There is still no consensus among different specialists on the subject of kinematic variation during the hemiparetic gait, including the main changes that take place during the gait cycle and whether the gait velocity changes the patterns of joint mobility. One of the most frequently discussed joints is the knee.
OBJECTIVESThis study aims to evaluate the variables found in the angular kinematics of knee joint, and to describe the alterations found in the hemiparetic gait resulting from cerebrovascular injury.
METHODSThis study included 66 adult patients of both genders with a diagnosis of either right or left hemiparesis resulting from ischemic cerebrovascular injury. All the participants underwent three-dimensional gait evaluation, an the angular kinematics of the joint knee were selected for analysis.
RESULTSThe results were distributed into four groups formed based on the median of the gait speed and the side of hemiparesis.
CONCLUSIONSThe relevant clinical characteristics included the important mechanisms of loading response in the stance, knee hyperextension in single stance, and reduction of the peak flexion and movement amplitude of the knee in the swing phase. These mechanisms should be taken into account when choosing the best treatment.
We believe that the findings presented here may aid in preventing the occurrence of the problems found, and also in identifying the origin of these problems.
The human gait should be efficient and economical, concurrently requiring a complex integration of both the nervous system and the musculoskeletal system. Clinical analysis of gait involves the measurement of essential biodynamic parameters and the interpretation of this information in conjunction with the therapeutic prescription or indication. This is widely used in the evaluation of pathologic gaits, where movements are frequently complex and difficult to evaluate with the naked eye.1
One of the most important gait alterations occurs as a result of cerebrovascular accidents (CVA). Craik and Oatis2 assert that approximately 70% of patients who survive a CVA recover their ability to deambulate. The hemiparetic gait is described as being slow, laborious and abrupt. This alteration is due to deficiencies in perception-cognition, motor control, joint mobility, strength and muscle tone.3 There is still no consensus among different specialists on the subject of kinematic variation during the hemiparetic gait; one of the most frequently discussed joints is the knee, including the main changes that take place during the gait cycle4–9 and whether the gait velocity changes the patterns of joint mobility.10
This study aims to evaluate the variables found in the angular kinematics of knee joint and to describe the alterations found in the hemiparetic gait resulting from a cerebrovascular accident.
METHODSThis study was comprised of 66 adult patients of both genders. The mean age was 45.4 ± 8.5 years (range 31– 60). Thirty-three patients were female; 33 were male. Mean weight was 67.6 ± 16 kg (range 44–110) and mean height was 161.3 ± 9.7 cm (range 136–189).
The inclusive criteria utilized included: diagnosis of a disability resulting from an ischemic cerebrovascular accident with right or left hemiparesia; having the lesion for not less than 12 months; being a community deambulator; not presenting the need for auxiliary means of accomplishing their gait; being able to walk barefoot; and having not previously been through orthopedic surgical procedures.
All subjects in the study had clinical indications for 3-D evaluation and were sent to the laboratory for medical prescriptions. As soon as the subjects met the inclusion criteria above, their consent to use the final report of their exams in this study was requested, as well as a signature of a signature on their declaration of consent.
The sample was collected at the gait laboratory of the Association for the Assistance of the Disabled Child [AACD] in the city of São Paulo.
After both the anamnesis and evaluation (inclusion criteria), the individuals were submitted to the protocol of anthropometric measurements for the execution of the 3-D gait exam, which was composed of height, weight, distance between both anterior and superior iliac spines, length of the lower limbs, diameter of knees and ankles, and measurement of the tibial torsion. The individuals were acquainted with the equipment and the procedure, and were instructed about the activities to be carried out. They were also trained for deambulating on the experimental track. All participants wore swimsuits, which enabled the placement of motion markers. Fifteen anatomic areas were selected and adopted as a reference for the fixation of the motion markers to the VICON 370 movement analysis system. Helen Hayes motion markers were used to estimate the position of the joint centers11 and to calculate the 3-D kinematics of the pelvis, hip, knee and ankle joints.12
The task was to walk on a track (which was 90 cm by 6 m and marked on the floor) 12 times or to perform 12 complete gait cycles. The individuals were instructed to deambulate at a comfortable velocity similar to their daily gait.
