Although individuals with Down syndrome have considerable oral disease, the prevalence of dental caries in this group is low. The present study aimed to compare known risk factors for dental caries development in children with Down syndrome and a matched population (siblings). In both populations, the number of acidogenic microorganisms, such as mutans streptococci, lactobacilli and Candida species, and the paraffin-stimulated pH, flow rate and IgA concentration in whole saliva were evaluated and compared.
METHOD:Saliva was collected, and the caries index was evaluated in 45 sibling pairs aged between 6 and 18 years old. The salivary IgA concentration was determined by immunoturbidimetry. Salivary mutans streptococci, lactobacilli and Candida species were quantified on mitis salivarius agar containing bacitracin and 20% sucrose, rogosa agar supplemented with glacial acetic acid and sabouraud agar supplemented with chloramphenicol, respectively.
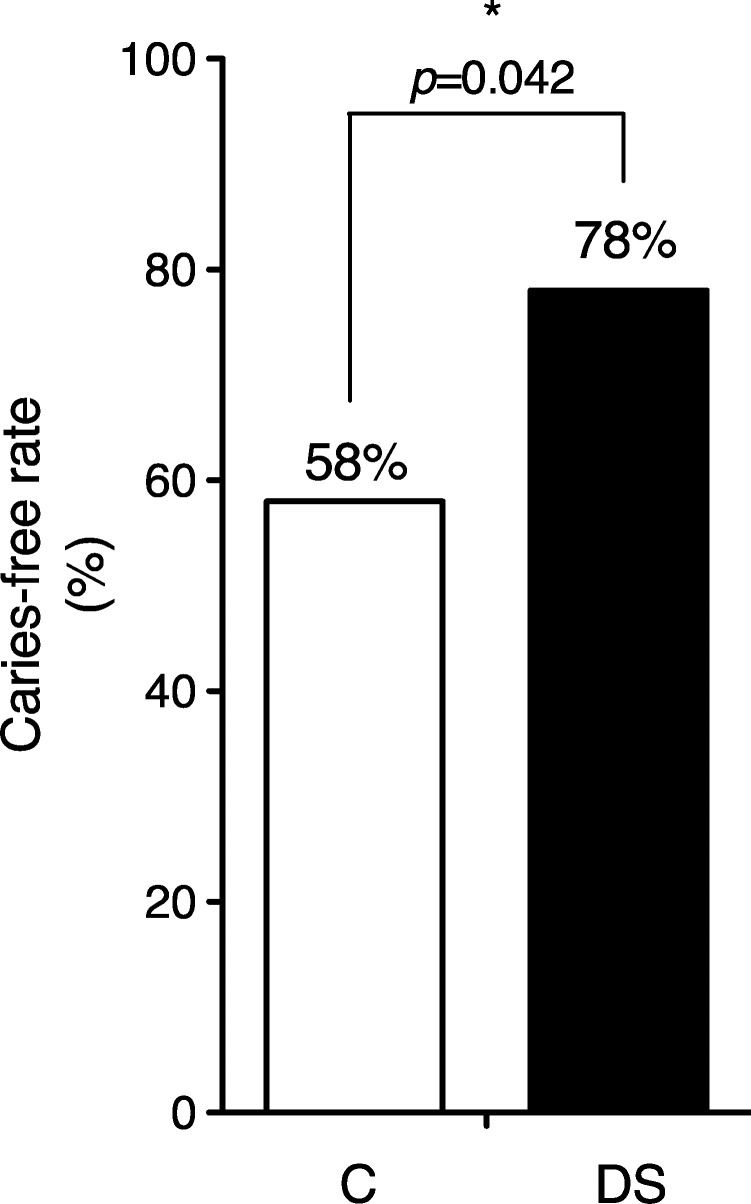
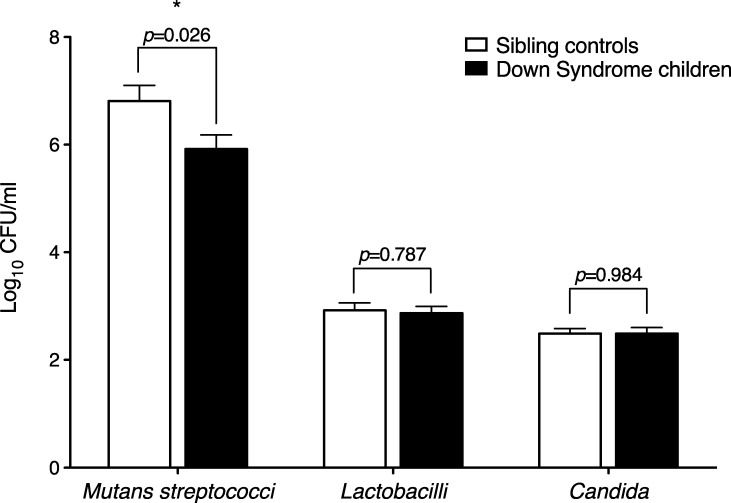
RESULTS:Down syndrome children had a higher caries-free rate (p<0.05) and lower salivary mutans streptococci counts (p<0.03) compared to their siblings. Similar numbers of lactobacilli and Candida species were found in both groups. Salivary flow rates were 36% lower in Down syndrome children compared to their siblings (p<0.05). The salivary pH did not differ between Down syndrome children and controls. The Down syndrome children had an IgA secretion rate 29% lower than that of their siblings, but this difference was not statistically significant.
CONCLUSIONS:In conclusion, the lower number of mutans streptococci in the saliva may be one of the factors contributing to the lower caries rate observed in Down syndrome children, despite evidence of hyposalivation.
Down syndrome (DS) is a genetic disorder resulting from a trisomy of chromosome 21 that leads to multiple oral abnormalities, including malformations of the small palate and maxilla, delayed tooth eruption and dental agenesis (1-3). DS individuals have a significantly higher prevalence of some oral diseases, including periodontal disease, which develops at early age and is rapidly progressive (4,5), and oral candidiasis (6). However, we (7) and others (8-11) have found a lower prevalence of dental caries in DS populations. Possible causes for the lower prevalence of caries may be dietary and other environmental factors, late tooth eruption, and altered salivary flow and/or composition. Mutans streptococci, including Streptococcus mutans and Streptococcus sobrinus, are the primary etiological agents of dental caries in most populations (12), and other acid-tolerant oral bacteria, such as Lactobacillus species, may also be implicated (12). Longitudinal studies have shown that an increase in the numbers of both mutans streptococci and lactobacilli in saliva or plaque over time is associated with caries onset and progression (13,14). Some studies have suggested a link between Candida species and dental caries, particularly in children, adolescents and young adults (15), with a possible active role for C. albicans in caries pathogenesis (16). In addition to microbial factors, several salivary components are also related to caries status. Salivary pH and flow play crucial roles in caries development (17). Secretory immunoglobulin A (IgA) in the saliva is also a local defense factor against caries (18).
The aim of the present study was therefore to compare known risk factors for dental caries development in children with DS and a matched population of siblings. In both populations, the number of acidogenic microorganisms, such as mutans streptococci, lactobacilli, and Candida species, and the paraffin-stimulated pH, flow rate, and IgA concentration in whole saliva were evaluated and compared.
MATERIALS AND METHODSAll DS children aged between 6 and 18 years old included in the Portuguese national database were invited to participate in the study. For each DS child, the sibling closest in age who was living in the same household was used as the matched control. The exclusion criteria included a lack of siblings, non-Caucasian ethnicity, systemic diseases other than DS, and current medication use. Informed consent was obtained from the participants’ parents, who were provided with detailed information on the study protocol. The ethics committee of Faculty of Dental Medicine of Porto University approved the consent form, and the research protocol was developed in accordance with the 1983 revision of the Helsinki Declaration. The present investigation was performed in accordance with European and Portuguese laws.
The final study sample consisted of forty-five Caucasian sibling pairs. Data were gathered using a questionnaire and clinical observations. The present or past history of institutionalization and the dietary habits were assessed. The parents answered the questionnaires of both children. Calibrated examiners carried out dental caries examinations using a mirror and explorer in accordance with the World Health Organization criteria and methods. The total number of decayed, missing and filled primary (dmft) and permanent (DMFT) teeth was recorded for each study patient and control to characterize the epidemiological history of caries in both groups.
