Corticosteroids have been used in bronchopulmonary dysplasia prevention because of their antiinflammatory effects. Among their effects is a decrease in the incidence of bronchopulmonary dysplasia. However, short- and long-term side effects have been detected in preterm newborns.
PURPOSETo analyze the effects of corticosteroids on bronchopulmonary dysplasia, length of stay, mortality, growth, as well as the adverse effects in very low birth weight newborns between 10 and 14 days of life and dependent on mechanical ventilation.
METHODSCohort study. All newborns with a birth weight under 1500 g, mechanical ventilation-dependent between 10 and 14 days of life, during the period January 2000 and June 2001 were included (n = 38). They were divided into 2 groups: Group I with corticosteroids (n = 16) and Group II without corticosteroids (n = 22). Dexamethasone administration: from the 10th day of life, d1 – d3, 0.3 mg/kg/d; d4 – d6, 0.2 mg/kg/d; d7 – d9, 0.1 mg/kg/d. Respiratory evolution, bronchopulmonary dysplasia (oxygen dependence at 28 days of life), growth pattern and the presence of adverse effects were analyzed.
RESULTSThe incidence of bronchopulmonary dysplasia was 6.5% (Group I) and 30% (Group II), P = .07. A decrease in growth was detected in Group I compared with Group II (change in weight: Group I — 47 g/week, Group II — 85.5 g/week, P = .06; change in head circumference: Group I — 0.75 cm/week, Group II — 1 cm/week, P = .05).
CONCLUSIONUse of corticosteroids in very low birth weight infants dependent on mechanical ventilation during the first 10 to 14 days of life did not affect the respiratory evolution and occurrence of bronchopulmonary dysplasia, but the velocity of growth was reduced.
Devido às suas ações anti-inflamatórias, os corticosteróides têm sido utilizados para prevenção de displasia broncopulmonar, sendo descrita, uma redução da incidência desta patologia. No entanto, efeitos adversos a curto e a longo prazo têm sido detectados, em recém-nascidos pré-termo.
OBJETIVOAnalisar os efeitos sobre a incidência de displasia broncopulmonar, duração de ventilação mecânica e de internação, mortalidade, crescimento, além dos efeitos adversos dos corticosteróides, administrados entre 10-14 dias de vida, em recém-nascidos de muito baixo peso, dependentes de ventilação mecânica.
MÉTODOSRealizou-se estudo de coorte, incluindo-se todos os recém-nascidos com peso de nascimento < 1500 gramas dependentes de ventilação mecânica, entre 10-14 dias de vida. Foram divididos em: Grupo I – receberam dexametasona (16) e Grupo II – não receberam dexametasona (22). Administrou-se dexametasona, a partir do 10° dia de vida, dias 1 a 3 – 0,3 mg/kg/d, dias 4 a 6 – 0,2 mg/kg/d, dias 7 a 9 – 0,1 mg/kg/d. Analisou-se o desenvolvimento de displasia broncopulmonar (dependência de oxigênio aos 28 dias de vida), efeitos sobre a evolução respiratória e sobre o padrão de crescimento, além da ocorrência de efeitos adversos.
RESULTADOSA incidência de displasia broncopulmonar não diferiu entre os grupos (GI – 62,5%; GII – 22,7%;p = 0,07). Detectou-se desaceleração do crescimento no GI em relação ao GII(D P = 47g/semana, GI e 85,5g/semana, GII; p = 0,06; D PC – 0,75 cm/semana GI e 1cm/semana, no GII; p = 0,05).
CONCLUSÃOO uso de corticosteróides, em recém-nascidos pré-termo, entre 10 – 14 dias de vida não reduziu incidência de displasia broncopulmonar e causou uma desaceleração do crescimento.
The use of surfactants and the introduction of new respiratory therapeutic modalities has changed the outcome for preterm newborns with respiratory distress syndrome, even though effects on the incidence of bronchopulmonary dysplasia (BPD) have not been observed.
