To determine the viruses and risk factors associated with hospital and intensive care unit (ICU) admissions in infants with acute bronchiolitis.
INTRODUCTION:Bronchiolitis is a major cause of morbidity in infants. Widespread use of molecular-based methods has yielded new insights about its etiology, but the impact of viral etiologies on early outcomes is still unclear.
METHODS:Seventy-seven infants with bronchiolitis who were under two years of age and visited an emergency unit were included. Using molecular-based methods, samples were tested for 12 different respiratory viruses. Logistic regression models were used to identify clinical and virological variables associated with the main endpoints: hospital admission and ICU admission.
RESULTS:We identified at least one virus in 93.5% of patients, and coinfections were found in nearly 40% of patients. RSV was the most common pathogen (63.6%), followed by rhinovirus (39%). Identification of RSV was only associated with an increased risk of hospital admission in the univariate model. Younger age and enterovirus infection were associated with an increased risk of hospital admission, while atopy of a first-degree relative showed a protective effect. Prematurity was associated with an increased risk of admission to the ICU. Coinfections were not associated with worse outcomes.
CONCLUSIONS:Molecular-based methods resulted in high rates of viral identification but did not change the significant role of RSV in acute bronchiolitis. Younger age and enterovirus infection were risk factors for hospital admission, while prematurity appeared to be a significant risk factor for admission to the ICU in acute viral bronchiolitis.
Acute viral bronchiolitis is one of the most frequent causes of hospitalization in infants worldwide.1 Aside from the significant financial burden it causes in developed countries,2 the condition is responsible for many deaths attributed to respiratory infections in developing nations.3
Respiratory syncytial virus (RSV) is the major etiological agent responsible for bronchiolitis and is identified in 70% of bronchiolitis cases during the fall and winter.1 Parainfluenza (PIV), adenovirus, and influenza are frequently described as occurring year-round.4
The recent widespread use of molecular-based methods has yielded new insights about the etiology of bronchiolitis and has suggested a significant role for other viruses, such as human metapneumovirus,5 rhinovirus,6 and coronaviruses, in bronchiolitis.7 Currently, controversy exists regarding whether the presence of these viruses increases the severity of RSV. Either way, the impact of coinfections, in general, is still unclear.8-10
Recent studies examining the etiology of bronchiolitis in infants have reported high rates of viral detection.10-12 However, these studies have been limited to hospital-admitted patients, which may lead them to over- or underestimate the true significance of the roles that different viral etiologies may play in determining the severity of bronchiolitis.
In this study, we investigated which viruses and risk factors are associated with hospital admission and admission to the intensive care unit (ICU) in infants with acute bronchiolitis.
PATIENTS AND METHODSThis study involved a prospective cohort of 77 infants seeking medical attention on business days between 7:00 AM and 5:00 PM at the Emergency Unit of Hospital Israelita Albert Einstein in São Paulo, Brazil, between March 1, 2006, and July 30, 2007. The Hospital Israelita Albert Einstein is a private institution that mostly treats people of a high socioeconomic status.
To be included in the study, patients had to be under two years of age and have exhibited symptoms of acute bronchiolitis for no longer than five days. Parents' agreement (informed consent) was also required. Bronchiolitis was defined as the first episode of wheezing associated with signs of an upper respiratory tract infection. Patients with previous wheezing episodes or previous use of beta-agonists were excluded. A structured questionnaire was given to parents or caregivers to obtain information regarding atopy in the family (i.e., asthma, allergic rhinitis, or atopic dermatitis), day care attendance, exposure to environmental tobacco smoke or pets, existence of siblings, and pre-existing conditions such as prematurity, cardiopathy, or gastroesophageal reflux.
Vital signs, including pulse oximetry (Nellcor OxiMax N-65, Covidien, Mansfield, MA, USA) and respiratory rate, were obtained from all patients by an attending nurse. A detailed clinical examination was performed by the pediatrician, who then filled out the Respiratory Distress Assessing Instrument (RDAI)13 and made all treatment decisions. The RDAI assesses wheezing and respiratory distress on a scale from 0 to 17, with higher scores indicating more severe illness, and has good interobserver reliability. Nasopharyngeal aspirates were obtained by a respiratory therapist by the instillation of 1 ml of saline in each nostril and gentle suction with a silicone catheter. The sample was immediately put on ice and sent to the laboratory for analysis.
