It has been recognized that patients with non-small cell lung cancer who are lifelong never-smokers constitute a distinct clinical entity. The aim of this study was to assess clinical risk factors for survival among never-smokers with non-small cell lung cancer.
METHODS:All consecutive non-small cell lung cancer patients diagnosed (n = 285) between May 2005 and May 2009 were included. The clinical characteristics of never-smokers and ever-smokers (former and current) were compared using chi-squared or Student's t tests. Survival curves were calculated using the Kaplan-Meier method, and log-rank tests were used for survival comparisons. A Cox proportional hazards regression analysis was evaluated by adjusting for age (continuous variable), gender (female vs. male), smoking status (never- vs. ever-smoker), the Karnofsky Performance Status Scale (continuous variable), histological type (adenocarcinoma vs. non-adenocarcinoma), AJCC staging (early vs. advanced staging), and treatment (chemotherapy and/or radiotherapy vs. the best treatment support).
RESULTS:Of the 285 non-small cell lung cancer patients, 56 patients were never-smokers. Univariate analyses indicated that the never-smoker patients were more likely to be female (68% vs. 32%) and have adenocarcinoma (70% vs. 51%). Overall median survival was 15.7 months (95% CI: 13.2 to 18.2). The never-smoker patients had a better survival rate than their counterpart, the ever-smokers. Never-smoker status, higher Karnofsky Performance Status, early staging, and treatment were independent and favorable prognostic factors for survival after adjusting for age, gender, and adenocarcinoma in multivariate analysis.
CONCLUSIONS:Epidemiological differences exist between never- and ever-smokers with lung cancer. Overall survival among never-smokers was found to be higher and independent of gender and histological type.
Lung cancer remains the leading cause of cancer mortality, accounting for more deaths than breast, colon, and prostate cancer combined. Smoking was established as a risk factor for lung cancer in the early 1950s.1–3 Moreover, smoking is associated with both lung cancer carcinogenesis and the prognosis of lung cancer patients.4 Due to the overwhelming etiological role of tobacco smoking, lung cancer is mainly considered a smoking-related disease; consequently, the never-smoker population is usually under-represented in lung cancer studies.5 Only recently has attention turned toward the small number of never-smokers with this disease.
The proportion of never-smokers with lung cancer is expected to increase in parallel with successful smoking prevention and smoking cessation programs. Although the incidence of lung cancer may be increasing among never-smoker patients, it is unclear whether this increase represents are real increase in the lung cancer incidence among never-smokers or arises from the increasing prevalence of never-smokers in the general population.6
It is noteworthy that differences in epidemiological characteristics and histological subtypes between smokers and never-smokers have been demonstrated, especially among Asian patients.7,8 This suggests that the pathogenesis of non-small cell lung cancer (NSCLC) in never-smokers might be different than in smokers. It was recently demonstrated that a subgroup of patients with NSCLC exhibits a specific activating mutation in the epidermal growth factor receptor gene associated with better responses to target drugs, such as tyrosine kinase inhibitor drugs, and overall survival.9
There is limited literature regarding never-smoker lung cancer patients in the Western hemisphere, especially in Brazil. The aim of this study was to assess the epidemiological characteristics of never-smoker patients with lung cancer, focusing on clinical risk factors and survival.
METHODSThe study described here was a prospective cohort study conducted between May 2005 and May 2009 using an electronic database. All consecutive patients with pathologically proven NSCLC who presented at our outpatient lung cancer clinic were eligible. The Institutional Review Board of our center approved this study. Patient consent was obtained for entry into the database.
Data were collected at diagnosis as part of routine clinical practices using a structured data collection format. The selected epidemiological characteristics included age, gender, and ethnicity.