Bronchopulmonary dysplasia is one of the main causes of morbidity in the neonatal intensive care unit (NICU), especially for very low birth weight infants, with reported incidences of up to 71% among the newborns with a gestational age of 25 weeks,1 30% among newborns with a birth weight under 1500 g,2 and up to 100% for birth weights between 500 and 750 g.1,3,4
A greater survival of very low birth weight infants with BPD results in higher costs, especially in for the NICU, in addition to longer hospital stays and, after the discharge, frequent re-hospitalizations.
Several clinical trials have described favorable effects with the use of corticosteroids in the neonatal period, including a reduction of the incidence of BPD,5–9 the duration of mechanical ventilation,6,10–13 and short-term improvement in pulmonary compliance.10,11
However, concomitant with favorable effects of corticosteroids in preterm infants who are dependent on mechanical ventilation, several adverse effects have also been described such as hyperglycemia,6,13–15 systemic arterial hypertension,10,13–16 interventricular septum hypertrophy,5,15,17 irritability,5 gastrointestinal perforation,18 intensification of prematurity retinopathy,19 suppression of the adrenal axis,20 alteration in mineral homeostasis21 and the bone growth,22 postnatal growth retardation,23,24 and an increase of infection incidence.25
In addition to these short-term adverse effects, a higher incidence of cerebral palsy and neurological alterations have been observed in preterm newborns that received corticosteroids in the neonatal period and underwent long-term evaluation.26,27
This study analyzed the effects of the use of dexamethasone beginning between 10 and 14 days of life in very low birth weight infants and dependent on mechanical ventilation. We analyzed the incidence of BPD and its evolution, the duration of mechanical ventilation and oxygen therapy, the adverse effects and mortality with the purpose of evaluating the hypothesis that this corticosteroid can benefit the respiratory evolution of these newborns even though its use also has some adverse effects.
METHODSA cohort study was performed that included 38 preterm newborns. These were selected according the the following inclusion criteria: gestational age <37 weeks, birth weight <1500 g, dependence on mechanical ventilation between the 10th and 14th days of life.
Newborns with congenital malformations and chromosomal alterations were excluded, as were newborns who presented one of the following diagnoses at the beginning of the study: infection, hyperglycemia, systemic arterial hypertension, or hemorrhagic syndromes.
Newborns selected for the study were divided into 2 groups: Group I — received dexamethasone and Group II — did not receive dexamethasone.
Dexamethasone was administered to newborns that fulfilled the inclusion criteria according to the following therapeutic schedule beginning on the 10th day of life: every 12 hours, for 9 days as follows: day 1 through 3 — 0.3 mg/kg/day; day 4 through 6 — 0.2 mg/kg/d; and day 7 through 9 — 0.1 mg/kg/d.
Gestational age was determined from the date of the last menstruation; this was confirmed by the New Ballard method.28 When a difference of 2 or more weeks in estimated gestational age occurred between the clinical evaluation through this method and the one determined by the date of last menstruation, the clinical evaluation was used. The newborns were classified as adequate for gestational age (AGA), when their birth weights were below percentile 90 and above percentile 10 of the Ramos Intrauterine Growth Curve,29 and small for gestational age when their birth weights were below percentile 10 of that curve.30
The development of BPD was analyzed as the factor of primary concern. As a secondary concern, the following were observed: duration of mechanical ventilation and oxygen therapy, mortality, length of stay in the neonatal unit, neonatal growth pattern during the hospital stay, and the occurrence of adverse effects including systemic infection, fungal infection, hyperglycemia, systemic arterial hypertension, persistence of ductus arteriosus, necrotizing enterocolitis, intracranial hemorrhage, retinopathy of prematurity, and metabolic bone disease.