For every circuit made on the experimental track, one single gait cycle was chosen using the mean values. Using the Vicon Clinical Manager program, the 3-D kinematics data of the pelvis, hip, knee and ankle joints were expressed graphically with the angular position of the joint versus time, resulting in twelve graphics per individual. From the total of twelve cycles, only one was selected for analysis. This selection was based on the internal evaluation protocol of the AACD gait laboratory, which has, as a parameter, the average value for the angular kinematics and the average gait velocity. This same cycle was used for data analysis of the knee angular kinematics on the sagittal plane. Based on the graph for the knee joint on the sagittal plane, 11 variables were defined: knee angular position in the initial contact (degrees); knee first flexion peak in the stance phase (degrees); time of knee flexion peak in the stance (% of stance); minimum value of the angular position after the flexion peak in the stance (degrees); time of minimum value in the stance (% of stance); angular position in the terminal stance (degrees); angular velocity in the terminal stance (degrees / % of the cycle); flexion peak in the swing (degrees); angular velocity in the swing (degrees / % of the cycle); time of flexion peak in the swing (% of the swing); and movement amplitude in the swing (degrees).
The comparison groups were formed based on the gait velocity parameter, considering the median (non-parametric distribution) of all velocities gauged. Two large groups were formed: a group with lower or equal velocity in relation to the median, and a group with higher velocity in relation to the median. Each of these groups was divided into two subgroups, according to the side affected by the hemiparesis, which was either right or left.
Four groups were formed: Group 1 - lower velocity and left hemiparesis (LVLH), comprised of 23 individuals; Group 2 - lower velocity and right hemiparesis (LVRH), comprised of 10 individuals; Group 3 - higher velocity and left hemiparesis (LVLH), comprised of 7 individuals; and Group 4 - higher velocity and right hemiparesis (LVRH), comprised of 26 individuals.
Possible changes among the groups, according to previously defined variables, were analyzed by the non-parametric test for Kruskal-Wallis independent samples. This was completed by a multiple comparison test as needed. The means were calculated, but the standard deviation was not calculated because variables found using the non-parametric test do not have a standard distribution. The rejection level for the null hypothesis was fixed to be less than or equal to 0.05 (5%).
This research was approved by the Ethics Committee of the Hospital das Clínicas.
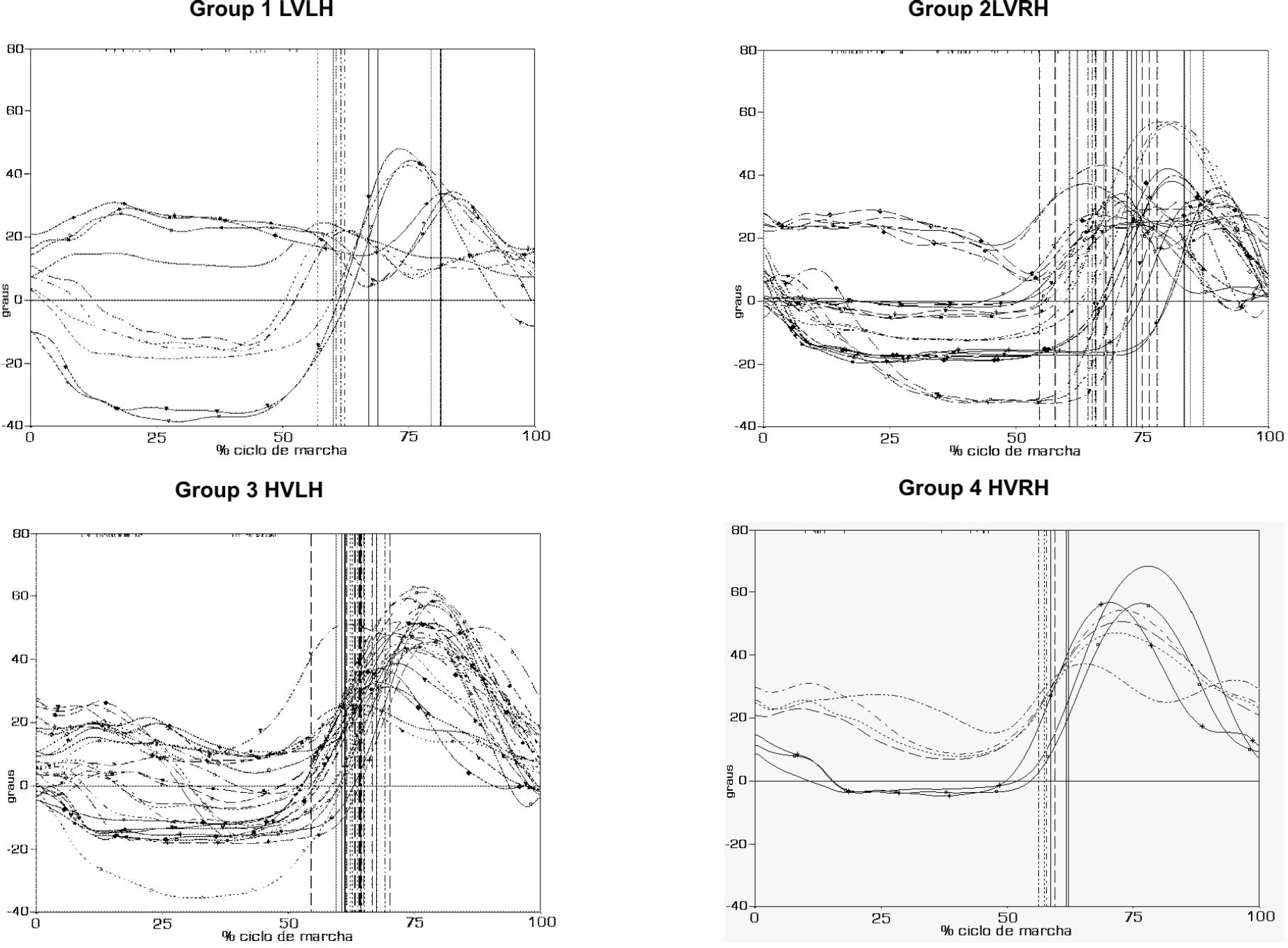
RESULTSThe angular kinematics results for each individual are presented in Figure 1 and are divided into the four groups according to the evaluation parameters, i.e., the relation to gait velocity median and the side of hemiparesis.
Knee angular kinematics of the patients with consequences of ICVA and hemiparesis. Group 1 (HVLH) - lower velocity than the median and left hemiparesis; Group 2 (HVRH) - lower velocity and right hemiparesis; Group 3 (HVLH) - higher velocity than the median and left hemiparesis; and Group 4 (HVRH) - higher velocity and right hemiparesis