NSCLC was further divided into three major histological subtypes: squamous cell carcinoma (SCC), adenocarcinoma and other types of carcinoma (large-cell carcinoma, poorly or undifferentiated carcinoma and not otherwise specified NSCLC). The vast majority of the lung specimens that were used for diagnosis were bronchial biopsies, and immunohistochemistry was employed to better identify the histological types when required. The American Joint Committee on Cancer (AJCC) staging system (sixth edition) was applied, and for analysis purposes, disease stage was categorized into two groups: early disease (Stages Ia to IIIa) and advanced disease (Stages IIIb and IV); moreover, among Stage IV patients, the database included whether metastases were intra- or extrathoracic.
Weight loss, comorbidity, the Karnofsky Performance Status Scale, smoking status and treatments were documented. Weight loss was categorized into two groups: 10% or more and less than 10%.
Comorbidities included one or more of the following conditions: hypertension, ischemic heart disease, diabetes mellitus, chronic obstructive pulmonary disease, asthma, and pulmonary tuberculosis sequelae.
Smoking status was classified into two levels: ever-smoker and never-smoker. Patients with any history of smoking (current and former smokers) were classified as ever-smokers, and the intensity of tobacco exposure was measured in pack-years. Never-smokers were defined as those who had never smoked in the past. Data related to passive exposure to environmental tobacco smoke and cooking fumes were not consistently available. Consequently, for the purpose of analysis, the never-smoker category did not account for passive exposure.
Treatment status was stratified into two categories: treated (surgery, chemotherapy, or/and irradiation) and best treatment support.
The date of the last follow-up or of death was collected for each patient. Overall survival was measured from the date of histological diagnosis to the date of death or the date that the patient was last known to be alive for censored observation.
Statistical analysesDifferences between the never- and ever-smokers were compared using the chi-squared test for categorical variables and Student's t-test for continuous data, as were the differences between the two genders within the group of never-smokers.
Survival curves and the five-year survival rates were calculated according to the Kaplan-Meier method, while a log-rank test was used to assess the differences in survival between the groups. A multivariate analysis (Cox proportional hazard regression) was evaluated by adjusting for known prognostic factors and potential confounders. The number of independent variables (factors) was limited by the occurrence of the event (death). The factors considered for inclusion were gender (female vs. male), smoking status (never- vs. ever-smoker), histological type (adenocarcinoma vs. non-adenocarcinoma), AJCC stage (early vs. advanced staging), and treatment (chemotherapy and/or radiotherapy vs. the best treatment support). The age at diagnosis and the Karnofsky Performance Status Scale were adjusted as continuous variables in the Cox regression model. Statistical analyses were performed using IBM's Statistical Package for the Social Sciences, version 19.0. All hypothesis tests were two-tailed, and the level of significance was set at 5%.
RESULTSTwo hundred eighty-five NSCLC patients diagnosed between May 2005 and May 2009 were included in our analysis. The majority were ever-smokers (76%). The median tobacco exposure was 41 pack-years (range: 1 to 210 pack-years). Among the never-smokers (56 patients), there were significantly more women (68%) and adenocarcinomas (70%). There were no significant differences between never- and ever-smokers with regard to age, the Karnofsky Performance Status Scale, weight loss, ethnicity, comorbidities, AJCC stage, and patient treatment. The majority of the patients presented with advanced disease, although no significant difference in the proportion of advanced disease or extrathoracic disease was observed between the groups (Table 1).
Characteristics of NSCLC patients grouped according to their smoking status at diagnosis.
