Suppressor of cytokine signaling 3, myxovirus resistance protein and osteopontin gene polymorphisms may influence the therapeutic response in patients with chronic hepatitis C, and an association with IL28 might increase the power to predict sustained virologic response. Our aims were to evaluate the association between myxovirus resistance protein, osteopontin and suppressor of cytokine signaling 3 gene polymorphisms in combination with IL28B and to assess the therapy response in hepatitis C patients treated with pegylated-interferon plus ribavirin.
METHOD:Myxovirus resistance protein, osteopontin, suppressor of cytokine signaling 3 and IL28B polymorphisms were analyzed by PCR-restriction fragment length polymorphism, direct sequencing and real-time PCR. Ancestry was determined using genetic markers.
RESULTS:We analyzed 181 individuals, including 52 who were sustained virologic responders. The protective genotype frequencies among the sustained virologic response group were as follows: the G/G suppressor of cytokine signaling 3 (rs4969170) (62.2%); T/T osteopontin (rs2853744) (60%); T/T osteopontin (rs11730582) (64.3%); and the G/T myxovirus resistance protein (rs2071430) genotype (54%). The patients who had ≥3 of the protective genotypes from the myxovirus resistance protein, the suppressor of cytokine signaling 3 and osteopontin had a greater than 90% probability of achieving a sustained response (p<0.0001). The C/C IL28B genotype was present in 58.8% of the subjects in this group. The sustained virological response rates increased to 85.7% and 91.7% by analyzing C/C IL28B with the T/T osteopontin genotype at rs11730582 and the G/G suppressor of cytokine signaling 3 genotype, respectively. Genetic ancestry analysis revealed an admixed population.
CONCLUSION:Hepatitis C genotype 1 patients who were responders to interferon-based therapy had a high frequency of multiple protective polymorphisms in the myxovirus resistance protein, osteopontin and suppressor of cytokine signaling 3 genes. The combined analysis of the suppressor of cytokine signaling 3 and IL28B genotypes more effectively predicted sustained virologic response than IL28B analysis alone.
Genetic and biological host factors such as the human leukocyte antigen, ancestry, cytokine polymorphisms, gender, liver fibrosis and insulin resistance are implicated in the effectiveness of interferon (IFN)-α therapy in chronic hepatitis C (1,2).
The interferon system is a crucial component of the natural immune response to infectious agents. Type I interferon induces numerous antiviral proteins, including the myxovirus resistance (MxA) protein, 2-5 oligoadenylate synthetase 1 and double-stranded RNA-dependent protein kinase. The MxA protein has selective activity against a number of viruses, and its levels are higher in chronic hepatitis C virus (HCV) patients during interferon treatment; the single nucleotide polymorphisms (SNPs) in the promoter region of MxA appear to be associated with the response of HCV-infected patients to interferon (3,4).
Osteopontin (OPN) is an extracellular matrix protein expressed in activated Kupffer and stellate cells that contributes to the migration of macrophages into the necrotic areas of the injured liver. OPN is an essential cytokine in initiating the Th1 immune response; recent research has shown that genetic polymorphisms in the OPN gene determine the magnitude of the immune reaction, and SNPs in the promoter region of human OPN may affect the necroinflammatory activity in chronic hepatitis C patients (CHC) (5).
The mechanisms by which HCV interferes with IFN signaling and attenuates antiviral efficacy are not fully elucidated. HCV infection leads to endogenous IFN production and increased expression of the suppressor of cytokine signaling 3 (SOCS3) via viral core proteins or IFN inhibitory factors (6). SOCS3 can suppress JAK-STAT signaling at the level of STAT1 (Signal Transducer and Activator of Transcription 1) phosphorylation by blocking the IFN-induced formation of interferon-stimulated gene factor 3 (ISGF3) (7). Previous studies have reported that an SNP (-4874nt A>G) in the promoter region of the SOCS3 gene is associated with a poorer treatment outcome (8).
IL28A, IL28B and IL29 are cytokines related to the lambda interferon family and have been structurally associated with the IL-10 family (9). SNPs located upstream of the IL28B gene are the most important genetic markers for predicting the response to combination therapy with pegylated IFN-α and ribavirin in HCV genotype 1 patients (10–12).
IFN-α acts through the well-characterized JAK-STAT pathway to up- or downregulate hundreds of genes that function in the immune response pathways (13). SNPs in the interferon-α pathway genes and interferon-induced genes within the MxA and OPN (4,14-16) promoter regions are associated with therapeutic outcome. High intrahepatic expression levels of interferon-stimulated genes are associated with lower antiviral response rates to interferon plus ribavirin in chronic hepatitis C patients (17).
The aim of this study was to evaluate the influence of host genetic heterogeneity mediated by SNPs in the promoter regions of MxA, OPN and SOCS3 in chronic hepatitis C patients treated with pegylated interferon (Peg-IFN) plus ribavirin combination therapy. The capacity of these protective genotypes to predict therapeutic response in HCV patients was compared with therapeutic response prediction using IL28B genotypes.
MATERIALS AND METHODSStudy design and patientsBetween January 2010 and March 2011, 181 adult outpatient clinic patients chronically infected with HCV genotype 1 who were treated with a combination of Peg-IFN alpha-2a or alpha-2b and ribavirin were consecutively screened. Of these patients, 143 were treated for the first time, whereas 38 had been previously treated with a combination of standard alpha-2a or alpha-2b interferon and ribavirin.
Inclusion criteriaWe included viral genotype 1 CHC patients who were older than 18 years old and were treated with Peg-IFN alpha-2a or alpha-2b plus ribavirin.
Exclusion criteriaThe exclusion criteria were hepatitis B or HIV co-infection and alcohol intake ≥40 g of ethanol/day or other concomitant chronic liver disease.
The patients were divided into the following 2 groups: the SVR (sustained virologic response) group (individuals who had achieved SVR), and the non-response (NR) group (individuals who were non-responders or relapsers to therapy). Other data were obtained from patient interviews and chart reviews. SVR was defined as undetectable HCV RNA in serum 24 weeks after the end of therapy. Patients received 48 weeks of treatment. Non-response to therapy was defined as an HCV viral load decline of less than 2 logs at week 12 during therapy or detectable serum HCV RNA at any time during therapy up to 48 weeks. Relapse was defined as undetectable HCV RNA in serum at the end of therapy followed by detectable HCV RNA after discontinuation of therapy.
Genotyping of allelic variantsGenomic DNA from the peripheral blood mononuclear cells was isolated using the DNA Blood MiniKit (Invitrogen, USA). We defined protective genotypes as those genotypes associated with a sustained virological response.
MxAThe biallelic polymorphism in the promoter region of MxA rs2071430 at position -88nt (G>T) and rs17000900 at position -123nt (C>A) from the transcription start site was determined by PCR-restriction fragment length polymorphism (RFLP), as described by Hijikata et al. (4). Amplification was conducted using Taq DNA Polymerase High Fidelity (Invitrogen, USA).
OPNThe SNPs in the OPN promoter region at position -616nt (rs2853744; G>T) and position -449nt (rs11730582; T>C) were analyzed by the direct sequencing of DNA fragments (5). The extracted genomic DNA was amplified using Taq DNA Polymerase High Fidelity (Invitrogen, USA). The direct sequencing was performed using BigDye Terminator v3.1 Ready Reaction Cycle Sequencing (Applied BioSystems, USA).
SOCS3 and IL28BThe SOCS3 and IL28B SNPs were genotyped by real-time PCR. Thermal cycling was performed on a Step One Real-Time PCR System (Applied BioSystems, USA). The fluorescence data were collected, and the genotypes were determined using Sequence Detection Systems software version 1.3.1 (Applied BioSystems). The genotyping of the SOCS3 promoter region at -4874nt (of rs4969170 A>G) was performed according to Persico et al. (8). The SNP for IL28B was rs12979860 (T>C).
Ancestry informative markers (AIMs)Genetic ancestries were determined by analyzing the ancestry informative markers (AIMs) for African, Amerindian and European populations. The allele*1 was defined as the presence of insertion or the lack of restriction enzyme site (18). The AIMs were selected based on previous studies that analyzed a panel of 48 ancestry markers in different populations (18–20). Seven AIMs were selected that had higher than 48% ethno-geographical differential frequencies for Amerindian/European, African/European and Amerindian/African ancestries.
The evaluated AIMs were as follows: African ancestry, AT3-I/D (rs3138521) and LPL (rs285); European ancestry, Sb19.3 (rs3138524), APO (rs3138522) and FY-Null (rs2814778); and Amerindian ancestry, PV92 (rs3138523) and CKMM (rs4884). Three SNPs (FY-null, LPL and CKMM) were determined using the Taqman assay (Applied BioSystems, USA); 3 Alu insertion polymorphisms (APO, Sb19.3 and PV92) and a polymorphic insertion of a 68-bp fragment at locus AT3-I/D were analyzed for specific alleles using PCR (19–21).
Statistical analysisThe statistical analysis was performed using the R projects software version 2.11.1 for Windows (http://www.r-project). The categorical data were analyzed using Fisher's exact tests or the χ2 test; the continuous data were analyzed using the non-parametric Mann-Whitney U test. The univariate factors with p-values less than 0.05 and confidence intervals of 95% were considered to be statistically significant.
The allele frequencies were estimated by the gene counting method using the GENEPOP online version 4.0.10 (http://genepop.curtin.edu.au) (22). The admixture proportions of each population sample were estimated by the ADMIX software version 2.0 software [www.genetica.fmed.edu.uy] (23,24). The parental population allele frequencies used were described by Shriver et al. (18).
EthicsThis study was approved by the ethics committee of the institution, and all of the patients provided written informed consent. All of the procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975 as revised in 1983.
RESULTSOf the 181 HCV individuals in the study, 52 achieved SVR, and 129 were non-responders/relapsers to the combined Peg-INF alpha-2a or alpha-2b and ribavirin therapy. The patient characteristics are described in Table 1. Severe fibrosis (Metavir F3 and F4) was observed more frequently in those patients who did not respond to the combination treatment (OR = 2.27; 95% CI: 1.11–4.65; p = 0.018) when compared to the sustained responders.
Comparison of the baseline characteristics of individuals infected with HCV by response to Peg-IFN-α/ RBV therapy.
| N | SVR N = 52 (%) | NR N = 129 (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 62 | 13 (21) | 49 (79) | 1.83 (0.89-3.78) | 0.066 |
| Male | 119 | 39 (32.8) | 80 (67.2) | ||
| Age (years) ± SD | 181 | 50±9.6 | 53±10.1 | 0.149 | |
| Liver fibrosis stage | |||||
| ≤F2 | 78 | 28 (63.6) | 50 (43.5) | 2.27 (1.11–4.65) | 0.018 |
| F3/F4 | 81 | 16 (36.4) | 65 (56.5) | ||
| ALT (U/L) | 166 | 117.42 | 113.08 | 0.733 | |
| Viral Load (IU/ml) | 73 | 5.6 | 5.9 | 0.270 |
SD = standard deviation; OR = odds ratio; 95% CI = 95% confidence interval.
Our studied population was admixed, and the genetic ancestry analysis revealed a tri-hybrid population with the following genetic ancestry contribution: African, 43.6%; European, 31.9% and Amerindian, 24.6%. We found that patients in the NR/R group had a higher contribution of African ancestry (46.6%) compared to European (27.2%) and Amerindian (26.1%) ancestries, whereas the patients in the SVR group had a greater contribution of European ancestry (40.4%) compared to African (35.6%) and Amerindian (24%) ancestries (p = 0.048).
Polymorphisms in the MxA promoterLinkage disequilibrium was observed between the MxA SNPs rs2071430 and rs17000900 (p = 0.001).
The polymorphisms in MxA at position -88 (rs2071430) were associated with patient response to Peg-INF and ribavirin therapy (Table 2). The G/G was significantly less frequent in the SVR group than in the NR/R group (11.2 vs. 88.8%, p<0.0001). G/T heterozygosis plus T/T homozygosis was present in 54% of the patients who achieved SVR and in 46% of the subjects from the NR/R group. The T allele had a significantly higher frequency in the patients with SVR (OR = 0.39; 95% CI: 0.21–0.71; p = 0.003).