Nasopharyngeal aspirate samples were volume-adjusted with saline to 3 ml, and six different aliquots were stored at -70°C. Nucleic acid extraction was performed with a QIAmp Viral RNA Mini Kit (Qiagen, Hamburg, Germany) according to the manufacturer's instructions. Reverse transcription was performed with a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions using 20 µl of the previously extracted RNA.
Identification of respiratory virusesThe beta-actin gene was amplified from cDNA for all samples14 for quality assurance of the sample collection and RNA extraction/cDNA synthesis. Detection of adenovirus,15 human bocavirus,16 human coronavirus (including HCoV-NL63 and HCoV-HKU1),17 and human metapneumovirus18 was performed by conventional PCR or RT-PCR according to previously published protocols. Identification of RSV,19 influenza A and B,20 parainfluenza 1, 2, and 3,20 rhinovirus, and enterovirus 21 was performed by real-time RT-PCR as previously described.19-21 All assays included positive (either wild-type virus or a plasmid carrying the target sequence) and negative controls (no DNA or cDNA added).
Conventional PCR and RT-PCR were performed in a PTC-200 (MJ Research, Waltham, MA, USA) or Mastercycler Gradient (Eppendorf, Hamburg, Germany) thermocycler; bands were visualized via 1.5% agarose gel electrophoresis and stained with ethidium bromide 0.5 mg/ml. Real-time RT-PCR was performed in an ABI 7300 (Applied Biosystems, Foster City, CA, USA).
Study endpointsA hospital admission was counted when the patient remained in the hospital for longer than 12 hours. Patients were hospitalized if their oxygen saturation on room air remained lower than 93% or if significant respiratory distress persisted after initial treatment in the emergency unit. Admission to the intensive care unit (ICU) was determined by clinical criteria, such as the need for assisted ventilation or close cardiorespiratory monitoring.
StatisticsData were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive analysis calculation of percentages, averages, standard deviations, medians, and ranges for demographic variables and virological results. Univariate and multivariate logistic regression models were used to identify clinical and virological variables associated with the main endpoints (hospital admission and admission to the ICU). Only variables with p<0.25 in the univariate analysis were included in the multivariate model. Results from logistic models were described by odds ratios and 95% confidence intervals.
Differences between means of continuous variables were calculated using ANOVA for normally distributed data and a Kruskal-Wallis test otherwise. A p-value < 0.05 was considered significant.
Approval for the study was obtained from the ethics committee of Hospital Israelita Albert Einstein. Fully informed parental consent was obtained for all subjects.
RESULTSTable 1 shows the patients' characteristics. All patients lived in an urban area, 65% were male, and the median age was 6±4 months; nearly half of the infants were younger than six months. Almost 3/4 of the patients had at least one close relative (mother, father, or brother) with reported atopy. Twelve patients were preterm (less than 37 weeks of gestation), most of whom (8 out of 12) were near-term (35 to 37 weeks) infants.
Demographic data of the 77 infants with bronchiolitis included in the study at the Hospital Israelita Albert Einstein in Sao Paulo, Brazil between March 2006 and July 2007.
| Characteristic | N | % |
|---|---|---|
| Male sex | 50 | 64.9% |
| Atopy in first-degree relative | ||
| none | 20 | 25.97% |
| 1 relative | 36 | 46.75% |
| > 1 relative | 20 | 25.97% |
| No siblings | 24 | 31.16% |
| Prematurity | 12 | 15.50% |
| Presence of cardiopathy | 3 | 3.90% |
| Environmental tobacco exposure | 15 | 19.40% |
| Day care attendance | 13 | 16.88% |
| Atopic dermatitis | 9 | 11.68% |
| Gastroesophageal reflux | 11 | 14.29% |
| Pets (dog or cat) | 14 | 18.18% |
On admission, most patients had bronchiolitis of moderate severity, with a median RDAI score of six, but no significant difference in the severity score was observed between age groups (0-6 months, 6-12 months, and >12 months). An oxygen saturation lower than 95% was observed in 21 patients (28.3%).
Treatment included nebulized beta-agonists in 83% and systemic steroids in 46% of the patients; 32 patents (41.5%) required hospital admission, and 8 were admitted to the ICU. Only two patients required non-invasive ventilation, and there were no deaths. The overall mean length of stay in the hospital was 3.9 days, but a significantly increased length of stay was observed among patients admitted to the ICU (6.4 days, p = 0.012, Kruskal Wallis test).
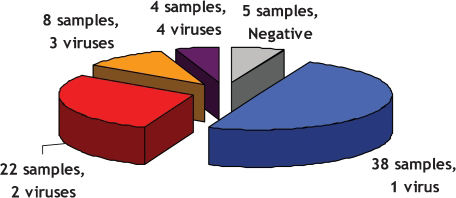
At least one viral agent was identified in 72 samples (93.5%); RSV was the most frequently detected agent, followed by rhinovirus, enterovirus, and human metapneumovirus (Table 2). Single agents (mostly RSV) were identified in 38 samples, whereas coinfections were observed in 34 cases (44%) and primarily involved rhinovirus and enterovirus. Up to four different agents were identified in some samples (Table 2 and Figure 1).
Respiratory viruses identified in nasopharyngeal aspirates of infants with bronchiolitis (n = 77).
| Virology result | Single infection | Coinfection | Total | % |
|---|---|---|---|---|
| Respiratory syncytial virus | 26 | 23 | 49 | 63.6% |
| Rhinovirus | 5 | 21 | 26 | 33.8% |
| Enterovirus | 1 | 15 | 16 | 20.8% |
| Human Metapneumovirus | 4 | 8 | 12 | 15.6% |
| Human Bocavirus | 1 | 8 | 9 | 11.7% |
| Parainfluenza 3 | 0 | 6 | 6 | 7.8% |
| Influenza A | 1 | 1 | 2 | 2.6% |
| Human Coronavirus | 0 | 2 | 2 | 2.6% |
| Parainfluenza 1 | 0 | 1 | 1 | 1.3% |
| Adenovirus | 0 | 0 | 0 | 0.0% |
| Negative samples | - | - | 5 | 6.5% |
After adjusting the multivariate model, the main variables associated with hospital admission were age, atopy in a first-degree relative, and the identification of enterovirus (Table 3). Identification of RSV showed a significant association in the univariate analysis, but this effect was not detected after including other variables in the model. Age had an odds ratio of 0.838, meaning that an older age led to a decrease in hospital admissions; atopy in the family showed a similar protective effect. Identification of enterovirus in the samples was associated with an increased hospital admission rate (Table 3). Gender, lack of siblings, prematurity, day care attendance, having relatives with asthma, tobacco exposure, and the identification of any other viruses were also investigated for possible associations with hospital admission rates. With regard to admission to the ICU, we investigated the same variables described above and found prematurity to be a significant risk factor, with an odds ratio of 24.512 (95% CI: [3.214 – 186.925], p = 0.002).
Logistic regression model results for hospital admission of infants with bronchiolitis (n = 77).
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | OR | 95% CI | p |
| Age | 0.935 | [0.840 - 1.039] | 0.212 | 0.838 | [0.718 - 0.979] | 0.026 |
| Male gender | 0.522 | [0.202 - 1.351] | 0.180 | 0.493 | [0.149 - 1.624] | 0.245 |
| Prematurity | 2.240 | [0.641 - 7.832] | 0.207 | 5.507 | [0.896 - 33.853] | 0.066 |
| No siblings | 1.249 | [0.471 - 3.313] | 0.655 | |||
| Relative w/ asthma | 1.453 | [0.580 - 3.644] | 0.425 | |||
| Relative w/ atopy | 0.436 | [0.151 - 1.259] | 0.125 | 0.202 | [0.052 - 0.785] | 0.021 |
| Smoker at home | 1.762 | [0.565 - 5.493] | 0.329 | |||
| Dog at home | 0.615 | [0.203 - 1.864] | 0.391 | |||
| Cat at home | 1.367 | [0.182 - 10.259] | 0.761 | |||
| Day care attendance | 1.220 | [0.367 - 4.051] | 0.746 | |||
| RSV | 4.145 | [1.432 - 11.998] | 0.009 | 2.116 | [0.614 - 7.288] | 0.235 |
| Rhinovirus | 0.645 | [0.242 - 1.714] | 0.379 | |||
| hMPV | 1.005 | [0.288 - 3.506] | 0.993 | |||
| Enterovirus | 2.124 | [0.696 – 6.479] | 0.185 | 6.033 | [1.224 - 29.726] | 0.027 |
| hBoV | 0.149 | [0.018 - 1.259] | 0.080 | 0.080 | [0.006 - 1.087] | 0.058 |
| Parainfluenza 3 | 0.683 | [0.117 - 3.978] | 0.672 | |||
An assessment of the impact of coinfections on hospital admissions for each viral agent was feasible only for RSV due to the high number of single infections; we did not find an association between coinfections and hospital or ICU admissions. When considering all viral infections, we found that coinfections did not result in an increase in hospital admissions.