| Variables | Never-smoker n = 56 | Ever-smoker n = 229 | p-value |
|---|---|---|---|
| Age mean (SD) | 61.6 (13.9) | 63.4 (11.2) | 0.31¥ |
| Karnofsky Performance Status mean (SD) | 81.6 (13.6) | 80.3 (13.8) | 0.51¥ |
| Female, n (%) | 38 (68) | 73 (32) | <0.001 § |
| Ethnicity | 0.52 | ||
| Caucasian | 42 (75) | 161 (70) | |
| African descent | 11 (20) | 60 (26) | |
| Asian | 3 (5) | 8 (4) | |
| Histological type, n (%) | 0.03 § | ||
| Squamous cell | 11 (20) | 87 (38) | |
| Adenocarcinoma | 39 (70) | 116 (51) | |
| Other | 06 (10) | 26 (11) | |
| Weight loss ≥10%, n (%) | 17 (30) | 99 (43) | 0.08 § |
| Comorbidity,n (%) | 14 (25) | 44 (19) | 0.34 § |
| Staging, n (%) | |||
| IIIb to IV (advanced disease) | 46 (82) | 169 (74) | 0.19 § |
| Extrathoracic metastasis, n (%) | 17 (30) | 56 (24) | 0.57 § |
| Treatment, n (%) | 40 (71) | 161 (70) | 0.87 § |
| Surgery | 07 (12) | 34 (15) | 0.65§ |
| Chemotherapy | 36 (64) | 139 (61) | 0.62§ |
| Radiotherapy | 07 (12) | 32 (14) | 0.77§ |
| Combined therapies | 13 (23) | 45 (20) | 0.60§ |
| Best support treatment | 16 (28) | 68 (30) | 0.87§ |
n = number of patients; SD = standard deviation.
Supplementary analyses were completed for the never-smoker group to examine the behavior of the variables analyzed above according to gender. The patients of both genders were similar with respect to age, the Karnofsky Performance Status Scale, the proportion of histological types, weight loss, comorbidities, extrathoracic metastasis, and patient treatment. However, female never-smokers exhibited a greater proportion of Stages IIIb and IV than their male counterparts (Table 2).
Subject characteristics of never-smoker patients by gender.
| Variables | Female n = 38 | Male n = 18 | p-value |
|---|---|---|---|
| Age mean (SD) | 62.8 (12.6) | 59.3 (16.4) | 0.39 ¥ |
| Karnofsky performance status mean (SD) | 81.3 (13.6) | 82.2 (13.9) | 0.82 ¥ |
| Histological type n (%) | 0.59 § | ||
| Squamous cell | 08 (21) | 03 (17) | |
| Adenocarcinoma | 27 (71) | 12 (68) | |
| Other | 03 (8) | 03 (17) | |
| ≥10% Weight loss n (%) | 13 (34) | 04 (22) | 0.36§ |
| Comorbidity n (%) | 09 (24) | 05 (28) | 0.74 § |
| Staging n (%) | |||
| IIIb to IV | 34 (90) | 12 (67) | 0.04 § |
| Extra thoracic metastasis n (%) | 10 (26) | 07 (39) | 0.20 § |
| Treatment n (%) | 27 (71) | 13 (72) | 0.93 § |
n = number of patients; SD = standard deviation.
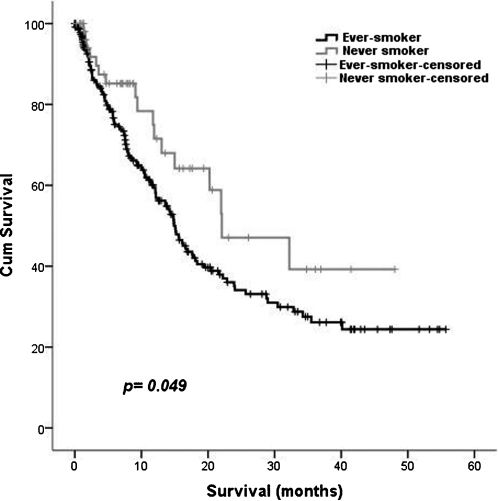
The overall five-year survival rates of never-smokers and ever-smokers were significantly different (p = 0.049) (Figure 1). The median overall survival was 15.7 months (95% CI: 13.2 to 18.2). The median survival time was 14.9 months (95% CI: 12.9 to 16.9 months) for ever-smokers and 22.1 months (95% CI: 9.5 to 34.6 months) for never-smokers.
To complement the survival study, a multivariate analysis was conducted using the Cox proportional hazard model. Being a never-smoker (vs. an ever-smoker), a higher Karnofsky Performance Status, early staging and treatment (vs. the best treatment support) were independent and favorable prognostic factors for overall survival after adjusting for age, gender, and adenocarcinoma (vs. non-adenocarcinoma) (Table 3).
