Cancer has been investigated using various pre-targeting techniques or models focusing on radiobombesin analogues; however, both are not offered together. In this study, nano-bombesin labeling by a pre-targeting system was undertaken to develop an alternative approach for prostate tumor treatment.
METHODSA two-step pre-targeting system utilizing a combination of streptavidin (SA), biotinylated morpholino (B-MORF), biotinylated BBN (B-BBN) with two different spacers (β-Ala and PEG), and a radiolabeled cMORF was evaluated in vitro and in vivo.
RESULTSFinal conjugation conditions consisted of a 1:1:2 ratio of SA:B-MORF:B-BBN, followed by addition of 99mTc-cMORF to compensate for free MORF. In vitro binding experiments with prostate cancer cells (PC-3) revealed that total binding was time-dependent for the Ala spacer but not for the PEG spacer. The highest accumulation (5.06±1.98 %) was achieved with 1 hour of incubation, decreasing as time progressed. Specific binding fell to 1.05±0.35 %. The pre-targeting biodistribution in healthy Swiss mice was measured at different time points, with the best responses observed for 7-h and 15-h incubations. The effector, 99mTc-MAG3-cMORF, was administered 2 h later. Strong kidney excretion was always documented. The greatest tumor uptake was 2.58±0.59 %ID/g at 7 h for B-βAla-BBN, with a region of interest (ROI) value of 3.9 % during imaging. The tumor/blood ratio was low due to the slow blood clearance; however, the tumor/muscle ratio was 5.95.
CONCLUSIONSThe pre-targeting approach with a peptide was a viable concept. Further evaluation with modified sequences of MORF, including less cytosine, and additional test intervals could be worthwhile.
Prostate cancer is the most common non-skin malignancy in men, leading to substantial morbidity and mortality. Rectal examination, prostate specific antigen (PSA) and imaging methods such as computed tomography, magnetic resonance imaging and transrectal ultrasound are all useful for diagnosis and staging of this disease. Yet, all of them suffer from accuracy limitations in early cancer, especially concerning differentiation between clinically insignificant lesions that should better be left alone, and those that deserve radical treatment.1 Functional and molecular radiopharmaceutical imaging offers the possibility of disclosing cellular processes, potentially representing an advance in the identification of invasive and metastasizing lesions.2
Nanoparticles or nanocarriers exhibit advantageous pharmacokinetics and bioavailability in the context of cancer diagnosis and therapy.3
Receptor-binding radiotracers for tumor diagnosis have been extensively studied including bombesin, a neuropeptide with affinity for prostate as well as breast tumors.4
Since Anastasi et al.5 bombesin (BBN), originally isolated from amphibian skin, has been mostly collected from porcine gastric tissue which expresses gastrin-releasing peptide (GRP), a similar molecule with potent gastrin releasing action.6
Bombesin has a structure closely related to that of several mammalian peptides, including the alluded to GRP, also known as BB2, neuromedin B (BB1), and additional BB3 and BB4 subtypes. Physiological effects are triggered by binding to specific cell receptors, including growth of some categories of tumor cells.7
BBN analogues have been radiolabeled with multiple radioisotopes and techniques, more recently focusing nanoparticles.3 Analogues like the truncated BBN[7-14]NH2 sequence have been labeled with 99mTc, 111In, 90Y, 64Cu,8177Lu, 18F or 68Ga.4 This fragment is convenient as a highly specific GRP-receptor–binding motif.9,10 Nevertheless, publications addressing the pretargeting system have not been identified in the literature.
Morpholinos (MORFs) are oligomers in which a phosphorodiamidate backbone replaces the sugar phosphodiester linkage in natural DNAs and RNAs. They resist degradative nucleases and are endowed with high efficacy and specificity of hybridization.11
Pretargeting agents are usually antitumor antibodies,12 whereas peptides have received very limited attention thus far. Carrier molecules are often employed to increase cellular uptake in connection with antisense tumoral targets, and also in other applications that require oligomers and transmembrane migration.13
The preparation of oligomers with a carrier by covalent conjugation is difficult. This trouble can be avoided by utilization of streptavidin, a convenient linker for biotinylated carriers and oligomers because it requires only simple mixing.14
Investigations in cancer dealing with various pretargeting techniques, as well as models focusing radiobombesin analogues are available, but not both together.