We elected to study stimulated saliva because the amount obtained without stimulation was insufficient for the planned biochemical and microbiological analyses. Saliva was collected in a quiet room over a 5-minute period between 8:00 AM and noon to minimize circadian rhythm effects and at least 2 h after eating, tooth brushing, or mouth washing. Salivary secretion was stimulated with paraffin pellets (Ivoclar Vivadent, NY, USA), and the children were asked to spit into a sterile tube. The total amount collected over a 5-minute period was registered, enabling the calculation of the stimulated salivary flow rate (ml/min). The salivary pH was measured immediately after saliva collection using pH indicator paper (5.0-8.0, Duotest, Germany). Saliva for IgA analysis was frozen directly at -80°C, whereas the saliva collected for microbiological analysis was mixed 1:1 with Brain Heart Infusion broth (Cultimed, Barcelona, Spain) with 15% glycerol and then frozen at -80°C until assayed. The saliva samples were rapidly defrosted in a 37°C water bath and mixed well. Salivary IgA was determined by immunoturbidimetry using an automatic analyzer (Pentra C200, Horiba ABX Diagnostics, Switzerland). The IgA secretion rates (μg/min) were calculated by multiplying antibody titers by the salivary flow rate (19).
For the microbiological analyses, the samples were serially diluted to 10-6 with 0.9% sterile NaCl solution and immediately plated in triplicate on the following culture media: mitis salivarius agar containing 0.2 units of bacitracin/ml plus 20% sucrose to detect mutans streptococci; rogosa agar supplemented with 0.13% glacial acetic acid to assess the number of lactobacilli; and sabouraud agar supplemented with chloramphenicol to evaluate the presence of Candida. Mitis salivarius agar and Rogosa agar were incubated anaerobically for seven days at 37°C, and Sabouraud agar was incubated aerobically for 48 h at 37°C. Colonies were counted, and the results were expressed in colony forming units per ml of saliva (CFU/ml). The lower limit of detection was 104 CFU/ml for mutans streptococci and 102 CFU/ml for lactobacilli and Candida.
Statistical analysisThe categorical variables were described as relative frequencies (%), and the continuous variables were described using the mean ± standard deviation (SD). When appropriate, the chi-square independence test or Fisher's exact test were used to analyze hypotheses regarding the categorical variables, and Student’s t-test was used for the continuous variables. A level of 0.05 was considered significant. The analyses were performed using the statistical analysis software SPSS® v.17.0 (Statistical Package for Social Sciences).
RESULTSThe mean age of the DS children was 12.7±4.0 years, and the mean age of the sibling controls was 12.8±3.7 years. The DS group included 49% males, whereas the sibling control group included 60% males. None of the observed children had been institutionalized. The ingestion of a cariogenic diet, i.e., frequency of consuming acidic and sweet foods, was found to be similar between DS children and their siblings (Table 1).
Dietary habits in Down Syndrome (DS) and sibling control (C) children, namely regarding the frequency of acidic or sweet food ingestion.
| C | DS | p-value | |
|---|---|---|---|
| How often did your children consume acidic foods between meals in the last several months? | 0.552 | ||
| Never or once a month | 25 (57%) | 21 (48%) | |
| Once a week | 18 (41%) | 20 (45%) | |
| Once a day or several times a day | 1 (2%) | 3 (7%) | |
| How often did your children consume sweet foods between meals in the last several months? | 0.218 | ||
| Never or once a month | 25 (57%) | 19 (43%) | |
| Once a week | 19 (43%) | 23 (52%) | |
| Once a day or several times a day | 0% | 2 (5%) |
Values are shown in absolute numbers (percentage). p-values were calculated using Fisher's exact test.
The caries-free rate was significantly higher in the DS group than in the sibling group (Figure 1). The epidemiological history of caries in both populations was evaluated based on the dmft and DMFT scores, as shown in Table 2.