DISCUSSIONWe identified a high rate of viruses and coinfections among patients presenting to our emergency department with bronchiolitis. RSV was the most significant single agent involved. We identified at least one virus in 93.5% of patients, and coinfections were found in nearly 40% of patients. These findings indicate that molecular-based approaches result in high rates of viral identification but do not change the significant role of RSV in this condition.
A high rate of hospital admission was found (40%), and younger age and the presence of enterovirus were associated with an increased risk of admission. Conversely, reported atopy in a close relative showed a protective effect for this same endpoint. Prematurity was also significantly associated with an increased risk of admission to the ICU. This high rate of hospital admissions may be related to the study design because all patients were seen in the emergency department and not as outpatients.
Recently published studies regarding the etiology of bronchiolitis have reported high detection rates of respiratory viruses ranging from 90% to 96%,9,10,12 but these studies have included only hospitalized patients.
Previous studies performed using different combinations of techniques have reported lower frequencies of coinfection.8 The high frequency of coinfections found in our study is probably related to the higher sensitivity of the molecular approach adopted in this study. This hypothesis is supported by Miron et al.,10 who used the same method for rhinovirus detection21 and reported a coinfection rate of nearly 30%.10 Another advantage of the RT-PCR approach employed in this study is its ability to identify the recently described genotype of human rhinovirus, namely rhinovirus C, which has been associated with a greater clinical impact.22,23
RSV was the most common virus found in single-virus infections, whereas rhinovirus, the second most frequently identified virus, was mainly found in coinfections; these results are in agreement with previous findings.10,12 Due to the large number of coinfections and the small number of children in each viral category of our study, a comprehensive analysis of the impact of each virus identified in coinfections was not feasible. However, we observed that the detection of RSV in either a single-virus infection or a coinfection did not differ between hospital and ICU admissions. Richard et al. 9 reported an increased risk of admission to the ICU when a dual viral infection was found, but the population in their study included many premature infants and children with underlying chronic illnesses. Marguet et al.12 did not find a difference in bronchiolitis severity in patients infected with single-virus RSV infections compared to those with RSV-rhinovirus coinfections. The only differences identified were a reduction in the length of stay when an associated rhinovirus infection was present and that patients with a single-virus rhinovirus infection had milder presentations.12
Interestingly, the identification of enterovirus, but not RSV, showed a significant association with hospital admissions in the multivariate model even though an enterovirus single-virus infection was found in only one patient. Jacques et al.24 reported a high rate of hospitalization among infants infected with enterovirus with a clinical diagnosis of bronchiolitis, but this finding may be biased by an etiological criterion in the inclusion criteria for the study.
The associations of younger age with an increased risk of hospital admissions and of prematurity with admission to the ICU were expected. The former partially justifies our high rate of hospital admissions because nearly half of our patients were younger than six months of age. Airflow resistance critically depends on airway caliber, so very young children are more prone to respiratory failure. This finding, also described by others,2 was not surprising.
Prematurity is a well-known risk factor for severe bronchiolitis in this population and is the main reason for administering prophylaxis with monoclonal anti-RSV antibodies.25 Besides the risk of apnea and the need for cardiorespiratory monitoring of these children, it has been shown that a significant reduction in expiratory flow is present in prematurely born children, and this flow limitation remains for at least the first two years of life.26 However, the prematurity risk we found (OR = 24) for ICU admissions is most likely overestimated due to the small number of infants admitted to the ICU during the course of our study.
The protective risk of familial atopy for hospital admissions, although also reported by others,27 may be related to the high socioeconomic level of the population studied.
In conclusion, we identified at least one virus in 93.5% of infants with bronchiolitis as well as a high rate of coinfections, mainly due to rhinovirus and enterovirus. Major risk factors for hospital admission included younger age and enterovirus infection, whereas familial atopy had a protective effect, and prematurity was a significant risk factor for ICU admission. The increasing recognition of coinfections in acute bronchiolitis may impact the design and sample size of future studies in this field.
We thank all of the pediatricians, nurses, physiotherapists, and technicians of the Emergency Unit of Hospital Israelita Albert Einstein for their kind assistance during data collection. We also thank Angela Tavares Paes for assistance with statistics and Marcia Triunfol of Publicase for manuscript suggestions and review.
FUNDING: This work was fully supported by the Sociedade Beneficiente Israelita Brasileira Hospital Israelita Albert Einstein.