By means of 99mTc, bombesin has been radiolabeled using tetradentate N3S, P2S2, N4 chelator systems or tricarbonyls combined with tridentate coligands.15,16 Conventionally the design of a peptide molecule with spacers or linkers has the objective to improve in vivo pharmacokinetics. Pegylation is one of these strategies.
Rogers et al.17 introduced a polyethylene glycol (PEG) linking moiety into the biomolecular targeting vector to prevent hindrance of the binding affinity of the BBN7–14NH2 motif with GRP receptors. Smith et al.,18 reported the flexibility of the lengh of the hydrocarbon spacer group.
β-Alanine (β-Ala) is a short amino acid spacer containing three carbon atoms which is coupled to the active site of BBN in order to achieve better in vitro and, particularly, in vivo performance. In the current study β-Alanine and polyethylene glycol were included in the molecular design. The system combined morfolino and it's complement (cMORF) as well biotin-streptavidin.
The objective was investigation of the radiochemical and biological profile of two BBN molecules administered by the pretargeting protocol.
MATERIAL AND METHODSMaterialsThe materials used in this study were:
- •
Biotinylated MORF and cMORF from Gene tools, OR, USA
MORF - 5’-TCTTCTACTTCACAACTA-3’ biotin )- MW 6381
cMORF - 5’-TAGTTGTGAAGTAGAAGA-3’-Primary amine – MW 6317
- •
Conjugated Biotinylated Bombesin Analog with two different spacers from Peptide International, KY,USA
Biotin-β-Ala-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 (MW 1237.52)
Biotin-dPEG-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 (MW 1766.16)
- •
S-acetylmercaptoacetyltriglicine (S-MAG3) - synthesized at University of Massachusetts, Worcester, MA, USA.
- •
MAG3-cMORF – conjugated at University of Massachusetts, Worcester, MA, USA
- •
Technetium-99m (99mTc) was retrieved from an alumina-based 99Mo/99mTc generator, supplied by Radiopharmacy Center of the Institute of Energetic and Nuclear Research (IPEN/CNEN)-São Paulo, Brazil
- •
MAG3-Ahx-BBN (Ahx – 6-aminohexanoic acid) – piChem, Austria.
- •
Reagents and solutions (Trypsin, Glycine, Cell culture solution RPMI with L-Glutamine) – Sigma-Aldrich and Merck, São Paulo, Brazil
- •
Streptavidin (SA) – Pierce Protein Research Product – Thermo Scientific, Rockford, IL, USA
- •
Human prostatic tumor cells PC-3, ATCC- Code CRL-1435, USA.
- •
Animals – Male Swiss mice (20-30g) and Male Nude mice (Balb/c) (15-20g) – Animal Facility of IPEN/CNEN-SP, Brazil
N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycine (NHS-MAG3) was synthesized and conjugation of MAG3-cMORF was executed at University of Massachusetts, as previously described. 19
Solution preparationSolutions of different molecules were prepared in 0.5 M NaCl/0.2M NH4OAc. Concentrations were: SA – 0.189 mM; B-MORF - 0.3 mM; B-βAla-BBN - 0.2 mM; B-PEG-BBN - 0.2 mM and MAG3-cMORF - 0.053 mM.
Conjugation of biotin-morfolino (B-MORF) with streptavidin (SA)Biotin-MORF was added stepwise to streptavidin and vortexed, at three different molar ratios (1:1, 1:2, 1:3).
Association of biotin-β-Ala-BBN and biotin-PEG-BBN with SAThe conjugated peptides were associated with SA adopting the same ratios as for SA:B-BBN.
Radiolabeling of complementary morpholino (MAG3-cMORF) with 99mTcComplementary morpholino was labeled with 99mTc via MAG3 procedure as previously described20. Briefly 4 μl (100 - 200 μCi) of sodium pertechnetate was added to a solution containing 10 μL de MAG3-cMORF (0.346 μg/μl in 0.25 M ammonium acetate solution, pH 5.1), 4 μl sodium tartrate dihydrated (50 mg/ml) pH 9.2 and 1.5 μl of SnCl2.2H2O (4 μ/μl) in 1 μg of sodium ascorbate/ μl of HCl 10 mM). The mixture was heated for 20 min/100°C.
Conjugation of the three molecules and radiolabeling- a.
SA + Biotin-MORF + Biotin-βAla-BBN + MAG3-cMORF-99mTc
- b.