We defined BPD as dependence on oxygen in preterm newborns at risk at 28 days of life confirmed by clinical and radiological alterations.31,32
Diagnosis of pneumonia was established in the presence of clinical deterioration, tachypnea, rales, and radiological signs of condensation and/or positive blood culture. Infection was defined as the occurrence of clinical worsening, poor peripheral perfusion, hypoactivity, and positive blood culture. Hyperglycemia was defined as a plasma glycemic level equal to or higher than 150 mg/dL.33 Systemic arterial hypertension was defined as a systolic pressure equal to or higher than 2 standard deviations from that reference.34 The diagnosis of persistence of ductus arteriosus was based on the presence of clinical manifestations such as systolic or continuous murmur in the second left intercostal space, pulsatile precordium or visible ictus, or wide pulses at the physical examination and confirmed by the echocardiogram. The diagnosis of necrotizing enterocolitis was made when the following clinical alterations were noted: abdominal distention, bloody stools, pain at abdominal palpation and/or radiological alterations, distended intestinal loops, edema in the walls of intestinal loops, and the presence of pneumatosis intestinalis.35 Diagnosis of intracranial hemorrhage was based on the result of the head ultrasonography as classified according to Papile.36 Perinatal asphyxia was diagnosed when the newborn presented an Apgar score <6 at the fifth minute of life.37
A chest x-ray was performed at admission at any time when clinically indicated, and at 28 days of life; head ultrasonography was performed in the first 7 days of life and at approximately 14 and 30 days of life; an echocardiogram was performed in the presence of a murmur in the second left intercostal space or wide pulses with visible ictus were detected; an ophthalmologic examination was performed at 4 weeks of life by a pediatric ophthalmologist using the international classification.38 The assistance to the newborn followed the guidelines in the NICU and was prescribed by the responsible assistant doctor, who was blinded to the study groups.
For the purposes of statistical analysis, a database was developed using the EPI-INFO 6.04 software. For continuous variable analysis, the t test and Mann-Whitney test were used, and for discrete variables, the chi square test was used. Statistical significance was declared when P <.05.
RESULTSAmong 38 very low birth weight newborn infants admitted between January 2000 and July 2001 were included, 16 were selected for Group I (dexamethasone treated) and 22 for Group II.
The groups did not differ regarding gestational age, birth weight, gender, color, size for gestational age, perinatal asphyxia, and surfactant administration (Table 1).
Characteristics and evolution of the studied population.
| Group I | Group II | P | |
|---|---|---|---|
| Gestational age (wk) | 28.9 ± 1.8 | 29 ± 1.7 | .99 |
| Birth weight (g) | 1082.18 ± 200 | 970 ± 264 | .29 |
| Gender (M/F) (%) | 43.75/56.25 | 50/50 | .95 |
| Color (white/nonwhite) | 62.5/37.5 | 68.2/32.8 | .98 |
| Adequate for gestational age (%) | 68.7 | 59.0 | .78 |
| Small for gestational age (%) | 31.2 | 41.0 | .78 |
| Perinatal asphyxia (%) | 66.6 | 35.0 | .15 |
| Surfactant (%) | 93.75 | 68.2 | .13 |
| Hospital stay (d) | 78.6 ± 40.2 | 64.8 ± 26.0 | .32 |
| Duration of oxygen | 20.1 ± 2.0 | 8.0 ± 8.2 | .05 |
| therapy (d) | |||
| Extubation (d) | 2.6 ± 2.2 | 3.0 ± 1.0 | .40 |
| Duration of mechanical ventilation (d) | 19.1 ± 5.0 | 12.9 ± 10.0 | .09 |
A tendency for a higher incidence of BPD occurred in Group I (Group I — 62.5%, Group II — 22.7%, P = .07). No effect of the use of dexamethasone on duration of mechanical ventilation was observed (Group I — 19 ± 14 days, Group II — 12.9 ± 10 days, P = .09). However, Group II used oxygen for a shorter period (Group I — 20 ± 10 days, Group II — 8 ± 4 days, P = .05).