Across the groups, no significant statistical differences was detected for several variables: knee angular position in the initial contact, knee flexion peak in the stance phase, minimum value of the angular position after the flexion peak in the stance, time of minimum value in the stance, angular position in the terminal stance, and angular velocity in the terminal stance (Table 1). The other variables did show significant statistical differences: time of knee flexion peak in the stance (p ≤ 0.05), knee flexion peak in the swing (p < 0.001), angular velocity in the swing (p = 0.01), time of flexion peak in the swing (p = 0.01), and the value of the movement amplitude in the swing (p ≤ 0.05) (Table 2).
Median velocity and mean values of the kinematic variables for knee joint of patients with ICVA consequences
| Group 1 (VMEE) | Group 2 (VMED) | Group 3 (VMAE) | Group 4 (VMAD) | |
|---|---|---|---|---|
| Velocity, median (cm/s) | ≤ 64.8 | ≤ 64.8 | > 64.8 | > 64.8 |
| Knee angular position in the initial contact (0) N.S. | 9.5 | 7.0 | 16.6 | 8.7 |
| Knee first flexion peak in the stance phase (0) N.S. | 10.50 | 11.17 | 17.06 | 10.24 |
| Time of knee flexion peak in the stance (% of the stance) * | 4.7 | 11.3 | 9.1 | 10.3 |
| Minimum value of the angular position after the flexion peak in the stance (0) N.S. | −9.1 | −9.8 | 4.0 | −3.8 |
| Time of minimum value in stance (% of the stance). N.S. | 56.0 | 66.6 | 61.7 | 57.4 |
| Angular position in terminal stance (0) N.S. | 17.0 | 20.0 | 19.0 | 17.0 |
| Angular velocity in terminal stance ( 0 / % of the cycle) N.S. | 3.0 | 2.7 | 2.5 | 2.6 |
| Flexion peak in swing (0) *** | 35.7 | 31.8 | 54.3 | 44.9 |
| Angular velocity in the swing ( 0 / % of the cycle) ** | 0.3 | 0.3 | −1.3 | −0.9 |
| Time of flexion peak in swing (% of the swing) ** | 25.5 | 11.8 | 33.7 | 29.0 |
| Movement amplitude in the swing (o) * | 26.3 | 24.8 | 37.7 | 36.5 |
Group 1 (LVLH) - lower velocity than the median and left hemiparesis; Group 2 (LVRH) - lower velocity and right hemiparesis; Group 3 (HVLH) - higher velocity than the median and left hemiparesis; Group 4 (HVRH) - higher velocity and right hemiparesis; N.S. – Non-significant;
Absolute values of the difference between mean values of patients in the four groups and the minimum significant differences
| Group 1, Group 2 | Group 1, Group 3 | Group 1, Group 4 | Group 2, Group 3 | Group 2, Group 4 | Group 3, Group 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VADMP | DMS | VADMP | DMS | VADMP | DMS | VADMP | DMS | VADMP | DMS | VADMP | DMS | |
| Time of knee flexion peak in stance (% do stance) | 14.52 | 19.18 | 12.14 | 21.86 | 12.90 § | 14.50 | 2,38 | 24.96 | 1,62 | 18.85 | 0,76 | 21.57 |
| Flexion peak in swing (o) | 5,46 | 19.18 | 28,40 § | 21.86 | 14.87 § | 14.50 | 33.86 § | 24.96 | 20.33 § | 18.85 | 13,53 | 21.57 |
| Angular velocity in the swing phase (o / % of the cycle) | 2,74 | 19.18 | 25,19 § | 21.86 | 15.24 § | 14.50 | 27.93 § | 24.96 | 17.98 § | 18.85 | 9.95 | 21.57 |
| Time of flexion peak in the swing (% of the swing). | 17.15 § | 19.18 | 17.72 | 21.86 | 7.28 | 14.50 | 34.87 § | 24.96 | 24.43 § | 18.85 | 10.44 | 21.57 |
