The present study was designed to further investigate the effect of amitriptyline, a classical tricyclic antidepressant, on carrageenan-induced paw edema in rats.
METHODS:First, amitriptyline was administered intraperitoneally (i.p.) at doses of 20, 40 and 80 mg kg-1, 30 min before subplantar injection of carrageenan. Second, amitriptyline was given intracerebroventriculary or intrathecally at doses of 25, 50 and 100 µg/rat, 30 min prior to carrageenan challenge. Third, the effect of adrenergic receptor antagonists such as propranolol (10 mg kg-1, i.p.), prazosin (4 mg kg-1, i.p.) and yohimbine (10 mg kg-1, i.p.) and an opioid receptor antagonist (naloxone, 4 mg kg-1, i.p.) on the anti-inflammatory effect of amitriptyline (40 mg kg-1, i.p.) was investigated.
RESULTS:Our data confirm that intraperitoneally administered amitriptyline exhibits a marked anti-inflammatory effect on carrageenan-induced paw edema in rats 4 h postcarrageenan challenge (P < 0.001). Intracerebroventricular (i.c.v.) administration of amitriptyline also reduced the development of paw edema at 4 h postcarrageenan (P < 0.001), but intrathecal (i.t.) application of amitriptyline failed to alter the degree of paw swelling. Furthermore, the applied antagonists did not modify the anti-inflammatory effect of amitriptyline.
CONCLUSION:These results support the view that amitriptyline has a considerable anti-inflammatory effect on carrageenan-induced paw edema in rats and suggest that at least a part of this property could be mediated through supraspinal sites. Moreover, it seems unlikely that the investigated adrenergic and opioid receptors have a significant role in this effect of amitriptyline.
Antidepressants, particularly conventional tricyclic antidepressants (TCAs) such as amitriptyline, nortriptyline, and doxepin, are commonly prescribed for alleviating various types of pain, involving both inflammatory (rheumatoid arthritis and fibromyalgia) and neuropathic pain (neuropathic pain and postherpetic neuralgia), besides their use in the treatment of mood disorders.1–3
Recently, the anti-inflammatory effects of some antidepressants such as fluoxetine, trazodone, and desipramine, have been established in several experimental models of inflammation.4–7 Imipramine and clomipramine induce apoptosis in human lymphocytes, and fluoxetine suppresses lymphocyte proliferation in a dose-dependent manner.8,9 These findings warrant more research into the anti-inflammatory effect of antidepressants for a number of reasons. First, the exact mechanism of analgesic activity of antidepressant medications is not completely understood. Thus, a greater knowledge of the anti-inflammatory effects of antidepressants may result in better insights into their antinociceptive mechanisms. Second, the etiology of depression is not well understood, and some studies suggest that dysregulation in the immune system functioning and the secretion of proinflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α may have a significant role in the pathogenesis of major depression.10 Therefore, if antidepressants alter the function of immune system, this effect may represent a new prospect for explaining the efficacy of antidepressants in the treatment of major depression.11–14 Third, major depression is a common disorder in the general population and is even more common in people who are suffering from inflammatory diseases such as Crohn's disease and asthma.15,16 Thus, information about the anti-inflammatory activities of antidepressants would be useful for determining the best drug in these situations.
Amitriptyline, a dual serotonergic and noradrenergic reuptake inhibitor (SNRI), is widely used in the management of major depression and different types of pain, including neuropathic pain or migraines.17-21 Abdel-Salam and co-workers found that amitriptyline produces an anti-inflammatory effect on carrageenan-evoked paw swelling in rats,4 but there is no information about the sites of this action. Therefore, the purpose of the present study was to investigate the anti-inflammatory properties of amitriptyline further and to understand the function of central sites in these actions. We also tried to determine the possible involvement of some adrenergic and opioid receptors in the anti-inflammatory effect of amitriptyline, using the antagonists of these receptors.
MATERIALS AND METHODSChemicalAmitriptyline hydrochloride was donated by Iran Daru Pharmaceutical Co. (Tehran, Iran) and was dissolved in isotonic saline. Carrageenan was purchased from Fluka Chemical (Switzerland) and was also dissolved in isotonic saline. Indomethacin (Sigma, USA) was suspended in aqueous carboxy methylcellulose (2% w/v). Propranolol, prazosin, yohimbine (Sigma, USA), and naloxone (Tolid Drau Co., Tehran, Iran), were dissolved in isotonic saline.
AnimalsMale Wistar rats (200–250 g) were obtained from the animal house of the Faculty of Pharmacy, Isfahan University of Medical Sciences, Iran. Animals were housed in standard polypropylene cages, four per cage, under a 12∶12 h light/dark cycle with free access to food and water. Following surgical implantation of an i.c.v. cannula, animals were housed one per cage to avoid possible dislocation of the cannula. The experiments were carried out in accordance with local guidelines for the care of laboratory animals of the Isfahan University of Medical Sciences.
Surgical proceduresThe animals were anesthetized with i.p. injection of a ketamine (50 mg kg-1) and xylazine (10 mg kg-1) mixture. Then, the animals were placed in a stereotaxic frame (Stoelting, USA), and an i.c.v. cannula was implanted with stereotaxic coordinates: Anterior-posterior (AP), -0.8 mm; L, 1.4 mm; and V, 3.3 mm, according to Paxinos and Watson.22 The animals were handled daily for five days before the experiment to acclimatize them to manipulation and minimize nonspecific stress responses. Rats with the i.c.v. cannulas were euthanized at the end of the experiments, and their brains were examined to confirm the correct implantation of the cannula.