Multivariate analysis of factors related to overall survival in 285 NSCLC patients, using Cox regression (-2 log likelihood = 1,265.5; chi-squared = 60.0; p<0.001).
| Variable | Coefficient | SE | Adjusted HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age # | -0.02 | 0.01 | 0.98 | 0.97-1.00 | 0.050 |
| Female | -0.25 | 0.19 | 0.77 | 0.53-1.13 | 0.180 |
| Never-smoker | -0.54 | 0.27 | 0.58 | 0.34-0.99 | 0.047 |
| KPS # | -0.03 | 0.01 | 0.97 | 0.96-0.99 | <0.001 |
| Adenocarcinoma | 0.08 | 0.18 | 1.09 | 0.76-1.56 | 0.650 |
| Early disease detection | 0.82 | 0.23 | 0.44 | 0.28-0.69 | <0.001 |
| Treatment | -0.57 | 0.21 | 0.57 | 0.38-0.85 | 0.006 |
KPS = Karnofsky Performance Status Scale; SE = standard error; HR = hazard ratio; CI = confidence interval for HR.
Moreover, a subgroup analysis of never-smokers revealed that the survival of the never-smoker-population did not show any influence of female gender (vs. male) or adenocarcinoma (vs. non-adenocarcinoma) on the Cox regression [-2log likelihood = 105,803; chi-squared = 2,027; p = 0.363).
DISCUSSIONOur main finding was that never-smokers had better five-year survival rates than ever-smokers. This confirms the results of the other studies, which have shown survival benefits associated with a never-smoking status in NSCLC patients. Never-smoking status has been reported as an independent predictor of improved survival at five years (16% for current smokers, 23% for never-smokers),10 and a poorer survival outcome in patients with a history of smoking was described in a retrospective analysis.4 However, there is controversy regarding this finding in the current literature. Another study did not find differences in survival between NSCLC patients stratified according to their smoking status.11
In our study the presence of comorbidities did not differ between ever or never-smokers, although it has been described with tobacco-related disease. Conversely, in other studies the presence of comorbidities justified the worst survival among lung cancer patients.12
For a more comprehensive analysis, we performed a Cox proportional hazards regression adjusted for known prognostic factors. This multivariate analysis also confirmed that never-smokers exhibited a decreased risk of dying. As expected, early disease diagnosis, patient treatment and higher Karnofsky Performance Status scores were also favorable, independent predictors of survival. Interestingly, neither female gender nor having adenocarcinoma reached the specified significance level in the multivariate survival analyses, although both variables accounted for a significantly higher proportion of never-smoker patients in the univariate analysis.
Never-smokers constituted 24% of the NSCLC patients in our population. The literature supports the assertion that several characteristics are more commonly seen in NSCLC patients who are never-smokers. Our study found that women were more likely than men to have non-smoking-associated lung cancer (68%). The risk of developing lung cancer among women who smoke has been described as higher than that of men who are exposed to the same smoking rate.13–18 These results are still controversial, and the possibility that women have an increased susceptibility to the effects of smoking is not yet clearly defined. However, the predominance of females among never-smokers with tumors, even without exposure to cigarette smoke carcinogens, has been previously described, suggesting that aspects related to hormonal factors may interfere with tumor carcinogenesis.6,8,15
The role of estrogen in the carcinogenesis of other types of tumors in women is well established. Growing evidence indicates the effects of estrogen on lung cancer cells. The presence of estrogen receptors (ERs) in pulmonary tumor cells suggests that this hormone plays a role in the carcinogenesis of lung cancer.19–21 There are two main types of ERs in humans: ER-alpha and ER-beta. ER-beta receptors are the major mediators of estrogen activity in lung cells. They are active receptors in lung tissue and can contribute to the growth of neoplastic cells. Although the prevalence of ERs in tumor cells is similar in men and women, gender differences in survival exist.22,23 The mechanism underlying these sex-based differences is unclear, but genetic and metabolic factors, hormonal influences, and the presence of specific isoforms of ER-beta may be involved.