The total numbers of decayed, missing and filled primary (dmft) and permanent (DMFT) teeth in Down syndrome (DS) children and sibling controls (C).
| C | DS | p-value | |
|---|---|---|---|
| Total | 1.84±3.13 | 1.02±2.42 | 0.167 |
| Decayed | 0.87±2.12 | 0.44±1.27 | 0.255 |
| Missing teeth | 0.04±0.21 | 0.16±0.67 | 0.293 |
| Filled teeth | 0.93±1.64 | 0.42±1.78 | 0.160 |
| DMFT | 1.42±2.11 | 0.71±1.79 | 0.080 |
| Decayed | 0.56±1.31 | 0.27±0.72 | 0.197 |
| Missing teeth | 0.04±0.21 | 0.11±0.44 | 0.359 |
| Filled teeth | 0.82±1.32 | 0.33±1.33 | 0.084 |
| dmft | 0.42±1.25 | 0.31±0.82 | 0.620 |
| Decayed | 0.31±0.92 | 0.18±0.61 | 0.423 |
| Missing teeth | 0 | 0.04±0.30 | 0.320 |
| Filled teeth | 0.11±0.61 | 0.09±0.47 | 0.847 |
Values are in means±SD.
Table 3 shows the mutans streptococci, lactobacilli and Candida species relative frequencies in saliva samples from the DS children and the sibling controls. All children in both groups had detectable mutans streptococci in the saliva, but some children did not have detectable lactobacilli or Candida. Compared to their siblings, DS children had lower mutans streptococci relative frequencies but similar numbers of lactobacilli and Candida species (Figure 2).
The mutans streptococci, lactobacilli or Candida relative frequencies in saliva samples from Down syndrome (DS) children and sibling controls (C).
| C | DS | p-value | |
|---|---|---|---|
| Mutans streptococci | 100% | 100% | 1.000 |
| Lactobacilli | 73.3% | 82.2% | 0.310 |
| Candida | 77.3% | 59.1% | 0.067 |
p-values were calculated using the chi-square test.
Mutans streptococci, lactobacilli and Candida salivary levels in Down syndrome children and sibling controls. The bars represent the means, and error bars represent the SD. p-values were calculated using the Student’s t-test. ∗Values are significantly different between DS and sibling controls.
Table 4 shows the pH, salivary flow rates and IgA concentrations of sibling controls and DS children. The salivary flow rate was 36% lower in DS children than in their siblings, but the pH did not differ between the two groups (Table 4).
The salivary pH, flow, and IgA concentration of Down syndrome (DS) children and sibling controls (C).
| C | DS | p-value | |
|---|---|---|---|
| pH | 7.33±0.30 | 7.40±0.41 | 0.282 |
| Salivary flow, ml/min | 0.47±0.29 | 0.30±0.24 | 0.046∗ |
| IgA, mg/l | 83.2±36.4 | 79.5±42.2 | 0.677 |
| IgA secretion rate, μg/min | 40.1±39.1 | 28.5±24.9 | 0.125 |
The values shown are the means±SD. p-values were calculated using the Student’s t-test. ∗Values are significantly different between DS and sibling controls.
Total salivary IgA was similar between the DS and sibling groups (Table 4). Although the DS group presented an IgA secretion rate 29% lower than that of their siblings, the difference was not statistically significant (Table 4).
DISCUSSIONThe present study was undertaken to elucidate the factors involved in the lower dental caries prevalence in Down syndrome (DS) children. Compared to their siblings, DS children showed lower counts of mutans streptococci in parallel with a higher caries-free rate, despite evidence of hyposalivation.
Although DS children had a lower dental caries prevalence compared with their siblings, the caries history, evidenced by DMFT/dmft scores, was not significantly different between the two groups.
Only a few previous studies have evaluated the number of Streptococcus mutans bacteria in DS children. Some authors have found non-statistically reduced levels (11), while others have found increases (20,21) in comparison to non-DS children. However because oral microbial colonization is strongly correlated with diet, oral hygiene, and familial pre-disposition, the recommended control group should be a non-DS sibling age-matched group, as used in the present study, rather than unrelated healthy children. In a previous study, we showed that oral hygiene habits were similar between DS children and their siblings (7). Given that the remaining methodology in the present work was similar to that of previous studies, the differences are likely to be related to the different control groups used.