There was a tendency for reduced mortality in the group that received dexamethasone (Group I — 6.5%, Group II — 30%, P = .07). Nevertheless, an influence by the use of dexamethasone on the hospitalization length was not observed (Group I — 78.5 ± 40 days, Group II — 64.8 ± 26 days, P = .32) (Table 2).
Adverse effects in the studied groups (%).
| Group I | Group II | P | |
|---|---|---|---|
| Pneumonia | 62.5 | 63.6 | .95 |
| Bacterial infection | 93.0 | 77.2 | .60 |
| Fungal infection | 0 | 4.5 | .90 |
| Persistence of ductus arteriosus | 50.0 | 54.5 | .95 |
| Interstitial emphysema | 25.0 | 22.0 | .99 |
| Necrotizing enterocolitis | 6.2 | 18.1 | .37 |
| Hyperglycemia | 6.2 | 0.0 | .43 |
| Blood hypertension | 0.0 | 4.5 | .99 |
| Gastrointestinal bleeding | 0.0 | 4.5 | .99 |
| Intracranial hemorrhage | 31.2 | 9.6 | .10 |
| Phosphorous deficiency | 37.5 | 13.6 | .12 |
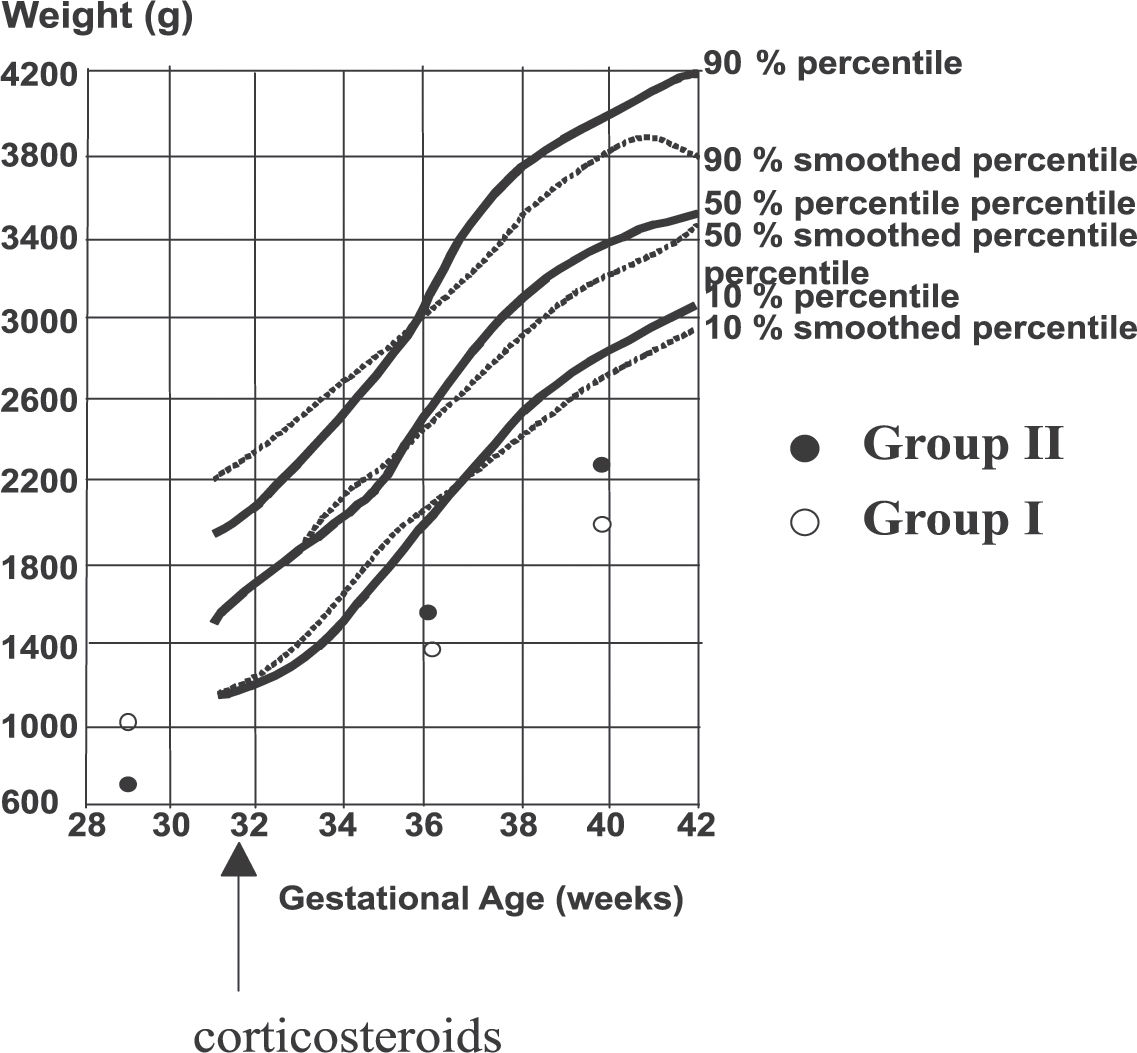
A reduced weight gain and growth velocity of the head circumference was detected between 28 and 36 weeks of corrected gestational age in Group I compared with Group II (change in weight: Group I — 47 g/week and Group II — 85.5 g/week, P = .06); change in head circumference: Group I — 0.75 cm/week and Group II — 1 cm/week, P = .05) (Figure 2). Regarding length, there were no observed differences between the groups (Figure 2).
Evolution of head circumference of included patients between 36 and 40 weeks of corrected gestational age in children treated with corticosteroids (Group I - open circles, treatment started at week 32), or untreated (Group II – closed circles). Observed values are compared to the 10, 50, and 90 percentile curves (percentile curves: full lines; smoothed percentile curves: dotted lines).
Weight gain (Figure 1) and growth velocity of the head circumference (Figure 2) between 28 and 36 weeks of corrected gestational age were reduced in Group 1 as compared to Group 2: weight gain was 47 g/week in Group I, 85.5 g/week in Group II, (P = .06); the head circumference increased by 0.75 cm/week in Group I, vs. 1.00 cm/week in Group II (P = .05)
