatis detection. Without it, clinical diagnoses would have been made in only 19 of 43 patients (44.2%) with positive DFAs, indicating that trachoma would not have been diagnosed in 55.8% of patients. These cases would have been treated as severe and refractory allergic conjunctivitis.
Despite its low sensitivity in this study, DNA amplification by PCR is still used for the detection of ocular chlamydial infection; more recently, however, nucleic acid amplification tests (NAATs) based on the amplification of rRNA have been developed, providing a potential advantage because bacterial rRNA is present at up to 10,000 times the copy numbers of genomic DNA and at up to 1,000 times the copy numbers of plasmid DNA. This development opens a field of new opportunities for the detection of chlamydial infections using these techniques.13,14
Although both trachoma and VKC have characteristic periods of waxing and waning even without treatment, the chronicity, the recurrent nature and the long duration of the diseases can be highly disabling. Patients are disabled not only by the severe symptoms in the acute phases but also by the more frequent occurrences of complications, such as lid abnormalities and conjunctival and corneal scars, which can cause reduced visual acuity, impairing the acquisition of reading skills in children.1,3
Trachoma is a self-limiting disease, resolving in approximately one year even without treatment. However, treatment is essential to avoid recurrences, scars, and other sequelae. Immunological diseases such as VKC are treated with anti-allergic and anti-inflammatory drugs such as corticosteroids that depress cell immunity and are implicated in the control of infections and trachoma. In contrast, trachoma is treated with antibiotics. Therefore, because the treatments differ for VKC and trachoma, it is essential to accurately determine their co-occurrence to initiate the appropriate treatment.
Based on the population studied and the applied methodologies, we report several findings. First, there was an association between VKC and Chlamydia trachomatis infection that was confirmed by positive DFAs in 49.4% of VKC patients and by positive PCRs in 20.0% of patients. Second, DFA detected Chlamydia trachomatis in a greater number of patients than did PCR, making it the more sensitive and specific test. Third, although the diagnosis of trachoma is essentially clinical, the disease may not be detected in some VKC patients. In conclusion, we suggest considering laboratory tests to routinely detect Chlamydia trachomatis by DFA in patients with severe and refractory VKC.
The authors wish to acknowledge Niro Kasahara, MD, PhD, from the Statistics and Epidemiology Service of the Department of Ophthalmology of Santa Casa in São Paulo, Brazil, for the statistical review and analysis provided, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support and Prof. Dra. Lia Mara Rossi, from Faculdade de Ciências Médicas da Santa Casa de São Paulo.
Statement about Conformity with Author Information: This study was reviewed and approved by the Bioethics Committee from Santa Casa in São Paulo, Brazil.
No potential conflict of interest was reported.
Nishiwaki-Dantas MC designed and conducted the study; collected, managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript. Abreu MT designed and conducted the study; managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript. Melo CM designed and conducted the study; collected, managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript. Romero IL designed and conducted the study; collected, managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript. Neto RBM designed and conducted the study; collected the data; prepared, reviewed, and approved the manuscript. Dantas PEC designed and conducted the study; managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript.