High-frequency neuromuscular electrical stimulation increases exercise tolerance in patients with advanced chronic obstructive pulmonary disease (COPD patients). However, it is conceivable that its benefits are more prominent in patients with better-preserved peripheral muscle function and structure.
OBJECTIVE:To investigate the effects of high-frequency neuromuscular electrical stimulation in COPD patients with better-preserved peripheral muscle function. Design: Prospective and cross-over study.
METHODS:Thirty COPD patients were randomly assigned to either home-based, high-frequency neuromuscular electrical stimulation or sham stimulation for six weeks. The training intensity was adjusted according to each subject's tolerance. Fat-free mass, isometric strength, six-minute walking distance and time to exercise intolerance (Tlim) were assessed.
RESULTS:Thirteen (46.4%) patients responded to high-frequency neuromuscular electrical stimulation; that is, they had a post/pre ΔTlim >10% after stimulation (unimproved after sham stimulation). Responders had a higher baseline fat-free mass and six-minute walking distance than their seventeen (53.6%) non-responding counterparts. Responders trained at higher stimulation intensities; their mean amplitude of stimulation during training was significantly related to their fat-free mass (r = 0.65; p<0.01). Logistic regression revealed that fat-free mass was the single independent predictor of Tlim improvement (odds ratio [95% CI] = 1.15 [1.04-1.26]; p<0.05).
CONCLUSIONS:We conclude that high-frequency neuromuscular electrical stimulation improved the exercise capacity of COPD patients with better-preserved fat-free mass because they tolerated higher training stimulus levels. These data suggest that early training with high-frequency neuromuscular electrical stimulation before tissue wasting begins might enhance exercise tolerance in patients with less advanced COPD.
There is growing evidence that chronic obstructive pulmonary disease (COPD) has systemic consequences, including a syndrome of skeletal muscle dysfunction.1 In this context, several authors have found substantial alterations in muscle structure,2 bioenergetics,3 nutritional status, 4 BODE index5 and function,6 especially but not exclusively in the appendicular muscles of the lower limbs.7
Exercise training has long been advocated as a useful rehabilitative strategy for this patient population.8 More recently, high-frequency neuromuscular electrical stimulation (hf-NMES) has been successfully used as a localized training modality in severely disabled patients who are unable to follow formal pulmonary rehabilitation and/or tolerate higher training intensities.9–12 In these studies, hf-NMES was applied to patients with the most severe muscle dysfunction and disease course. However, previous data from other populations suggest that the effectiveness of hf-NMES is modulated by the anatomical and functional integrity of the muscle apparatus; that is, subjects with better-preserved muscle mass and strength were more responsive to NMES.13–16 Moreover, the force evoked by electrical stimulation largely depends on the subject's tolerance to the applied current,14 which might be higher in less-disabled patients who are more accustomed to the subjective stress associated with exertion. These issues suggest that the potential for improvement with hf-NMES was fully realized in previous studies with end-stage COPD patients.9–12
Baseline characteristics (N = 30).
| Variables | Mean ± SD |
|---|---|
| Demographic and anthropometric | |
| Age (years ) | 63.7 ± 7.3 |
| Gender (m/f) | 26/4 |
| BMI (kg/m2) | 23.8 ± 4.2 |
| FFM (kg) | 46.9 ± 7.1 |
| Lung function | |
| FEV1 (L) | 1.42 ± 0.48 |
| FEV1 (% predicted) | 49.7 ± 13.4 |
| FEV1/FVC | 48.2 ± 8.5 |
| TLC (% predicted) | 114.2 ± 16.3 |
| RV (% predicted) | 189.9 ± 52.5 |
| RV/TLC | 53.8 ± 10.0 |
| DLCO (% predicted) | 56.9 ± 19.0 |
| Exercise capacity | |
| VO2 peak (% predicted) | 70.4 ± 16.1 |
| Endurance time (s) | 488.4 ± 190.5 |
| 6MWD (m) | 495.6 ± 72.9 |
| Peripheral muscle function | |
| Isometric strength (Nm) | 132.4 ± 36.5 |
Definition of abbreviations: BMI – body mass index; FFM – fat-free mass; FEV1 - forced expiratory volume in 1 second; RV - residual volume; TLC - total lung capacity; DLCO – lung diffusing capacity for carbon monoxide; VO2 - oxygen uptake; 6MWD – six-minute walking distance
Therefore, we performed a prospective trial to determine whether the potential for exercise capacity improvement with hf-NMES in COPD patients might be associated with phenotype characteristics indicative of better-preserved muscle structure and function. We reasoned that a confirmation of this hypothesis would have practical relevance, implying that hf-NMES could be an appropriate early rehabilitative strategy to use before severe muscle atrophy and weakness develops in patients with COPD.