MxA, OPN, SOCS3 and IL28B polymorphism genotype and allele frequencies by therapeutic outcome in patients with chronic hepatitis C.
| SVR N = 52 (%) | NR N = 129 (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| MxA (rs2071430) | ||||
| Genotypes | ||||
| G/G | 12 (11.2) | 95 (88.8) | 0.10 (0.05-0.22) | <0.0001 |
| G/T + T/T | 40 (54) | 34 (46) | ||
| Allele frequency | ||||
| T | 0.471 | 0.258 | 0.39 (0.21–0.71) | 0.003 |
| G | 0.529 | 0.742 | ||
| MxA (rs17000900) | ||||
| Genotypes | ||||
| C/C | 20 (17.4) | 95 (82.6) | 0.22 (0.11-0.44) | <0.0001 |
| C/A + A/A | 32 (48.5) | 34 (51.5) | ||
| Allele frequency | ||||
| A | 0.346 | 0.140 | 0.20 (0.09–0.41) | 0.001 |
| C | 0.423 | 0.860 | ||
| OPN (rs2853744) | ||||
| Genotypes | ||||
| T/T | 9 (60) | 6 (40) | 0.23 (0.07-0.69) | 0.013 |
| G/T + G/G | 43 (25.9) | 123 (74.1) | ||
| Allele frequency | ||||
| T | 0.221 | 0.087 | 0.30 (0.12-0.73) | 0.009 |
| G | 0.779 | 0.913 | ||
| OPN (rs11730582) | ||||
| Genotypes | ||||
| T/T | 36 (64.3) | 20 (35.7) | 0.08 (0.03-0.17) | <0.0001 |
| T/C + C/C | 16 (12.8) | 109 (87.2) | ||
| Allele frequency | ||||
| T | 0.750 | 0.417 | 0.23 (0.12-0.43) | <0.0001 |
| C | 0.250 | 0.583 | ||
| SOCS3 (rs4969170) | ||||
| Genotypes | ||||
| G/G | 23 (62.2) | 14 (37.8) | 0.15 (0.07-0.33) | <0.0001 |
| A/G + A/A | 29 (20.1) | 115 (79.9) | ||
| Allele frequency | ||||
| G | 0.577 | 0.443 | 0.56 (0.32-0.99) | 0.065 |
| A | 0.423 | 0.557 | ||
| IL28B (rs12979860) | ||||
| Genotype | ||||
| C/C | 20 (58.8) | 14 (41.2) | 0.20 (0.09–0.47) | 0.0008 |
| C/T + T/T | 24 (22.9) | 81 (77.1) | ||
| Allele frequency | ||||
| C | 0.648 | 0.458 | 0.45 (0.25–0.80) | 0.009 |
| T | 0.352 | 0.542 | ||
OR = odds ratio; 95% CI = 95% confidence interval.
The SNP at position -123 (rs17000900) was associated with a therapeutic response. As shown in Table 2, non-responders/relapsers had a higher frequency of C/C homozygotes than did the sustained virological responders (82.6% vs. 17.4%, p<0.0001), which is similar to the frequency of G/G homozygotes for rs2071430.
Polymorphisms in the OPN promoterThe direct sequencing of the DNA fragments between nt -733 and -236 in 181 patients revealed 2 SNPs in the promoter region of OPN, located at nt -616 (rs2853744) and -443 (rs11730582). The prevalence of these 2 SNPs is shown in Table 2. The T/T genotype for the SNP at rs2853744 was associated with a sustained virological response to antiviral Peg-IFN-α therapy (60% vs. 40%, p = 0.013). The T allele occurred more frequently in the SVR group compared to the NR/R group (OR = 0.30; 95% CI: 0.12 - 0.73; p = 0.009).
Patients with the T/T genotype for the SNP at rs11730582 (64.3%) had a significantly higher SVR rate than patients with the T/C or C/C genotypes (12.8%). T/C + C/C was associated with non-response/relapse (12.8% vs. 87.2%, p<0.0001). The T allele had a strong association with the SVR rate (OR = 0.23; 95% CI: 0.12 - 0.43; p = 0.001). No linkage disequilibrium was noted between the SNPs.
