Results from our laboratory have demonstrated that intracerebroventricular administration of sildenafil to conscious rats promoted a noticeable increase in both lumbar sympathetic activity and heart rate, with no change in the mean arterial pressure. The intracerebroventricular administration of sildenafil may have produced the hemodynamic effects by activating sympathetic preganglionic neurons in the supraspinal regions and spinal cord. It is well documented that sildenafil increases intracellular cGMP levels by inhibiting phosphodiesterase type 5 and increases cAMP levels by inhibiting other phosphodiesterases.
OBJECTIVE:To examine and compare, in conscious rats, the hemodynamic response following the intrathecal administration of sildenafil, 8-bromo-cGMP (an analog of cGMP), forskolin (an activator of adenylate cyclase), or dibutyryl-cAMP (an analog of cAMP) in order to elucidate the possible role of the sympathetic preganglionic neurons in the observed hemodynamic response.
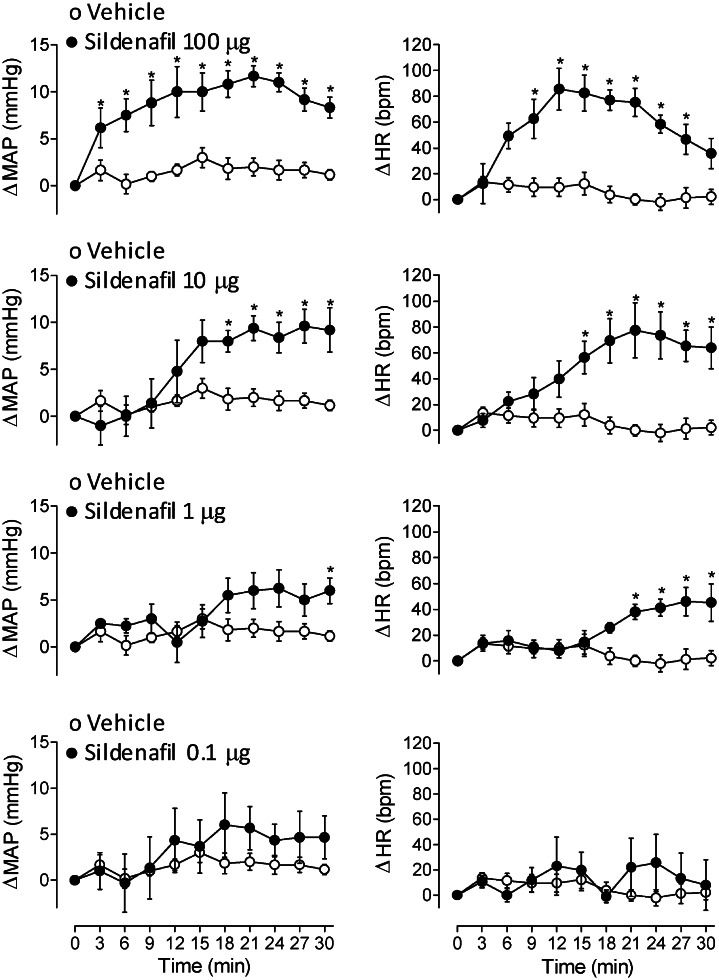
RESULTS:The hemodynamic responses observed following intrathecal administration of the studied drugs demonstrated the following: 1) sildenafil increased the mean arterial pressure and heart rate in a dose-dependent manner, 2) increasing doses of 8-bromo-cGMP did not alter the mean arterial pressure and heart rate, 3) forskolin did not affect the mean arterial pressure but did increase the heart rate and 4) dibutyryl-cAMP increased the mean arterial pressure and heart rate, similar to the effect observed following the intrathecal injection of the highest dose of sildenafil.
CONCLUSION:Overall, the findings of the current study suggest that the cardiovascular response following the intrathecal administration of sildenafil to conscious rats involves the inhibition of phosphodiesterases other than phosphodiesterase type 5 that increase the cAMP level and the activation of sympathetic preganglionic neurons.