Evolution of weight of included patients between 36 and 40 weeks of corrected gestational age in children treated with corticosteroids (Group I - open circles, treatment started at week 32), or untreated (Group II – closed circles). Observed values are compared to the 10, 50, and 90 percentile curves (percentile curves: full lines; smoothed percentile curves: dotted lines).
Corticosteroids have been used with the aim of reducing the inflammatory responses due to antiinflammatory reactions39,40 triggered by several factors, such as hyperoxia,41 infection,42 and mechanical ventilation43,44 among others, upon the developing lung. These inflammatory responses may contribute to reduced alveolarization,41–44 which constitutes one of the main characteristics of BPD.45
Favorable effects on the incidence BPD as a result of the use of corticosteroids in preterm newborns dependent on mechanical ventilation at 10 to 14 days of life were not observed in this study. Neither did a reduction of the duration of ventilation, nor of the hospital stay result from the use of corticosteroids, in contrast to what has been observed by others.7,9,10,12,13 However, we found a tendency for reduced mortality among newborns that received corticosteroids as described.7,9,10,12,13 It is possible that because of the design of the study, in which the inclusion criterion was children dependent on mechanical ventilation between 10 and 14 days of life, newborns with severe pulmonary diseases may have been selected for inclusion, and consequently, evidence of favorable effects of corticosteroids upon the respiratory function in this selected group may not have become evident. However, it is important to note that the newborns included in the treated group had a tendency for a lower mortality rate, which indirectly could be suggesting that despite the severity of their conditions, certain benefits may have occurred.