Polymorphisms in the SOCS3 promoterOur data show that the SNP located at nt -4874 (rs4969170) in the promoter region of the SOCS3 gene is associated with the antiviral treatment response. As shown in Table 2, the A/G + A/A genotype was more frequent among the subjects in the NR/R group compared to the SVR group (79.9% vs. 20.1%, p<0.0001). The G/G genotype frequency was much higher in the SVR patients than in the NR/R patients (62.2% vs. 37.8%, p<0.0001).
The alleles were not associated with the antiviral therapy response (OR = 0.56; 95% CI: 0.32 – 1.00; p = 0.065).
Analysis of the combination of protective genotypes at the MxA, OPN and SOCS3 promoter regionsA higher number of protective genotypes present in a patient indicated a higher probability of achieving sustained virological response (Figure 1). The patients who had 3 or more protective genotypes had a greater than 90% probability of attaining SVR (OR = 0.01; 95% CI: 0.001 - 0.10; p<0.0001). There were few patients with higher numbers of protective genotypes.
Polymorphisms in the IL28B geneIndividuals with the C allele in the IL28B polymorphism at rs12979860 had a greater probability of achieving SVR, whereas patients with the T allele had a greater probability of non-response (OR = 0.45; 95% CI: 0.25–0.80; p = 0.009). The C/C genotype was more frequent in the SVR group, whereas the C/T and T/T genotypes were more frequent in the NR/R group (OR = 0.20; 95% CI: 0.09–0.47; p = 0.0008) (Table 2).
Polymorphisms in the MxA, OPN and SOCS3 promoter genes and prediction of therapy response when analyzed in combination with IL28B geneThe comparisons of the separate analysis of each protective genotype in the OPN and SOCS3 genes to the IL28B C/C genotype (rs12979860) detected an association between SVR and those polymorphisms. The protective genotypes from MxA were not associated with SVR compared to IL28B.
The combined analysis of the MxA, OPN and SOCS3 genotypes with IL28B C/C revealed that patients with 3 or more protective genotypes had a greater probability of attaining SVR compared to patients with only the IL28B C/C genotype (OR = 0.07; 95% CI: 0.02 - 0.20; p<0.0001) (Figure 1).
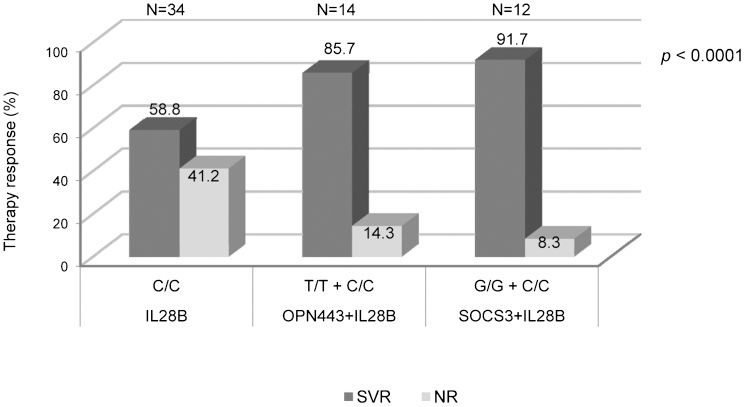
In this population, analysis of only the isolated protective IL28B genotype (C/C) was associated with SVR in 58.8% of patients. Analyzing the IL28B C/C with the protective OPN genotype (T/T) at rs11730582 increased the SVR proportion to 85.7% (OR = 0.23; 95% CI: 0.11-0.46; p<0.0001). The combination of the IL28B C/C with the SOCS3 protective genotype (G/G) achieved a predictive SVR rate of 91.7% (OR = 0.12; 95% CI: 0.05-0.28; p<0.0001) (Figure 2).