METHODSSubjectsThe study group consisted of thirty patients who were consecutively evaluated at the Federal University of São Paulo's outpatient COPD clinic. All patients had been diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria;17 that is, they presented with a forced expiratory volume in one second (FEV1) of <70% of the predicted value and a FEV1/forced vital capacity (FVC) of <0.70. They also presented with chronic breathlessness (Medical Research Council dyspnea scale I-III).18 No participant had been previously enrolled in a pulmonary rehabilitation program. All participants were at a stable phase of the disease, which was indicated by no change in medical therapy (including oral steroid use) or exacerbation of symptoms within the preceding four weeks. Exclusion criteria were long-term use of supplemental oxygen; associated locomotor or neurological conditions; malignancy; cardiac failure; distal arteriopathy; α1-antiprotease deficiency; recent surgery; a severe endocrine, hepatic or renal disorder; use of anticoagulant medication and regular physical activity. Before entering the study, all patients had their pulmonary function optimized with long-acting bronchodilators and inhaled steroids when appropriate.
The Research Ethics Committee of the Federal University of São Paulo, São Paulo, Brazil (UNIFESP-EPM), approved the study (Protocol No. 1455/03). All the procedures and any associated risks were described in detail to the participants, and written informed consent was obtained. Data from an ad-interim analysis of a subgroup of patients (N = 17) who had undergone muscle biopsies were previously published.19
Design and proceduresThis study was a prospective, randomized, crossover, and sham-controlled study. Patients were assigned to a six-week, home-based hf-NMES program followed by sham stimulation or vice-versa. A two-week wash-out period was included between conditions. After randomization, the patients were not told which sequence they would follow. Each evaluation consisted of a three-day protocol: Day 1, pulmonary function and six-minute walking tests; Day 2, body composition assessment and incremental and submaximal cardiopulmonary exercise tests; and Day 3, isokinetic knee strength assessment. The evaluation team was blinded to the patients' treatment sequences.
Training protocolThe hf-NMES training protocol was based on a schema previously proposed by Neder et al.,9 who used a portable, dual-channel NME stimulator (Dualpex® 961 Sport, Quark Produtos Médicos, Brazil). The following training protocol was chosen to minimize the effects of fatigue on muscle contractility: (i) symmetrical, biphasic, square-pulsed current at 50 Hz; (ii) duty cycle: 2 s on and 10 s off (16%) in the first week, 5 s on and 25 s (18%) off in the second week, 10 s on and 30 s off (25%) in the third and fourth weeks, 10s on and 20 s off (33%) thereafter; and (iii) pulses 300- to 400-μs wide, using the highest tolerable amplitude (15-20 mA at the start of the training session, increasing up to 60 mA). This training protocol was applied to each leg of the sequence (15 minutes at a time the first week, 30 minutes at a time the second week and 60 minutes at a time thereafter) five times per week for six weeks. The sham stimulation protocol consisted of (i) a symmetrical, biphasic, square-pulsed current at 50 Hz; (ii) a duty cycle: 2 s on and 10 s off (16%); (iii) pulses fixed at 200 μs; and (iv) a constant current intensity of 10 mA. We certified that these settings were not too high to elicit effective muscle contraction in all patients. The sham stimulation was applied to each leg for 15 minutes at a time, three times per week for 6 weeks.
To familiarize the patients with the equipment and detect possible side effects, active and sham hf-NMES protocols were initially applied under the guidance of a qualified and experienced physiotherapist in an outpatient hospital setting during the first week. During the home-based training phase, each participant kept a daily user diary that included his/her impressions during the training sessions. In addition, the patients came to the laboratory once a week to adjust the stimulator settings, provide feedback about the previous week and receive instructions to further adjust the settings at home.