Over the past decades, methods have differed as to the beginning of administration of corticosteroids: (i) from the first hours of life (early),6,7–9,16,45–47 (ii) from 7 to 14 days of life (moderately early),12,23,48 (iii) after 14 days of life (late)10,13,49,50. Doses and the length of treatment (from 9 to 42 days) are also reported.
Despite this great variability in the method of administration of corticosteroids, favorable effects have commonly been detected. A reduction in the incidence of BPD due to the therapeutic schedules that started either in the first hours of life or between the 7th and 14th day of life7,9,48,51 have been reported, in addition to a reduction in the duration of mechanical ventilation and great success with extubation in all schedules.7,9,48,51,52
Nevertheless, common adverse effects have also been described in the short-term, such as hyperglycemia,6,9,13,14,15,48,51 systemic arterial hypertension,9,10,13–16,51,52 hypertrophy of the myocardium,5,15,17,51 gastrointestinal bleeding,9,51 and an elevation in the incidence of infections.25,51,52
In this study the use of corticosteroids did not alter most of the factors described above. However, an increase in the incidence of hyperglycemia occurred that was similar to that found in other studies. In addition, we observed a decrease in the growth between 28 and 36 weeks of corrected gestational age. Others have described this influence in the postnatal growth. Papile et al.23 detected a reduction in the head circumference growth of newborns during corticosteroid therapy.
Mataloun et al.24 observed a decrease in growth during corticosteroid therapy in a study that used higher doses over a prolonged period; growth resumed after the suspension of treatment.
At present, long-term follow-ups of children who received corticosteroids during the neonatal period have been revealing neurological alterations and an increase of the incidence of cerebral palsy.9,52 It is important to emphasize that these long-term neurological alterations were found in children who received corticosteroids in the first 96 hours of life and after 14 days. Additionally, these studies are heterogeneous regarding the dosage and length of therapeutic schedule used. The studies that detected increased incidence of cerebral palsy were of children who received therapeutic schedules of longer duration. However, Shinwell et al.53 showed a 3-fold increase in the incidence of cerebral palsy with a therapy schedule beginning early and of short duration (1 to 3 days) in a multicentric study. These results showed that neurological effects may also occur following therapy schedules of short duration.
Based on the literature, the use of corticosteroids in preterm infants who are dependent on mechanical ventilation and are at risk for development of BPD in the neonatal period is not advised.54,55
In spite of these recommendations, there are still doubts regarding the long-term adverse effects of corticosteroid use due to the fact that these investigations are heterogeneous. In addition, there is an unsolved contradiction in that the neurological effects have not been observed during long-term follow-up when the corticosteroids were introduced between 10 to 14 days of life. These results raise doubts as to the inevitability of long-term neurological effects.
Although dexamethasone, the most commonly used corticosteroid in major studies, produces these side effects, other corticosteroids such as beclomethasone may not.56 This issue requires further study.
Some neonatal units, including many in Brazil, continue to use dexamethasone with restricted indications57 despite existing recommendations. Therefore, new studies to analyze the neurological evolution of the newborns that received corticosteroids in the neonatal period, mainly between 10 and 14 days of life, is necessary.
The results of this study do not favor the use of corticosteroids in preterm newborns dependent on mechanical ventilation between 10 and 14 days of life because there was no evidence of favorable effects upon the respiratory evolution of these newborns, although a tendency for lower mortality was detected; on the other hand, unfavorable effects upon growth were detected.
Moreover, recent investigations regarding the pathophysiological mechanisms involved in “new” BPD emphasize the great importance of perinatal development and suggest beginning the use of mechanical ventilation earlier and continuing for a period longer than 7 days. Therefore, beginning treatment with corticosteroids between 10 and 14 days of life could be too late for prevention of BPD, in addition to increasing the risk of unfavorable effects on growth and adverse effects.