Prediction of therapeutic response based on the combined analysis of protective OPN (rs11730582) or SOCS3 genotypes with the protective IL28B genotype. The protective IL28B genotype (C/C) alone was associated with SVR in 58.8% of the patients. The IL28B C/C genotype combined with the protective OPN genotype (T/T) at rs11730582 increased the SVR proportion to 85.7%. The combination of IL28B C/C with the SOCS3 protective genotype (G/G) achieved a predictive SVR rate of 91.7%.
For the 181 patients who were treated for chronic HCV, the frequencies of the protective genotypes were 30.9% for OPN T/T (rs11730582), 20.4% for SOCS3 G/G and 18.8% for IL28B C/C. The frequencies of the combinations of protective genotypes were 6.6% for the IL28B C/C plus OPN T/T and 6.07% for the IL28B C/C plus SOCS3 G/G.
DISCUSSIONSNPs may be associated with the therapeutic outcome of treatment with Peg-INF and ribavirin, and IL28B genotypes have been reported to be significant markers in recent trials (10). In this study, we found that the combined presence of more than 3 protective genotypes from the OPN, MxA and SOCS3 genes was as effective at predicting the therapeutic response as was CC IL28B alone. We verified that grouping the SOCS3 and IL28B protective genotypes increased the capacity to predict SVR.
Interferons exert their biological effects primarily through the JAK-STAT pathway, which regulates gene transcription and mediates antiproliferative, antiviral and immunomodulatory responses (25). SOCS3 interacts with the JAKs, resulting in impaired STAT1 and STAT3 phosphorylation, reduced levels of STAT1, impaired binding to the interferon-sensitive response element (ISRE) and decreased interferon-stimulated gene (ISG) expression (26). Persico et al. (8) reported that the SOCS3 rs4969170 A/A genotype was strongly associated with a failure to respond to HCV antiviral therapy. In our study, the G/G genotype was more frequently found in the sustained virological responders, whereas the A/G + A/A genotype was most frequent in NR/R subjects. Persico et al. (8) found that the A/A genotype carriers had significantly higher SOCS3 mRNA and protein expression levels, suggesting that high SOCS3 expression in the liver, as observed in the non-responders, may be associated with non-response to HCV therapy (27). The SOCS3 rs4969170 A>G polymorphism is located within the binding region for 2 transcription factors, RUNX1 and PURα. The allelic G form binds both factors, whereas the allelic A form only binds RUNX1 (28). The SOCS3 A allele could predispose patients to overproducing SOCS3 and to resistance to antiviral therapy (29). The MxA and OPN proteins could be downregulated by SOCS3 overexpression, suppressing IFN-α-induced STAT activation and the expression of antiviral proteins.
The SNPs in OPN may be crucial in stimulating diverse Th1 immune responses to HCV by regulating OPN expression in the liver. The Th1 response is involved in inflammation in chronic hepatitis C; HCV-infected hepatocytes are eradicated by the Th1 response during IFN-based therapies. The T/T genotype in the OPN polymorphism at rs11730582 was significantly associated with SVR, whereas the T allele was less frequently found in the NR/R group; this observation is in agreement with the data from Naito et al. (16). The SVR group had a higher frequency of the T/T genotype at the OPN SNP rs2853744, although this finding was not confirmed by other authors (5,16). OPN SNPs may affect OPN expression in the liver because they are located just upstream of the cis-acting enhancing element of human OPN (29).
We evaluated the MxA gene at rs17000900 and noted linkage disequilibrium with rs2071430. Therefore, only rs2071430 was analyzed. The G/T and T/T genotypes were present more frequently in the SVR patients, whereas the G/G genotype was associated with NR/R, suggesting that the T allele has a protective effect. This assertion is supported by an in vitro study that reported higher transcriptional activity when stimulated by interferon-alpha and by the fact that the T allele polymorphism at rs2071430 increases the homology of its encompassing sequence element to an ISRE (4). Patients with the G/G genotype may produce a suboptimal MxA response from treatment with interferon-alpha. Our data agree with the findings that indicated a statistical association between heterozygosis and SVR.