MeasurementsBody compositionHeight was obtained while the subjects stood barefoot and was determined to the nearest 0.5 cm. Body mass was measured with the subjects wearing light clothing and was established to the nearest 0.1 kg. Total body bone- and fat-free mass (FFM, kg) was measured using dual-energy x-ray absorptiometry (DEXA) (Hologic QDR-4500A™). The subjects laid on a specially designed table for 10 to 20 min wearing only a standard hospital gown. The DEXA scanner performed a series of transverse scans from head to toe at 1-cm intervals, using the pencil-beam mode. Data were collected in 120-pixel elements per transverse scan, with each pixel measuring approximately 5 × 10 mm. FFM depletion was defined as an FFM index (FFMI = fat-free mass/height2) ≤15 kg/m2 for females or ≤16 kg/m2 for males.20
Pulmonary function testsSpirometric tests were performed using the CPF System™ (Medical Graphics Corporation-MGC, St. Paul, MN, USA) with airflow measured by a calibrated pneumotachograph. The subjects completed at least three acceptable maximum forced expiratory maneuvers before and after taking 400 μg of inhaled salbutamol. A computer-based automated system (PF-DX System™; Medical Graphics) was used to measure static lung volumes (total lung capacity [TLC] and residual volume [RV]) by body plethysmography. Carbon monoxide diffusing capacity (DLCO) was measured using the modified Krogh technique (single breath). The subjects performed two acceptable and reproducible tests, with results within 10% or 3 ml/min/mmHg.
Six-minute walking testThe six-minute walking distance (6MWD) was measured according to a standardized protocol.21 Subjects were instructed to walk at their own pace along a 34.5-m corridor from one end to the other, covering as much ground as possible in the allotted time. The technicians encouraged subjects with the standardized statements “You're doing well” or “Keep up the good work.” Subjects were allowed to stop and rest during the test, but they were instructed to resume walking as soon as they felt able to do so. Dyspnea (Borg scale), pulse oximetry (oxyhemoglobin saturation, SpO2, %), and heart rate (HR, bpm) were assessed at the beginning and end of the test. The subjects were also asked at the end of the walk whether they experienced any of the following symptoms: dyspnea, chest pain, lightheadedness, or leg pain.
Cardiopulmonary exercise testing (CPET)The exercise tests were carried out on an electromagnetically braked cycle ergometer (CPE 2000, Medical Graphics Corporation-MGC, St. Paul, MN, USA) with gas exchange and ventilatory variables analyzed breath-by-breath using a calibrated, computer-based exercise system (MGC-CPX System, Medical Graphics Corp.). Cardiac electrical activity (CardiO2 System, Medical Graphics Corp.) and SpO2 (Ohmeda Biox 3740, USA) were continuously recorded.
The incremental exercise test consisted of (a) 2 min at rest; (b) 2 min of real zero-external intensity exercise obtained with the use of an electrical system that moved the ergometer flywheel at 50 rpm; (c) an incremental phase; and (d) a 3-min recovery period. Power (W) was continuously increased in a linear ramp pattern (5 to 20 W•min-1) such that the incremental exercise test duration was greater than 8 min and lower than 12 min for all patients. The average pulmonary oxygen uptake (V˙ O2, L/min) for the last 15 s of the ramp was considered representative of the subject's peak V˙O222. After 40 minutes, the patients performed a constant load test at 75-80% of their peak work rate to their limit of tolerance (Tlim, min) on the same ergometer. Tlim was defined as the time point at which the patients signaled to stop exercising or failed to maintain the required pedaling rate for 10 seconds, despite being encouragement from the investigators.
Peripheral muscle strengthConcentric isokinetic knee-extensor strength on the dominant side was measured using an isokinetic dynamometer (Con-Trex™, CH 8046, Zurich, Switzerland). After mild warm-up exercise (typically walking around the room), the subjects were positioned and stabilized according to a standard procedure. All patients performed two trials of an isometric strength test (IS) against a fixed-resistance pad at 60 ° (Nm). The highest value was selected for analysis.
Statistical analysisThe Statistical Package for the Social Sciences (SPSS, Chicago, IL, Version 13.0, 2004) statistical software was used for the data analysis. Symmetrically distributed baseline characteristics were summarized using the mean ± standard deviation (SD). Nonsymmetrical data were expressed by the median and range. Differences between groups were assessed with the unpaired Student's t-test and Mann-Whitney test, as appropriate. The paired Student's t-test was used to compare within-patient variations after either hf-NMES or sham stimulation. We anticipated a heterogeneous response to hf-NMES;11–14 therefore, to identify exercise “responders” to hf-NMES, we assumed that a 10% increase in ΔTlim (post-pre/pre) with no improvement after sham stimulation would indicate a true physiological response. This cut-off was based on test-retest data and previous experience with interventions in COPD patients. 9,23 We then performed a logistic regression analysis to determine the independent predictors of a positive Tlim response to hf-NMES. A p-value less than 5% was considered statistically significant for all tests.