Previous results from our laboratory have demonstrated that the administration of sildenafil into the left lateral ventricle of conscious rats elicited a marked increase in the lumbar sympathetic activity associated with tachycardia but no change in the arterial pressure.1 The intracerebroventricular administration of sildenafil may have produced sympathetic overactivity in the spinal cord, thereby activating sympathetic preganglionic neurons (SPNs) in addition to supraspinal areas, such as the rostral ventrolateral medulla, circumventricular organs (subfornical organ, organum vasculosum lamina terminalis, and area postrema) and hypothalamic sites controlling the sympathetic drive.2–5 Thus, the increase in lumbar sympathetic activity and tachycardia observed in conscious rats following the administration of sildenafil into the left lateral ventricle may be explained by an effect in the spinal cord that is probably mediated by SPN activation.1 Therefore, in order to test the hypothesis that sildenafil is able to directly activate the SPNs and cause a hemodynamic response, the first goal of the present study was to examine, in conscious rats, the effects of sildenafil on the mean arterial pressure (MAP) and heart rate (HR) following intrathecal administration.
Sildenafil increases intracellular cGMP levels via inhibition of phosphodiesterase type 5 (PDE5).6–11 Moreover, sildenafil can inhibit other phosphodiesterases, including those that preferentially degrade cGMP, cAMP, or both depending on the dose. The inhibition of phosphodiesterases leads to an increase in the intracellular cAMP level.12–15 In anesthetized rats, a dose-dependent increase in the MAP following the intrathecal administration of an cGMP analog (8-bromo-cGMP) has been observed. A 8-bromo-cGMP-dependent increase in the MAP suggests that the mechanism underlying this increase involves cGMP.16
As the hemodynamic response caused by sildenafil may involve a lack of cGMP degradation, the second goal of this study was to evaluate the hemodynamic effects of the intrathecal administration of 8-bromo-cGMP in conscious rats and to compare the results with the sildenafil-induced hemodynamic response. Moreover, several studies have proposed that sildenafil, by itself, increases the levels of cAMP.13–15 After taking this idea into account, the final objective of the present study was to examine the cardiovascular response of conscious rats following the intrathecal administration of forskolin, a direct activator of adenylate cyclase that increases the intracellular levels of cAMP, and dibutyryl-cAMP, an analog of cAMP, and to compare the results with the sildenafil-induced response.
MATERIAL AND METHODSAnimalsAll experiments were performed on male Wistar rats (320±30 g) housed individually with free access to food and water. The rats were maintained on a 12:12 h light-dark cycle. The experimental protocols used in this research were approved by the Committee of Ethics in Animal Research of the School of Medicine of Ribeirão Preto, University of São Paulo (protocol 199/2008).
Surgical proceduresThe rats were anesthetized with tribromoethanol (250 mg/kg, i.p., Sigma Aldrich) and underwent a two-stage surgery. Initially, a polyethylene cannula PE-10 (∼15 cm long, total volume 10 μL) filled with heparinized saline (pH 7.4) was inserted through the intervertebral space (L5-L6) into the spinal subarachnoid space in a cranial direction (2 cm). The cannula was then implanted using the method reported by Prado et al.17 with a slight modification developed by Storkson et al.18 Once the procedure was completed, the animals were allowed five days to recover. On the day before the experiment, polyethylene tubing was inserted into the left femoral artery for direct measurement of the arterial pressure. The experiments were performed in conscious rats 24 hours after femoral artery catheterization.
Measurement of arterial pressureDuring the experiments, silence was maintained to avoid undue stress on the animals. The pulsatile arterial pressure was recorded by connecting the arterial catheter to a pressure transducer (P23Gb, Statham, Hato Hey, PR) and was continuously sampled (2 kHz) using an IBM/PC equipped with an analog-to-digital interface (DI-220 Dataq Instruments, Akron, OH). The pulsatile arterial pressure recordings were then analyzed using the CODAS software (Dataq Instruments, Akron, OH) to obtain the MAP and HR values.