Another interesting finding is the predominance of adenocarcinoma among never-smoking patients, which is consistent with the results of previous studies.7,24,25 Although this histological variant is most commonly found in women, never-smoking men also showed a higher proportion of adenocarcinoma in our study, suggesting that a factor unrelated to sex is responsible for the predominance of adenocarcinomas in the never-smoking population. Several genetic alterations have been described that may contribute to the development of adenocarcinoma in nonsmokers. Two main pathways for the development of lung adenocarcinoma have been described: the KRAS and the epidermal growth factor receptor (EGFR)20 pathways. KRAS mutations are generally linked to tobacco consumption, and the EGFR pathway is generally associated with nonsmokers.26–28 Recent studies have indicated that patients with mutations in the EGFR gene respond better to treatment with EGFR tyrosine kinase inhibitors.29,30 Although KRAS mutations are historically considered to represent a tumorigenic pathway in smokers, their prevalence has been reported to be similar in smokers and nonsmokers; however, differences in mutation type have been reported.31 Though this is still controversial, molecular differences between groups may be responsible for distinct clinical manifestations and responses to treatment. As biomarkers may be used for risk stratification and treatment selection, new pathogenic pathways are being studied.32,33
In contrast to studies in Asian populations, we did not find differences in the age of diagnosis among never-smokers compared with ever-smokers.34 Our findings are consistent with the results of studies performed in Europe and the United States, where this disease occurs mainly in older adults. These differences may be explained by the hypothesis that indoor air pollutants, such as cooking fumes, play a role in lung carcinogenesis in developing countries, although there is some controversy surrounding this issue.35 Exposure to cooking fumes is common in Brazil; however, we lacked information regarding our participants' exposure levels to such fumes.
As lung cancer is considered a disease of smokers,36,37 never-smoker patients may experience either late presentation or late diagnosis on the part of physicians. The majority of our patients presented with the disease at later stages; however, when the group of never-smokers was analyzed for gender associations, we found that women were more likely than men to have lung cancer diagnosed at a more advanced stage. It is noteworthy that we did not find differences in extrathoracic disease between never-smokers and ever-smokers. Consequently, the clinical threshold for investigating symptomatic never-smokers must be lower.
The limitations of our study are related to the lack of information about passive smoking and cooking fume exposure as well as molecular analyses of the tumors. However, the epidemiological behavior of our never-smoker sample confirmed that even a racially varied population, as found in Brazil, follows the same model as that of never-smokers in other parts of the world.
In conclusion, the vast majority of never-smoker lung cancer patients were female and exhibited adenocarcinoma as the predominant histological type. Additionally, the female never-smoker patients showed a higher proportion of advanced disease, although the proportion with extrathoracic metastasis was similar to that of male never-smokers. Among NSCLC patients, after adjusting for age, female gender and adenocarcinoma, being a never-smoker with early treatment of the disease and having a higher Karnofsky Performance Status Scale were associated with a better prognosis. Lung cancer in never-smokers has a different clinical profile, with a distinctly lower mortality rate compared to lung cancer among smokers, which reflects a singular clinical behavior and natural history.
Financial disclosure:
Prof. Ilka Lopes Santoro and Dr. Sérgio Jamnik are coinvestigators in a Phase III research project supported by AstraZêneca®, Boehringer®, Abbott® and Bristol®. They are also involved in a Phase II project supported by Merck Sharp Dome®.
The other authors do not have any financial disclosures to declare.
No potential conflict of interest was reported.
Santoro IL and Fernandes ALG conceived and designed the study, were responsible for the analysis and interpretation of the data, critical revision, and final approval. Ramos RP, Franceschini J, and Jamnik S were responsible for the collection, analysis and interpretation of data, and draft of the article.