Regarding dietary habits, many epidemiological studies have linked the incidence of caries and food habits, identifying both protective and exacerbating factors. The consistency, viscosity, cooking process, and even the time of ingestion of food containing simple carbohydrates change the cariogenicity of a food. Although the main meals were shared between DS and their siblings, the healthy controls could have different dietary habits because they can more easily buy candy or snacks independently. Interestingly, the consumption of sweet and acidic foods was similar between the DS children and their siblings. Therefore, diet cannot be responsible for the differences found regarding the caries prevalence in these two groups.
Furthermore, higher C. albicans counts have been reported in DS children in comparison to healthy controls (22), as have candidal infections (6). Again, the differences we found could be related to the control group used. We, like Mathias et al. (21), did not find any differences in Lactobacillus counts between DS children and healthy controls.
There are conflicting reports regarding the salivary pH of individuals with DS in comparison to controls; no difference (8,10,11,23), higher pH (24) and a lower pH (25) have been observed. We believe that the conflicting reports in the literature about the pH levels in DS may be attributed to different measurement methods. The present study, similar to most studies, measured the pH of saliva collected intra-orally and analyzed extra-orally. Some authors believe that this method may be inaccurate because whole saliva does not represent the intra-oral micro environment, the buffering system may change once saliva is removed from the oral cavity, and the pH level of the salivary film covering the soft and hard tissues may not correspond precisely to that of the secreted saliva (8,11,23,26). However, measuring pH intra-orally, especially in cognitively and physically impaired children, such as DS children, is challenging and also raises ethical issues. Further studies evaluating the different pH measurement methods available for saliva are needed.
The stimulated salivary flow of the sibling controls was lower than the common mean value for stimulated salivary flow in adults (∼1.2 ml/min) (27). This result could be explained by the wide range of values characteristic of salivary flow and by the fact that children present lower salivary flow rates than adults (28). In DS individuals, most other researchers (19,25), although not all (8), have also reported reductions in salivary flow. The lower salivary flow of DS children could in theory increase their caries susceptibility (12,17), but other factors clearly favor these children in a caries-protective manner. In addition, the lower salivary flow observed in DS children prompted us to study stimulated saliva instead of unstimulated saliva, given that the amount obtained without stimulation would have been insufficient to perform all laboratory analyses.
Salivary IgA may play role in the immune defense against dental caries (29). IgA antibodies may neutralize extracellular enzymes and reduce the initial adherence of bacteria (30) by inhibiting sucrose-independent or sucrose-dependent streptococcal accumulation on tooth surfaces (31). Chaushu et al. (19) reported deficient secretory immunity in the oral cavity of DS individuals. In the present study, the DS children had less secreted salivary IgA, but the difference was not statistically significant. This result may reflect an early phase of immunodeficiency in young DS individuals. Thus, although DS children present with hyposalivation and a lower IgA concentration, they have a lower rather than a higher caries prevalence, suggesting that the low counts of mutans streptococci may contribute more to reduced caries prevalence than do the blunted salivary flow or lower IgA secretion.
In conclusion, the lower number of mutans streptococci in the saliva may be one of the factors contributing to the lower caries rate observed in DS children, despite evidence of hyposalivation. Further studies are clearly indicated.
The Faculty of Dental Medicine of the University of Porto, Portugal, supported this investigation.
No potential conflict of interest was reported.
Areias C designed the clinical study and the laboratory approach; performed the clinical oral evaluation and the laboratory procedures; discussed the results and wrote the manuscript. Sampaio-Maia B designed the clinical study and the laboratory approach; performed the laboratory procedures and the statistical analyses; discussed the results and wrote the manuscript. Melo P designed the clinical study and the laboratory approach and performed the clinical oral evaluation. Andrade C designed the clinical study and the laboratory approach and discussed the results. Pereira ML performed the statistical analyses and discussed the results. Azevedo A performed the statistical analyses. Scully C discussed the results and wrote the manuscript.