| Movement amplitude in the swing (o) | 4,15 | 19.18 | 13,86 | 21.86 | 11,83 § | 14.50 | 18.01 § | 24.96 | 15.98§ | 18.85 | 2,03 | 21.57 |
VADMP - Absolute value of the difference between the means; DMS - Minimum significant difference;
The first characteristic found in the assessed groups shows that all groups place the knee in the initial contact in accordance with the literary description.13,14 Burdett et al.4 have described an increase in knee flexion during initial contact at 'normal’ velocity. Olney,15 however, asserts that the velocity variation interferes with knee flexion, leading to an increase in flexion when the velocity is higher. In papers by and Richards,5 Knutsson, 6 Lehmann et al.,7 Cozean et al.,8 and Intiso et al,9 the values found in this study for knee position in the initial contact are different from the previous studies, occurring from a decrease in knee flexion in the initial contact. The literature shows discrepancies.
In this study, the values presented for the first knee flexion peak in the stance phase, which relates to the “loading response” event, are in agreement with the findings of Burdett et al.,4 Olney,15 and Kerrigan.16 These authors also demonstrated smaller values for the knee position at this phase of the gait cycle in patients who had hemiplegia, as compared to those individuals with no neurological lesion, which is in agreement with our findings. Nevertheless, Perry13 disagrees with this because he asserts that there is a difference in the knee angular position in the loading response when the gait velocity is increased or decreased.
The loading response contributes to smoothing rough changes in the trajectory arcs of the center of gravity; in other words, this smoothes the arc through which the body’s center of gravity moves. The change in this mechanism reduces both the quality and the quantity of shock absorption against the floor.17
The changes of this gait phase may have several causes: quadriceps muscle weakness with increasing knee flexion; spasticity of the quadriceps muscle, which leads to a torn quadriceps at the initial moment of flexion; hypertonia, which leads to premature knee extension; and excess plantar flexion (plantar flexion contracture and soleus muscle spasticity), which prevents the initial contact with the heel and causes the tibial bearing over the foot to be inhibited or blocked.4,17–19
The time of the knee flexion peak in the stance was assessed; it presented a statistically significant difference across the four groups studied. Although the groups presented statistical differences in flexion time, their mean values varied from only 4.7% to 10.3% in the gait cycle. Such values are inferior to the standard average gait values found in the literature, where the gait flexion peak takes place more than 15% of the way through the gait cycle.13,20
The minimum value for the angular position after the flexion peak in the stance, and the time of minimum value in the stance, were not statistically different across the groups. These variables show a trend that an increasing knee extension occurs, lasting longer during the stance in every group.