SamplingWe estimated the sample size in a pilot study, in which we calculated a standardized effect size (E/S) for a Tlim of 0.7, defined as the above-described effect size divided by the standard deviation of ΔTlim (72 sec) for α (one-sided) = 0.05 and β = 0.20. For this calculation, the estimated sample size was 27 patients.
Role of the funding sourceThis study was supported by a Research Grant from Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP - No. 01/09866-3), Brazil (LEN).
RESULTSSample characteristicsAs expected from the inclusion criteria, based on the GOLD classification, patients who had a moderate to severe airflow obstruction of 17/30 (56.6%) were considered Stage III, and the remaining patients were classified as Stage II.17 Of note, no patient walked less than 350 m in 6 minutes (a threshold for severe disability);24 in fact, 16/30 (53.3%) patients walked more than 500 m. Moreover, 20/30 (66.6%) patients had BMI values above 21 kg/m2, and only 5/30 (16.6%) of them were considered mildly FFM depleted.25 Therefore, according to the multi-dimensional BODE index (Body-Mass Index; Airflow Obstruction; Dyspnea; Exercise Capacity), 24/30 (80%) were in the less-severe quartiles of dysfunction (BODE 0-4). 24
hf-NMES training in COPD patientsThere were no relevant side effects (e.g., skin lesions or muscle pain) related to hf-NMES application. Based on patient reports (diaries), the treatment was well-tolerated, and all participants completed it. Nine COPD patients (30%) presented with an acute exacerbation during the study, eight of them during hf-NMES (see below). All patients remained on hf-NMES, even during exacerbations.
There was a substantial increase in the self-adjusted intensity of stimulation throughout the training period: the average current amplitude increased from 30.3 ± 5.8 mA in the first week to 48.6 ± 8.3 mA at the end of the treatment (mean increase = 60.4%). Therefore, the training workload increased markedly over the 6 weeks, as the duty cycle increased from 16% to 33% and the total session duration increased from 15 min/leg to 60 min/leg (i.e., the effective stimulation time increased from approximately 2 min to 20 min/session).
Physiological responses to hf-NMESThere were no significant differences in baseline anthropometric, lung function, muscle strength and exercise capacity characteristics between patients who initially received hf-NMES or sham stimulation (data not shown). We also found no significant differences in the physiological effects of hf-NMES compared to sham stimulation in the group as a whole (Table 2). As anticipated, however, there was large variability in the effects of hf-NMES on Tlim, with 13/28 (46.6%) subjects considered responders (mean ΔTlim = 36.1%; range = 11.1 to 411.1%). These positive changes in Tlim, however, were not followed by similar improvements in other physiological tests, including IS, 6MWD, and peak VO2 (p>0.05; data not shown). Due to technical problems, the VO2 peak and Tlim were evaluated in 26 and 28 patients, respectively.
Changes (Δ) in exercise capacity and muscle strength in COPD patients after hf-NMES and sham stimulation.
| Variables | hf-NMES | p value | Sham | p value | Hf-NMES x Sham p value |
|---|---|---|---|---|---|
| Δ VO2 peak (ml/min)# | - 13.0 ± 136.4 | 0.61 | -37.7 ± 132.3 | 0.15 | 0.792 |
| Δ Tlim (s)## | 129.5 ± 554.5 | 0.22 | - 15.5 ± 278.5 | 0.77 | 0.339 |
| Δ 6MWD (m) | 10.2 ± 28.6 | 0.06 | 9.5 ± 37.9 | 0.18 | 0.944 |
| Δ Isokinetic strength (Nm) | 0.24 ± 11.2 | 0.90 | 1.6 ± 11.8 | 0.46 | 0.669 |
Definition of abbreviations: VO2 - oxygen uptake; Tlim - time to exercise intolerance; 6MWD – six-minute walking distance
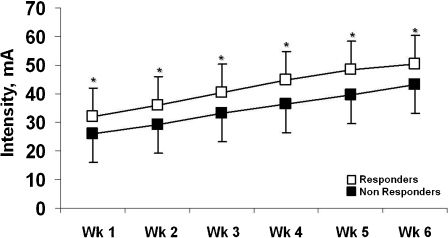
We found that responders trained at systematically higher stimulation intensities than nonresponders (Figure 1). The mean stimulation intensity was significantly related to FFM only in responders (r = 0.65; p<0.01). In addition, compared with their counterparts, responders had higher baseline values of FFM, 6MWD and, marginally, IS (p = 0.07) (Table 3). Also, importantly, 6/8 (75%) patients who had exacerbations during the hf-NMES period were nonresponders. In a logistic regression analysis that evaluated FFM, 6MWD, IS and the presence/absence of an exacerbation, FFM was the single independent predictor of a positive Tlim response to hf-NMES (odds ratio [95% CI] = 1.15 [1.04-1.26]; p<0.05).