We detected a lower frequency of the C/C genotype (18.8%) in the IL28B polymorphisms (rs12979860) than did a previous study (35%) (10), possibly because of the high African contribution in our admixed population. In separate analyses of the 143 naïve patients and the 38 previous NR/Rs to standard interferon-based therapy patients who were retreated with Peg-INF plus ribavirin, the results remained identical for all of the analyzed SNPs (data not shown), indicating that the low frequency of C/C IL28B may be a result of the high African contribution in our population (30). In our study population, the C/C genotype frequency was highly associated with the SVR group (58.8%), which is in agreement with the literature; Ge et al. found that the protective IL28B genotype is associated with SVR in approximately 55% of African Americans (10).
The MxA, OPN and SOCS3 protective genotypes exhibited individual associations with the therapeutic response; when analyzed together, the power to predict an SVR response increased progressively. The SVR response rate was 93.3% in patients with combinations of MxA, OPN and SOCS3 protective genotypes, whereas 58.8% of patients with the C/C IL28B genotype experienced SVR. The frequency of the combined MxA, OPN and SOCS3 protective genotypes in our study population was 10%.
We evaluated the combination of the MxA, OPN and SOCS3 protector genotypes with C/C IL28B to increase the probability of predicting the therapeutic response but found significant associations only with the OPN and SOCS3 genes. The frequency of SVR for the OPN and IL28B (T/T + C/C) combination of protective genotypes was 85.7%. The combined genotypes were present at a frequency of 6.6%, whereas the individual genotype frequencies were 30.9% (T/T OPN) and 18.8% (C/C IL28B).
The frequency of SVR for the SOCS3 and IL28B (G/G + C/C) combination of protective genotypes was 91.7%, which is higher than the individual frequencies of G/G SOCS3 (62.2%) and C/C IL28B (58.8%). The combined genotype (G/G + C/C) frequency was 6.07%, which is lower than the individual G/G SOCS3 (20.4%) and C/C IL28B (18.8%) frequencies. Although the frequency of the combined protective genotypes is low, its predictive power is reasonably accurate and is superior to the predictive power of C/C IL28B alone. A plausible biological explanation for this finding is that a less active SOCS3 allows IFN-lambda and IFN-alpha to signal more effectively via the JAK-STAT pathway to transcribe anti-viral proteins.
A previous study of HCV genotype 1 patients examined the effect of the IL28B genotype; ancestry analysis indicated that patients with the C/C genotype had an SVR rate of approximately 80% (10). Self-classified European-Americans had an SVR rate slightly higher than 80%. The authors explained that European ancestry is associated with a higher frequency of the IL28B C allele, which results in a greater probability of SVR. In our study, the group with a combination of the C/C IL28B and G/G SOCS3 genotypes had a greater SVR rate than the group with only the C/C IL28B genotype. Our results suggest that the association between the protective genotypes G/G SOCS3 and C/C IL28B is a more powerful predictor of SVR.
Our study population was admixed; the genetic ancestry analysis revealed a tri-hybrid population. It appears that our study population is more ethnically heterogeneous than populations evaluated in European and North American studies. We found that the patients in the NR/R group had a higher contribution of African ancestry, whereas the patients in the SVR group had a greater European contribution.
In conclusion, the combination of the MxA, OPN and SOCS3 protective genotypes was more frequent among the SVR patients. The combined analysis of the SOCS3 and IL28B protective genotypes (G/G + C/C) had greater power than IL28B to predict SVR. The limitations of the study must be clarified. Our study population was relatively small, and most of the combinations of SNPs that predicted treatment outcomes more effectively than did the IL28B genotype alone were present in less than 10% of the patients. New studies involving combination polymorphism analysis should be encouraged to validate our findings.
AUTHOR CONTRIBUTIONSAngelo AL performed the majority of this work. Cavalcante LN provided the data collection. Angelo AL, Machado TB, Lemaire DC, Malta F and Pinho JR provided the DNA extraction and genotyping. Angelo AL, Abe-Sandes K and Cavalcante LN provided the analytical tools. Lyra AC and Lyra LG edited and wrote the manuscript. Angelo AL, Lemaire DC, Cavalcante LN, Lyra AC and Lyra LG conceived the study.
This study was supported in part by the FAPESB (grant number 6081/2010) and by the FAPESP (grant number 2010/10549-1).
No potential conflict of interest was reported.