The values found by Kerrigan16 demonstrate results that vary from exceeding extension in stance to exceeding flexion at the same moment. Mulroy18 asserts that in the first six months after a lesion, in patients who deambulate at a very slow velocity and in those who deambulate at a higher velocity, there is generally excess extension in the stance. However, beyond six months after a lesion, the problems found at this cycle moment were no longer relevant within this population.
The literature presents findings similar to those of this study, along with possible reasons and counterbalances. Kerringan21 believes that the excess extension may be related to weakness of the quadriceps, spasticity of the ankle plantar flexor, or contracture of the plantar flexor. As a result, the primary problems for these patients are the potential risks for articular capsule and ligament structure lesions in the posterior knee area, which may lead to pain, feeble ligaments and osseous deformity.
Dietz22 believes the cause of hyperextension may be spasticity of the plantar flexor, and this may alter the mechanical properties of the muscle and increase its resistance. An increase in ankle plantar flexion at the initial contact, without concomitant changes in muscle forces, causes the knee to hyperextend. Intrinsic force-length-velocity properties of the muscle (particularly the gastrocnemius and vastus) diminished the effect of equinus posture alone, causing the abnormal knee extension to be less pronounced.23
For Perry,24 the assertion is that part of the desired hip flexion is injured because of the increase in knee extension. Furthermore, the stride length is decreased by the quantity of hip flexion that was lost.
Malezic25 and Morris26 assert that the knee hyperextension pattern in the terminal stance hinders the average standard and knee extension during the initial stance, although subjects frequently perform the initial contact with the knee flexed and quickly evolve to complete extension or hyperextension. This also corroborates our findings about the knee angular position in the initial contact, the knee first flexion peak in the stance phase, and the time of knee flexion peak in the stance.
Although the knee hyperextension is a counterbalance, it presents several undesirable biomechanical effects, especially when it continues up to the terminal stance. Knee hyperextension usually obstructs an effective impulse.27 In this case, the difficulty in flexing the knee causes the subject to perform the swing phase with the member extended, performing a circumduction7 or an ipsilateral hip elevation.28 None of these maneuvers provide suitable energy generation for the plantar flexor or hip flexor. This mechanism leads to a large loss, as this generation produces around 40% of the total gait consumption.15
The variable angular position in the terminal stance showed no statistically significant differences across the groups LVLH, LVRH, HVLH and HVRH. These values were higher than the standard values for normal gait29 and were not the same as those found by Burdett et al.4 and Olney,15 who described a reduction of the knee flexion in the terminal stance.
Our findings were similar that described by Mulroy18 as one of the items that characterize the pattern of the hemiplegic gait. Those values varied according to the gait velocity. The group that had a very low-velocity deambulation presented hyperextension in the terminal stance, and in the group with middle velocity, hyperextension was also noted. In the group with near-normal velocity, the values were also close to the normal pattern, while the groups with limited velocity deambulation presented excess flexion. The findings of that study corroborate the values presented here.
Even when compared to control subjects walking at slow speeds, ankle plantar flexor work during pre-swing was greatly reduced in the hemiparetic subjects. Differences in hip and knee moment work partially offset the reduction in ankle work, but net joint moment work was still significantly reduced.30
The angular velocity in the terminal stance had no statistically significant difference across the four groups selected for relation to the median velocity and the side of hemiparesis. Notably, the angular velocity in the terminal velocity proved to be within the parameters of normal gait.13,29
The knee flexion peak in the swing revealed a statistically significant difference across the four groups. All groups presented mean values that were inferior to what was expected; groups 1 and 2, with left and right hemiparesis and lower velocity than the median, presented significantly lower values than what was described for the normal gait. Groups 3 and 4 also show inferior values, although they are closer to what was expected for normal gait.