SA + Biotin-MORF + Biotin-PEG-BBN + MAG3-cMORF-99mTc
The three species were put together very slowly with constant agitation in molar ratios of 1:1:3 and 1:1:2, followed by radiolabeling.
Evaluation of conjugation and radiochemical labeling by size-exclusion High Performance Liquid Chromatography (HPLC)All solutions were evaluated by size-exclusion HPLC to define retention time and conjugation purity.
Superose-12 column (Amersham Pharmacia, NJ, USA) was used with acetonitrile 20% in NaCl 1 M and NH4OAc 0.1 M eluant at a flow rate of 0.6 ml/min. The effluent was monitored by in-line UV detector set at a wavelengh of 265 nm. Radioactivity was monitored by in-line NaI(Tl) scintillation detector.
Cell CulturePC3 human prostate adenocarcinoma cells expressing bombesin receptor were cultured adherently in RPMI 1640 supplemented with 10% fetal calf serum at 37°C. Cells were detached from culture bottles by trypsinization (0.24% trypsin/0.05% EDTA) and seeded into 12-well plates for binding studies containing 106 cells/well.
In vitro binding experimentsConcentrations used in each well were:
cMORF -58 pM (380 pg); B-MORF – 196 pM (1.25 ηg); B-MORF/cMORF = 3.4 and B-βAla- BBN or B-PEG-BBN 362 pM (446.67 pg).
The medium was aspirated and the cells washed twice with culture medium (RPMI). In well-plate 1 (in triplicate) it was added: 100 μl of mixture (SA+B-MORF+B-Ala-BBN or SA+B-MORF-PEG-BBN) + 900 μl culture medium. In well-plate 2 (in triplicate) it was added: 100 μl of mixture (SA+B-MORF+B-Ala or PEG-BBN) + 100 μl MAG3-Ahx-BBN (1 μM- unlabeled ligand for specific binding) + 800 μl culture medium.
The well-plates were incubated at 37°C for three periods of time (1, 3 and 5 hours). Afterwards the medium was aspirated and the cells were washed twice with the culture medium. Then it was added to all wells 100 μl 99mTc-MAG3-cMORF + 900 μl culture medium. The plates were further incubated for 10 minutes, then the supernatant was collected. The wells were washed with cold PBS and the material mixed together with the previous supernatant. Glycine was added to the wells and then collected, and finally wells were washed again with PBS, NaOH was added, and the resulting fluid collected.
In vivo studies: cell inoculation in animals, biodistribution and imagesAll animal studies were performed in accordance with the Guidelines for the Care and Use of Research Animals established by the Local Animal Welfare Committee.
The induction of tumor xenografts in Nude mice (age of 8 weeks) was done with PC-3 cells (5 × 106 cells/mouse) subcutaneously injected on the upper right part of the back of the animal. PC3 cells were allowed to grow in vivo for about 10 days post inoculation, thus forming tumors with a diameter of approximately one cm, adequate for experiments with the radiotracer.
Animals were sacrificed at different times by cervical dislocation. The main organs were removed and together with samples of muscle and bone were weighed and counted in a NaI (Tl) well counter (Cobra II, automatic γ-counter, Packard Instrument Company, USA), and biodistribution data were calculated as percent of injected dose per gram (% ID/g), using injected dose as standard.
Samples of blood were also taken along with stomach and intestines which were emptied before the measurements. Whole blood volume as well as total muscle mass were assumed to be 7% and 40% of body weight respectively, for the purpose of calculating %ID.
Biodistribution studies were conducted in three steps:
- 1.
Evaluation of 99mTc-MAG3-cMORF in healthy Swiss mice: It was conducted 1, 2 and 3 hours post injection.
- 2.
Pretargeting system in healthy Swiss mice
50 μl of the mixture SA+B-MORF+B-βAla-BBN or SA+B-MORF+PEG-BBN in the ratio (1:1:2) was injected in the tail vein of the animals. Two hours after the scheduled times of 3, 5, 7, 15 and 24 hours the effector 99mTc-MAG3-cMORF was administered by the same route. After two more hours the animals were sacrificed and biodistribution performed as described above (Scheme 1).
Pretargeting system in Nude mice bearing tumor cells
The same procedure of healthy animals was adopted but restricted to two incubation times (7 and 15 hours). Effector injection (99mTc-MAG3-cMORF) and assessment of biodistribution followed similar routines.