Baseline characteristics of body composition, pulmonary function, exercise capacity and peripheral muscle strength in responders and nonresponders to hf-NMES according to changes in exercise endurance (Tlim).
| Variables | Responders n = 13 | Nonresponders n = 15 | p values |
|---|---|---|---|
| Anthropometric | |||
| BMI (kg/m2) | 24.5 ± 4.5 | 23.3 ± 4.2 | 0.48 |
| FFM (kg) | 50.5 ± 6.4 | 44.5 ± 6.8 | 0.04 |
| Lung function | |||
| FEV1 (L) | 1.61 ± 0.4 | 1.35 ± 0.5 | 0.16 |
| FEV1 (% predicted) | 53.0 ± 13.0 | 48.5 ± 13.9 | 0.39 |
| FEV1/FVC | 62.7 ± 10.0 | 62.6 ± 11.6 | 0.98 |
| RV/TLC | 52.1 ± 9.6 | 54.2 ± 9.3 | 0.56 |
| Exercise capacity | |||
| VO2 peak (% predicted) | 73.3 ± 17.6 | 70.2 ± 14.6 | 0.61 |
| Tlim (s) | 460 ± 79 | 513 ± 251 | 0.47 |
| 6MWD (m) | 529 ± 64 | 468 ± 73 | 0.02 |
| Peripheral muscle function | |||
| Isometric strength (Nm) | 146.2 ± 39.6 | 122.0 ± 28.4 | 0.07 |
Data are presented as the mean ± SD.
Definition of abbreviations: BMI – body mass index; FFM – fat-free mass; FEV1 - forced expiratory volume in 1 second; FVC – forced vital capacity; V˙O2 - oxygen uptake; 6MWD – six-minute walking distance
This study seems to be the first sham-controlled study to evaluate the effects of high-frequency neuromuscular electrical stimulation (hf-NMES) on mildly systemically impaired outpatients with COPD. Contrary to previously reported data from more severe patients,9–12 hf-NMES was no more effective than sham stimulation in the group as a whole. However, exercise capacity (Tlim) was significantly improved in a subgroup of “responders” who had a better-preserved FFM and a higher tolerance for hf-NMES. From a practical point of view, these data indicate that stimulus intensity (the amplitude of the current) is a crucial training paradigm during hf-NMES for these patients. Our results also suggest that early training with hf-NMES before muscle wasting begins might be useful for enhancing exercise tolerance in COPD patients who are not yet severely disabled.
Functional effects of hf-NMES in COPD patientsElectrical stimulation has long been used in different clinical populations to improve patients' mobility and tolerance of effort.13,25–26 Recently, this strategy has been successfully tested in patients with advanced COPD,9–12 and several systematic reviews have concluded that hf-NMES is a promising rehabilitative option for this patient population.27–29 However, previous investigations in COPD patients have found some response heterogeneity.9–12 As previously cited, part of this heterogeneity might be related to the phenotype characteristics of the enrolled patients, as most of them showed evidence of FFM depletion and systemic disease.9–12 In fact, the present study is the first to explore the effects of hf-NMES in mildly systemically disabled patients, a population that might be intrinsically more “trainable” as a result of more rapid neural adaptations, intact excitation-contraction coupling, lower thresholds for motor unit activation and/or a higher tolerance of training stimuli.7,15,30–31 In fact, most of the beneficial effects of hf-NMES are likely to be related to neural adaptations, as true hypertrophy is rarely found in patients with COPD.9–12 A biopsy-based preliminary study from our group also showed a marked dissociation between functional and structural changes after hf-NMES in this patient population.19
There is a paucity of data regarding the physiological effects of specific NMES training regimens.16, 30–32 However, it is worth noting that the training protocol used in the present study is unique in that the stimulation period and the duty cycle (on:off ratio) were maintained for relatively long periods. We can, therefore, speculate that although hf-NMES has been more commonly associated with strength and hypertrophy,16,30–32 this muscle-conditioning regimen provided endurance-like training that impacted patients' whole-body exercise tolerance (Tlim). In addition, an improved endurance capacity may have further increased patients' mobility, thus potentiating the training effects. Therefore, future studies with NMES should look specifically at whether this strategy is actually able to positively impact patients' activities of daily living.