Experimental protocolsThe animals received an single intrathecal dose (10 μL) of sildenafil (100 μg, n = 7; 10 μg, n = 6; 1 μg, n = 4; 0.1 μg, n = 3), 8-bromo-cGMP (0.03 μg, n = 3; 0.47 μg, n = 6; 1.4 μg, n = 7), forskolin (100 μg, n = 7), dibutyryl-cAMP (100 μg, n = 4) or vehicle (1% DMSO, n = 6). The drugs and vehicle (10 μL) were administered via the intrathecal cannula using a Hamilton microsyringe. After drug administration, an additional 10 μL of aCSF was injected to clear the catheter of the drug solution. At the end of the experiment, 10 μL of Evan's blue was injected into the subarachnoid space. The rats were then anesthetized with tribromoethanol (250 mg/kg, i.p.) and perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffered saline. Finally, a necropsy was performed to confirm the position of the intrathecal catheter and to determine how far the dye spread within the subarachnoid space.17,19
DrugsThe drugs used in the current study were sildenafil citrate (Pfizer), 8-bromo-cGMP (8-bromoguanosine 3′,5′-cyclicomonophosphate sodium salt), dibutyryl-cAMP (N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt), and forskolin (Sigma-Aldrich). The vehicle used was DMSO (dimethyl sulfoxide). The components of the aCSF were 127 mM NaCl, 1.9 mM KCl, 1.2 mM KH2PO4, 2.4 mM CaCl2, 1.3 mM MgSO4, 26 mM NaHCO3 and 10 mM D-glucose (Sigma-Aldrich). All drugs were dissolved in 1% DMSO immediately before administration, and the pH of the drug solutions was adjusted to 7.4.
Statistical analysisThe results are expressed as the mean ± SEM. Comparisons were performed using one-way and two-way analysis of variance (ANOVA) tests followed by a post-hoc Newman-Keuls test. The statistical differences were considered significant at p<0.05.
RESULTSBasal MAP and HR.The baseline MAP and HR in rats that received an intrathecal administration of sildenafil, 8-bromo-cGMP, forskolin, dibutyryl-cAMP, or vehicle (DMSO) were similar (Table 1).
Effects of intrathecal administration of sildenafil on the MAP and HRIntrathecal sildenafil administration elicited a dose-dependent increase in the MAP and HR (Figure 1). Although the maximal pressor and tachycardic response induced by 100 and 10 μg of sildenafil were similar, there was a considerable delay in the maximal response with the lower dose.
Effects of intrathecal administration of 8-bromo-cGMP on the MAP and HR.The intrathecal administration of different doses of the membrane-permeable analogue of cGMP, 8-bromo-cGMP or its vehicle, DMSO, did not affect the MAP or HR (Figure 2).
Effects of intrathecal administration of forskolin and dibutyryl-cAMP on the MAP and HR.The administration of forskolin did not affect the MAP but did increase the HR, while the membrane-permeable cAMP analog, dibutyryl-cAMP, induced a rapid and significant increase in both the MAP and HR (Figure 3).
DISCUSSIONThe major findings of this study indicate that the intrathecal administration of sildenafil in conscious rats results in a dose-dependent increase in the MAP combined with tachycardia that is unrelated to cGMP action as the administration of a cGMP analog (8-bromo-cGMP) had no effect. In addition, a direct activator of adenylate cyclase (forskolin) increased the HR but did not affect the MAP. In contrast, a cAMP analog (dibutyryl-cAMP) increased the MAP and HR, similar to the increase observed following the administration of 100 μg sildenafil.
One possible explanation for the results observed following the intrathecal administration of sildenafil is the activation of SPNs located in the intermediolateral column of the spinal cord that predominantly innervate the heart and blood vessels. The hemodynamic response obtained with 10 and 1 μg of sildenafil indirectly support this hypothesis, as the increase in the MAP and HR was delayed when compared to results observed after the administration of 100 μg of the drug. The magnitude of the hemodynamic response was comparable even though the doses differed. A possible explanation for this delay may be that lower doses of sildenafil require a longer time to achieve the effective concentration required for the activation of SPNs located deep inside the spinal cord. In fact, an increase in sympathetic activity has been observed in humans after only a single dose of sildenafil.20,21 In addition, a study recently conducted in our laboratory1 has indicated that the intracerebroventricular administration of sildenafil in rats causes a noticeable increase in lumbar sympathetic activity without an accompanying change in the MAP.