Imaging of tumor-bearing mice was done in gamma-camera with horizontally-placed anesthetized animals (Mediso Imaging System, Budapest, Hungary), employing a low-energy high-resolution collimator. Images were acquired with 256×256×16 matrix size and 20% energy window set at 140 keV for a period of 180 s.
Statistical AnalysisTissue/organ distributions are presented as mean ± SD. For differences between the two experimental groups Student's t-test was selected, to a significance level of 5% (P<0.05).
RESULTSHPLC analysis confirmed conjugation of morpholino and it's complementary molecule, as well as the bombesin analogs which were combined with streptavidin.
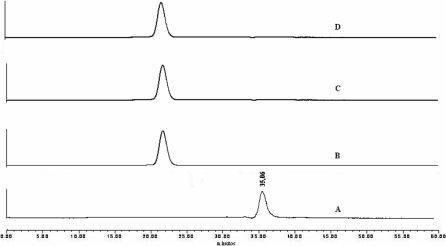
Figure 1 shows that commercial MORF is not totally biotinylated (only 60%) when using SA nanoparticles at different ratios. However, for both bombesin analogs 100% of the conjugated peptide was attached to biotin when different ratios were used for conjugation with streptavidin, showing a single peak (Fig. 2 and Fig. 3). Radiochemical purity of 99mTc-MAG3-cMORF was 99.6% (Fig. 4), with Rt of 26.6 min. In order to compensate for free MORF, final conditions were 1:1:2 and 1:1:3 SA/B-MORF/B-BBN. HPLC analysis with radioactivity detection following addition of 99mTc-cMORF confirmed unimpaired hybridization (Fig 4). Radioactivity recuperation was in the range of 73 to 82%.
Streptavidin possesses four biotin binding sites so Biotin-MORF and Biotin-BBN competed for them. Due to this competition, ratio between SA:B-MORF:B-BBN was evaluated (Scheme 2).
Specific cell- binding (SB) was calculated by the difference between values for well-plate 1 (total binding/T) and well-plate 2 (non-specific binding/NSB). For the radiolabeled βAla mixture (SA+B-MORF+B-βAla-BBN), total binding was confirmed to be time dependent (Figure 5). The highest accumulation (5.06±1.98%) occurred with 1 hour of incubation, decreasing on later times. Specific binding fell to 1.05±0.35%. This phenomenon did not hold true for the pegylated molecule (Fig 6), the highest value being reached at a later time (3 hours).
A superiority (87% more) in cell accumulation for B-β-BBN as compared to B-PEG-BBN can be appreciated in Figure 7.
Table 1 summarizes uptake in different organs and tissues of 99mTc-MAG3-cMORF in healthy Swiss mice. Kidneys came first, followed by intestine. Fast blood clearance was documented, and an intermediate time point (2 hours) was chosen for further studies.
Biodistribution of 99mTc-MAG3-cMORF in Swiss mice.
| Time (hours) Organs/Tissues | 1 | 2 | 3 |
|---|---|---|---|
| Blood (%ID/ml) | 0.41 ±0.05 | 0.18 ± 0.02 | 0.09 ± 0.02 |
| Heart | 0.20 ± 0.03 | 0.10 ± 0.02 | 0.07 ± 0.02 |
| Lung | 0.53 ± 0.08 | 0.25 ± 0.03 | 0.17 ± 0.02 |
| Kidneys | 8.19 ± 2.43 | 4.64 ± 0.96 | 2.93 ± 0.06 |
| Spleen | 0.21 ± 0.04 | 0.12 ± 0.03 | 0.17 ± 0.01 |
| Stomach | 0.28 ± 0.08 | 0.38 ± 0.05 | 0.55 ± 0.13 |
| Pancreas | 0.30 ± 0.08 | 0.14 ± 0.02 | 0.12 ± 0.03 |
| Liver | 0.44 ± 0.06 | 0.52 ± 0.09 | 0.41 ± 0.04 |
| Large Intestine | 0.37 ± 0.03 | 0.33 ± 0.10 | 1.33 ± 0.38 |
| Small Intestine | 1.71 ± 0.41 | 0.65 ± 0.07 | 0.35 ± 0.12 |
| Muscle | 0.13 ± 0.01 | 0.18 ± 0.03 | 0.33 ± 0.03 |
| Bone | 0.35 ± 0.01 | 0.18 ± 0.03 | 0.23 ± 0.02 |
Data are expressed as %ID/g (n = 6 mean values ± S.D)
Pretargeting biodistribution in healthy Swiss mice was done at multiple times (Table 2 and 3) in order to define the best schedule for the subsequent phase, namely pretargeting of tumor-bearing Nude mice.