It should be recognized that Tlim was assessed in response to a high-intensity, constant-work-rate exercise, a test paradigm that elicits a substantial contribution of force-generating Type II fibers.33 Given that hf-NMES has been associated with the selective hypertrophy of Type II fibers in some of these patients,19 it is conceivable that the test protocol was particularly suited to identify the physiological benefits of these micro-structural changes. A point of concern, however, relates to the lack of external validity of these changes, as even the Tlim responders did not walk further after training. Nevertheless, it should be noted that most of the patients walked more than 500 m at baseline, leaving little room for improvement in the 6MWD after training.
Predictors of improvement after hf-NMES in COPD patientsIn the present study, we identified a clear physiological response in a subgroup of responders with a better exercise tolerance and FFM at baseline compared with nonresponders (Table 3). These data indicate that patients with less-deteriorated muscle apparatus are likely to exhibit a more prominent exercise response. Although nonvolitional measures of skeletal muscle function and systemic markers of disease activity (e.g., proinflammatory and prooxidant markers) were not measured, it can be speculated that the training effects of hf-NMES could have been negated by the more-impaired muscle and systemic milieu in patients with more severe COPD.3
Additionally, it is crucial to note that the responders were able to train at consistently higher stimulation intensities than nonresponders (Figure 1). Although the responders had higher FFMs (Table 3), it is likely that either they tolerated higher training intensities and/or a given exercise stimulus was able to provoke the recruitment of a larger number of muscle fibers. 14,29,34 In fact, Lieber & Kelly found that a muscle's ability to generate tension (muscle efficiency) is directly related to the individual's intrinsic tissue properties, which might include muscle mass. 15 Conversely, the nonresponders' lower tolerance of hf-NMES could be related to decreased subcutaneous tissue thickness;35 however, there were no significant between-group differences in fat mass (data not shown), and skinfold thickness was not obtained in the present study. It seems that the aforementioned individual characteristics may have collectively contributed to the more impressive training response observed in patients with better-preserved muscle mass.
Clinical implicationsA recently published systematic review of the effects of electrical stimulation on peripheral muscle function in COPD patients concluded that NMES has the potential to be used as an adjunct to rehabilitation,28 especially because passive training is associated with low metabolic and ventilatory demands.36 The strategy was found to offer no special advantages over volitional training in less-disabled patients.28 However, the authors recognized that only a single study evaluated patients with less-severe COPD,19 and the specific COPD phenotypes that might benefit from NMES remain controversial. The present study adds novel evidence that muscle bulk should be taken into consideration when NMES is used in nondepleted patients. Nevertheless, it should be recognized that these patients could be more easily trained with volitional strength programs. Therefore, from a more practical point of view, our results offer a novel perspective for deciding whether to use hf-NMES simultaneously or separately with voluntary exercise in mildly impaired COPD patients.32
Study limitationsThis study has some relevant limitations. The two-week wash-out period may not have been long enough to reverse the effects of hf-NMES in patients who received the active intervention before the sham stimulation. However, at least in healthy subjects, this time frame was sufficient to reverse most of the putative neural adaptations associated with hf-NMES.16,30 Our results reflect the influence of hf-NMES for six weeks, and we cannot exclude the possibility that, with a longer treatment period, the most impaired patients might respond to this intervention. Additionally, we did not include a control group treated with standard rehabilitation to compare with COPD patients treated with hf-NMES. Additionally, we did not control the subjects for such variables as inflammatory markers or nutritional status; however, disease exacerbations and oral steroid usage were more common during active treatment than sham stimulation. Given that the patients with exacerbations were typically nonresponders, the potential for improvement with hf-NMES may have been mitigated by the increased proinflammatory burden and/or the deleterious effects of corticosteroids on the skeletal muscles.37
CONCLUSIONShf-NMES improved the tolerance for high-intensity, constant-work-rate exercise in mildly impaired COPD patients with a better-preserved FFM and a higher tolerance of transcutaneous stimulation. These data suggest that early training with hf-NMES, before progressive tissue wasting develops, may be clinically useful in enhancing exercise tolerance in nondepleted COPD patients.
This work was supported by a Research Grant from Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP – No. 01/09866-3), Brazil (LEN).
LMN received a Doctoral Fellowship Grant from CAPES, and SDC received a Post-Doctoral Fellowship Grant from FAPESP (No. 10060-3).