To fully understand the implications for human health, the hemodynamic effects caused by the intrathecal administration of sildenafil in conscious rats observed in this study necessitate comparison with similar studies in humans. In a study by Philips et al.20 in which healthy males received oral sildenafil, sildenafil had no effect on the MAP and HR but did elicit a significant increase in muscle sympathetic nerve activity. Despite the absence of hemodynamic changes (arterial pressure and heart rate), the findings of the previous researchers20 demonstrate that sildenafil was able to promote sympathetic activation. However, a study recently conducted by our laboratory1 demonstrated that intracerebroventricular administration of sildenafil (100 μg/5 μL) results in a noticeable increase in lumbar sympathetic activity without an accompanying change in the MAP. This contrasts with the significant tachycardia observed in the aforementioned Philips study.20
In the current study, the MAP and HR were unaffected by the intrathecal administration of increasing doses of 8-bromo-cGMP. Conversely, Malik et al.16 performed intrathecal administration of 8-bromo-cGMP in anesthetized rats, with the same doses used in the present study, and observed a dose-dependent increase in the MAP. However, it should be noted that the results of the current study were obtained using conscious rats, without the use of anesthesia and its associated detrimental effects. One possible explanation for the absence of any cardiovascular effects following the 8-bromo-cGMP injection is that this compound can act directly on high conductance potassium channels in the cell membrane, thereby promoting hyperpolarization of the cell and decreasing their excitability.22 Therefore, the possible excitatory effect of 8-bromo-cGMP could be antagonized by its direct action on potassium channels, masking any possible activation of SPNs and blunting the hemodynamic response. Finally, it is reasonable to hypothesize that the increase in the MAP and HR caused by the intrathecal administration of sildenafil are not mediated by cGMP. There is evidence that sildenafil can interfere with cAMP activity and inhibit the activity of other PDEs.12–15 It is well known that drug selectivity, dose and route of administration, distribution, pharmacokinetics and the extent of activation of the NO-cGMP pathway are important mechanisms determining the functional role of sildenafil.23 Bischoff12 observed that sildenafil has different inhibitory activity against the 11 families of PDEs. It is possible that the dose of sildenafil used in the present study inhibited not only PDE5 but also other PDEs, including those PDEs that preferentially degrade cGMP, cAMP, or both. Therefore, the additional treatments used in the current study aimed to verify a possible role of cAMP in the hemodynamic response promoted by sildenafil. To accomplish this, the rats were intrathecally injected with a direct activator of adenylate cyclase (forskolin) or a membrane-permeable analogue of cAMP (dibutyryl-cAMP). The intrathecally injected forskolin had no effect on the MAP but increased the HR. However, the intrathecally injected dibutyryl-cAMP increased the MAP and the HR, an effect similar to sildenafil, suggesting that cAMP may be involved in the mechanism underlying the cardiovascular effects of sildenafil. Uckert et al.14 observed that both forskolin and sildenafil administered independently caused the relaxation of isolated human corpus cavernosum tissue. However, this effect was reversed by PKA-I (a cAMP-dependent kinase inhibitor) and PKG-I (a cGMP-dependent kinase inhibitor) in both cases. These findings suggest that cross-regulation may exist between the cyclic nucleotides cAMP and cGMP. The results of the current study are in line with those of Stief et al.,24 who observed that the administration of increasing concentrations of sildenafil in isolated human corpus cavernosum tissue did not modify the cGMP levels in the tissue, although it did increase the cAMP levels. Similarly, increasing concentrations of sildenafil administered to isolated human heart muscle did not alter the cGMP levels, although an increase in the cAMP levels was evident.24 In addition, the findings of Stief et al.24 demonstrate that milrinone, a PDE3-specific inhibitor, causes an increase in the intracellular cAMP levels without altering the cGMP levels, as PDE3 preferentially degrades cAMP. In addition, Botha et al.25 verified that while the serum levels of cAMP increase after the administration of two different doses of sildenafil and milrinone, the serum levels of cGMP are not altered.
Overall, the results of the current study strongly suggest that the pressor response and tachycardia observed after the intrathecal administration of sildenafil to conscious rats involves the inhibition of non-PDE5, cAMP-increasing phosphodiesterases that activate sympathetic preganglionic neurons. The hemodynamic response elicited by the intrathecal administration of sildenafil to conscious, freely moving rats may aid in understanding the cardiovascular outcomes associated with the clinical use of this drug.
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).