For reduced flexion peaks, where the fastest group obtained higher values than the ones obtained by the group with the lower velocity, Olney15 described results that were very close to the ones described above. Inferior values to the ones described by the normality pattern were also published by Knutsson and Richards,5 Knutsson,6 Lehmann et al.,7 Cozean et al,8 Intiso et al,9 Kerrigan et al,28 and Mulroy et al.18
This gait pattern has historically been known as a stiff-legged gait and has been attributed only to quadriceps spasticity.31–33 Other causes, apart from abnormal quadriceps function, may include a dynamic weakness of the hip flexor33–35 and a lack of control of the ankle during gait.33,36 Kerrigan33 established relationships between the knee flexion reduction and the inappropriate activity of the hamstrings, and also between the knee flexion reduction and the lateness in removing the foot from the stance. Kerrigan34 also showed that by simulating an increase in the hip flexion in a substantial model, there is an increase in knee flexion in some cases. Riley and Kerringan35 have confirmed the importance of the hip flexion using a more sophisticated approximation model. They have also described a complex contribution of the rectus femuris and hamstrings to a model that increases knee flexion and limits hip flexion. Riley and Kerrigan36 have demonstrated that torque over the hip, ankle and knee joints affects the knee in this event, using a somewhat new analysis technique to produce the acceleration described by Kepple.37
In addition, Kerrigan38 noticed a mean of 17o (seventeen degrees) of knee flexion peak reduction when healthy individuals walked on their toes compared to when they walked normally. Such observation suggests that ankle function is sometimes related to knee flexion in the swing.
The authors Knutsson4 and Olney15 revealed the presence of several gait patterns for people with hemiparesis. Within this large number of patterns, there is a uniformity of some patterns, such as for knee flexion reduction in the swing.39
Gait analysis was also performed in subjects who had a stroke; imposed hip extension evoked a brief reflexive response in the quadriceps, followed by a heightened level of sustained activity. The initial response was velocity-dependent and was larger in the stroke group than in the control group. In contrast, the prolonged response was not velocity-dependent, was significantly greater in the rectus femuris and vastus lateralis in subjects with stroke, and, importantly, was correlated to decreased swing-phase knee flexion. Hyperexcitable heteronymous connections from hip flexors to knee extensors appear to elicit prolonged quadriceps activity and may contribute to altered swing-phase knee kinematics following stroke.40
The knee flexion peak in the swing, the angular velocity in the swing, the time of knee flexion peak in the stance and the movement amplitude in the swing have presented statistically significant differences across all of the assessed groups. By observing the mean values demonstrated in Table 1, it is observed that groups 1 and 2 presented lower values for the flexion peak, higher angular velocity, shorter time to the flexion peak and inferior movement amplitude when compared to groups 3 and 4.
This relationship between the mean values can be understood by considering that the assessed subjects presented lesion-superior motoneuron and consequent elastic hypertonia, resulting in a velocity-dependent tone. The faster the muscle is torn, the higher the possibility of the spindle being torn and enabling a blocking or limitation of the movement. As a result, the knee tends to stop flexing and begin extending, making the time of the flexion peak occur earlier, limiting the flexion peak, and reducing the movement amplitude.
The limitations found in this study and in other studies carried out with neurological patients involve the sample uniformity, despite our attempt to select patients who had both the same disease and the same topographic consequence. We cannot be certain the neurological disease is the same. Even with a more accurate diagnosis through the aid of imaging, we could not have better defined our population since we know that neural plasticity has several variables and influences. Therefore, we have attempted to select patients with a diagnosis of advanced lesion and with similar consequences, i.e., hemiparesis with upper limb prevailing, and to assess the biomechanical characteristics of these patients.
Dividing assessed groups according to velocity has been discussed by other authors.27,41 The division in relation to the hemicorpus taken was not aimed at assessing hemispherical lesion influences, as we did not consider the dominance and extension of the brain lesion when including the subjects.
The knee joint was chosen because of its important biomechanical function during gait. In the swing phase, it is fixed and functionally reduces the lower limb, enabling the lower limbs to advance freely without touching the ground. In the swing, it flexes smoothly, enabling impact absorption, saving energy and transmitting strength to the lower limbs. Undoubtedly, the changes found here may illustrate secondary, not primary, changes; therefore, further assessment of this joint associated with the ankle, hip, pelvis and spine joints, in addition to the kinetics and electromyography evaluation, must be carried out so we can detect whether our findings are the cause or the consequence of the problem.
CONCLUSIONTo sum up, there were several relevant clinical characteristics found here that should be taken into account when choosing the best treatment. The important mechanisms include a loading response in the stance, knee hyperextension in the single stance, reduction of the flexion peak and movement amplitude of the knee in the swing phase, as previously discussed.
We believe that our findings may aid in preventing the occurrence of the problems found, and also in finding the origin of these problems.