Biodistribution of (SA+B-MORF+B-βAla -BBN) plus 99mTc-MAG3-cMORF by pretargeting system in healthy animals.
| Time (hours) Organs/Tissues | 3 | 5 | 7 | 15 | 24 |
|---|---|---|---|---|---|
| Blood ( %ID/ml) | 5.98 ± 1.26 | 7.96 ±1.04 | 8.89 ± 0.52 | 1.70 ± 0.12 | 0.76 ± 0.34 |
| Heart | 2.23 ± 0.65 | 2.49 ±0.16 | 2.91 ± 0.23 | 0.71 ± 0.17 | 0.28 ± 0.10 |
| Lung | 3.65 ±1.31 | 3.94 ±1.35 | 6.47 ± 1.46 | 1.12 ± 0.42 | 0.67 ± 0.25 |
| Kidneys | 12.66 ± 4.29 | 18.14±5.6 | 17.63 ± 2.02 | 13.9 ± 9.18 | 7.51 ± 1.05 |
| Spleen | 1.44 ± 0.35 | 1.61 ± 0.2 | 2.37 ± 0.31 | 1.01 ± 0.56 | 0.43 ± 0.28 |
| Stomach | 2.35 ± 0.89 | 2.35 ± 0.3 | 2.91 ± 0.38 | 0.65 ± 0.08 | 0.47 ± 0.07 |
| Pancreas | 0.90 ± 0.24 | 1.36 ± 0.2 | 2.04 ± 0.47 | 1.20 ± 1.09 | 0.62 ± 0.52 |
| Liver | 1.97 ± 0.34 | 2.85 ± 0.3 | 3.36 ± 0.61 | 0.85 ± 0.15 | 0.74 ± 0.25 |
| Large Intestine | 2.26 ± 1.27 | 3.4 ± 1.91 | 3.41 ± 0.76 | 0.77 ± 0.12 | 0.77 ± 0.36 |
| Small Intestine | 2.7 ± 1.07 | 3.8 ± 1.04 | 7.69 ± 1.40 | 3.28 ± 0.96 | 2.54 ± 0.38 |
| Muscle | 1.01 ± 0.62 | 1.33 ± 0.7 | 0.92 ± 0.19 | 0.39 ± 0.08 | 0.19 ± 0.03 |
| Bone | 1.98 ± 0.50 | 2.16 ± 0.3 | 2.19 ± 0.06 | 1.67 ± 0.53 | 0.66 ± 0.08 |
Data are expressed as %ID/g (n = 6 mean values ± S.D)
Biodistribution of (SA+B-MORF+B-PEG -BBN) plus 99mTc-MAG3-cMORF by pretargeting system in healthy animals.
| Time (hours) Organs/Tissues | 3 | 5 | 7 | 15 | 24 |
|---|---|---|---|---|---|
| Blood ( %ID/ml) | 7.15 ± 1.98 | 10.90 ± 2.98 | 9.05 ± 4.15 | 1.64 ± 0.07 | 1.22 ± 0.18 |
| Heart | 2.62 ± 0.36 | 2.83 ± 0.75 | 2.76 ± 1.19 | 0.63 ± 0.04 | 0.40 ± 0.10 |
| Lung | 4.63 ± 0.34 | 6.37 ± 3.08 | 5.01 ± 2.63 | 1.01 ± 0.20 | 0.79 ± 0.44 |
| Kidneys | 18.21± 3.69 | 17.76 ± 8.60 | 15.98 ± 8.1 | 9.06 ± 2.11 | 11.89 ± 2.82 |
| Spleen | 1.72 ± 0.18 | 2.38 ± 0.60 | 2.57 ± 0.94 | 0.47 ± 0.01 | 0.48 ± 0.12 |
| Stomach | 2.31 ± 0.73 | 3.18 ± 0.77 | 4.12 ± 1.93 | 0.80 ± 0.34 | 0.72 ± 0.15 |
| Pancreas | 1.36 ± 0.24 | 3.49 ± 0.22 | 3.97 ± 2.39 | 0.50 ± 0.02 | 0.48 ± 0.06 |
| Liver | 2.25 ± 0.07 | 3.09 ± 0.76 | 3.25 ± 1.97 | 0.62 ± 0.06 | 0.79 ± 0.12 |
| Large Intestine | 2.16 ± 0.32 | 6.17 ± 2.93 | 3.55 ± 1.26 | 0.74 ± 0.06 | 1.03 ± 0.13 |
| Small Intestine | 4.76 ± 1.11 | 8.02 ± 3.52 | 6.81 ± 3.67 | 2.47 ± 0.44 | 3.89 ± 1.03 |
| Muscle | 1.46 ± 0.61 | 1.03 ± 0.11 | 0.98 ± 0.46 | 0.26 ± 0.01 | 0.18 ± 0.02 |
| Bone | 3.01 ± 0.50 | 2.35 ± 1.01 | 2.55 ± 1.55 | 0.82 ± 0.06 | 0.69 ± 0.04 |
Data are expressed as %ID/g (n = 6 mean values ± S.D)
In healthy animals blood radioactivity was high for both molecules decreasing only at 15 h (Tables 2 and 3, values in %ID/ml). The numerical increase of blood uptake for both mixtures β-Ala and PEG from 3 to 5 h (P = 0.236 and P = 0.288) as well from 5 to 7 h (P = 0.404 and P = 0.695) was not statistically confirmed. The only difference materizalized for β-Ala from 3 to 7 h (P = 0.028).
Renal excretion was remarkable for both conjugates, without difference (P = 0.69) between them. No difference either could be demonstrated for pancreas uptake of β-Ala and PEG by 7 h (P = 0.33) and 15 h (P = 0.45) (Tables 2 and 3). Lung as well intestinal distribution were also noteworthy for both mixtures.
Uptake by most of organs and tissues was smaller in tumor-bearing animals, (Figures 8, 9) when compared to healthy ones.
Blood uptake of β-Ala decreased at 7 h (P = 0.008) but not of PEG, although a tendency could be perceived (P = 0.08). Kidney changes were not confirmed (P = 0.15).
Highest tumor uptake for βAla was registered at 7 h (2.58±0.59 %ID/g) compared to just 0.97±0.08 %ID/g for PEG (P = 0.013) (Figure 8). Measurements for 15 h were not different from the former ones (Figure 9) (P = 0.22 and P = 0.39) or between them (P = 0.16).
Tumor/blood ratios are low in contrast with tumor/muscle ratios, and both increase after 15 h (0.84 and 0.50 for B-βAla-BBN and B-PEG-BBN respectively). Tumor/muscle ratio for B-βAla-BBN at 7h was 5.95 and for B-PEG-BBN, 2.45, with a difference of 58.8% that remained rather stable till 15 h.
Scintigraphic studies at 7 h unveiled a ROI of 3.9% for B-βAla-BBN and of 1.47% for PEG (Figure 10). Images taken at 15 h were associated with a reduced ROI (1.37% and 1.22 % respectively).
DISCUSSIONThe main focus for using pretargeting system instead of conventional radiotherapeutic techniques is to improve radioimaging and eventually radiotherapy.21 Usually this system is used for large biomolecules such as antibodies due their high molecular weight and slow clearance. To the best of our knowledge, this was the first attempt to combine together a nanoparticle and the morpholino oligomer, aiming at prostate cancer that exhibits receptors for bombesin analogs.
Preliminary HPLC evidence was not favorable, as conjugation of the commercial biotin-morpholino complex was incomplete upon addition of streptavidin. Only 60% was biotinylated. Such finding demanded different molecular ratios in order to achieved adequate results.
Previous studies have already addressed possible strategies to optimize the pretargeting process, such as manipulating the times for both administration of the pretargeting agent and the radiolabeling effector.21
Calculation of MORF and radiolabeled cMORF ratio is another concern. The amount of cMORF must be low for complete hybridization. In this investigation we considered a ratio between 3 and 6 (more precisely 5.65), based on reports of the literature.12
Human prostate tumor PC-3 cells exhibit a large number of GRP receptors on the cell surface. Reile and co-workers22 and Montet et al, 23 have shown that there are as many as 44,000 bombesin receptor sites on PC-3 cells. Nevertheless, high uptake is not granted despite such abundance. In healthy animals the uptake in major organs was low to moderate (<4% ID/g), with the exception of blood, lung, kidneys and intestines, that took up higher proportions.
The increase in blood uptake from 3 to 7 h for conjugated β-Ala was unexpected, but could be due to fluctuations in glomerular filtration, as peptides and small proteins can be reabsorbed in the proximal tubule.24 The length and composition of the spacer group in the molecule can influence clearance of the radiopeptide by either the urinary or hepatobilary system. Its role as known is to keep the metal center and corresponding ligand framework some distance away from the active site of the biomolecule, in order to maintain reactivity in vivo.9 In our study the few difference between the two spacers, nominally β-Ala and PEG, were limited to healthy animals.
High kidney accumulation could be attributed to the large number (six) of cytosines in the base sequence.25 Unfortunately documentation of radioactivity in urine and bladder was lacking because given the long observation period, animals weren’t anesthesized.
No influence by different spacers was observed on renal and intestinal uptake. Usually when hydrocarbon chain length increases, renal excretion decreases, while clearance via the hepatobiliary pathway is enhanced.26
Inhibition of renal uptake can be achieved by a combination of lysine and arginine27 or using a cytosine-free cMORF.25 Such maneuvers could be advantageous for prostate imaging, as they tend to attenuate overlapping activity of the urinary bladder.
The pancreas is another interesting goal for BBN. Schuhmacher et al.28 report that the GRP-receptor density in mice is in the following order: pancreas>tumor>intestine. In our study, pancreatic uptake in healthy animals reached the highest value at 7 h (3.64 ± 1.89 %ID/g for PEG and 2.04 ± 0.47 %ID/g for β Ala). Values were less encouraging in tumor-bearing rodents, but further optimization should be carried out.
The 7 h investigation time was based on pancreatic uptake in healthy animals at 7 h, however by that time blood concentration was already falling. By 15 h tumor/blood ratio was low as well, suggesting that injections earlier than 7 h should be the priority for the future.
La Bella et al,29 labeled BBN with 999mTc-carbonyl, with low uptake in PC-3 tumor-bearing mice (0.89 ± 0.27 %ID/g) but high uptake into the pancreas (7.11 ± 3.93 %ID/g), confirming that discrepancies may exist, thus requiring additional testing.
The highest tumor accumulation, observed for B-βAla- BBN in cell culture was confirmed by highest uptake in tumor-bearing animals for this same spacer.
As a rule, Nude mice displayed lower uptake in main organs and tissues than Swiss controls. Maina et al.30 states that BBN peptides may have different structure-function relationships in different species.
In our previous experience with a standard technique9 findings were roughly comparable to those here achieved. With different isotopes, spacers and chelating agents, even higher uptake than with pretargeting has been occasionally announced.15,18,26,29,31 Nevertheless species differences are not rare,30 and in the case of human cancer irregular responses are quite common,31 with false positives and negatives. Consequently, interpretation of numerical results does not always indicate the true clinical impact of the method. Pretargeting procedures may improve tumor-to nontumor (T/NT) ratio therefore studies with this modality are important and should be continued.14,21,25
Metastases were not part of this protocol, however preliminary observations with 99mTc-bombesin suggest good diagnostic ability in humans.31,32 Indeed nanoparticles seem to exhibit highly desirable features for lymph node imaging. Though currently emphasized agents are liposomes, quantum dots, dendrimers and magnetic nanoparticles, bombesin analogues administered by the pretargeting technique might prove an option as well.33
CONCLUSIONSStreptavidin nanoparticles intended for MORF/cMORF pretargeting may be prepared with peptides such as bombesin as pretargeting agents.
Competition between B-MORF and B-BBN for the 4 sites of Streptavidin was observed. The best combination of the mixture was achieved using the ratio 1:1:2 for SA/B-MORF/B-BBN respectively.
Encouraging tumor uptake was documented at both 7 h and 15 h. The study demonstrates that the pretargeting approach with a peptide is a viable concept, and deserves further evaluation with appropriate modifications in the sequence of MORF, including less cytosine, different pretargeting intervals, and also metastasis models.
AcknowledgmentsThis investigation was supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (Grant 2007/57616-2). Part of this study was conducted at the University of Massachusetts/UMass, Worcester, MA, USA , and appreciation is expressed for the guidance and support of all professors and staff of the Department of Radiology, UMass. Natanael Gomes da Silva was responsible for technical imaging, Angelica Garbuio and Roselaine Campos Targino for cell culture and Maria Neide Ferreira Mascarenhas for animal facilities.