Intrathecal injectionDrug injections at the lumbar level of the spinal cord were performed according to the method previously described by Mestre et al.23 Briefly, the animals were gently constrained by the hands of experimenter, and a 27-gauge needle was carefully inserted between the L5-L6 spaces. A flick of the tail indicated that the spinal cord channel was reached, and the injections were given in a volume of 10 µl. As a rule, we did not try the spinal injection twice, and the animals that did not show the tail reflex at the first injection effort were discarded. All intrathecal injections of amitriptyline or vehicle (control group) were made 30 min before the carrageenan injection.
Carrageenan-induced paw edemaThe rats received a subplantar injection of 100 µl of a 1% (w/v) suspension of carrageenan lambda in the right hind paw.24 The volume of the paw was measured by a Plethysmometer (Ugo Basile, Italy) immediately prior to carrageenan injection and then 4 h afterwards. The data were expressed as the variation in the paw volume (ml) and were compared to preinjection values.
Experimental designIn the first series of experiments, the effect of i.p. amitriptyline (20, 40 and 80 mg kg−1, n = 6) on paw edema was studied. Amitriptyline was given 30 min before subplantar injection of carrageenan. The control group received only vehicle (n = 6; i.p.). A group of animals that was pretreated with indomethacin (n = 6; 10 mg kg-1) was used as the positive control.
In the second series, we used the i.c.v. route to evaluate the possible involvement of the supraspinal level in the anti-inflammatory effect of amitriptyline. The drug was administered smoothly for 1 min through the injection cannula (25, 50 and 100 µg per rat, n = 8) 30 min prior to carrageenan in a volume of 10 µl. The control group received vehicle (n = 8; i.c.v).
In the third series, amitriptyline was injected into the space between L5-L6 (25, 50 and 100 µg per rat, n = 6) 30 min prior to carrageenan in a volume of 10 µl. The control group received vehicle (n = 6; i.t.).
In the fourth series of experiments, we assessed the possible involvement of opioid and some important adrenergic receptors in the inhibitory activity of amitriptyline on carrageenan-induced paw edema. The animals were pretreated with propranolol (10 mg kg-1, i.p., a nonselective β-adrenoreceptor antagonist), yohimbine (10 mg kg-1, i.p., an α2-adrenoreceptor antagonist) and prazosin (4 mg kg-1, i.p., an α1-adrenoreceptor antagonist) 30 min prior to i.p. injection of amitriptyline (40 mg kg-1). Naloxone (4 mg kg-1, i.p., a nonselective opioid receptor antagonist) was coadministrated with amitriptyline. These doses were chosen based on previous studies.25–27
Statistical analysisThe data are expressed as the means ± S.D. The differences between the control and treatment groups were tested by one-way analyses of variance (ANOVA) followed by the Tukey post-hoc test, using SPSS 13.0 software. A probability of P< 0.05 was used as the significance threshold for all comparisons.
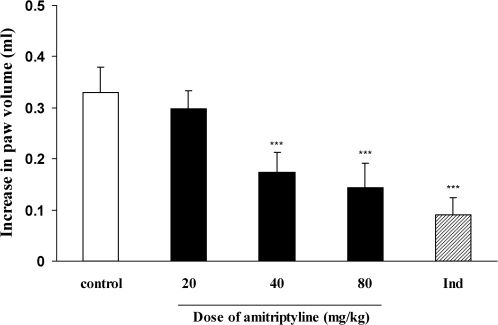
RESULTSEffect of i.p. injection of amitriptyline on carrageenan-induced paw edemaAs illustrated in Figure 1, i.p. injection of amitriptyline at doses of 40 and 80 mg kg-1 significantly inhibited the development of paw edema 4 h after the induction of inflammation (P < 0.001) as compared to the control group. As expected, the reference drug, indomethacin (10 mg kg-1), caused a significant inhibition of edema 4 h post-carrageenan (P < 0.001).
Effect of i.p. injection of amitriptyline on carrageenan-induced paw edema in rats. Amitriptyline (20, 40 and 80 mg kg-1), indomethacin (10 mg kg-1) and the vehicle were administrated 30 min prior to carrageenan (1%) injection, and the rats were evaluated for paw edema 4 h post-carrageenan injection. The values represent the mean variation in the paw volume ± S.D. (n = 6, ∗∗∗ P < 0.001). Ind: indomethacin.
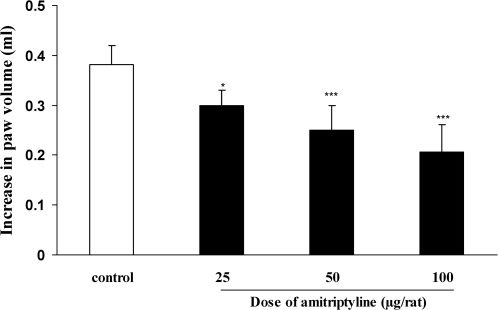
As shown in Figure 2, i.c.v. application of amitriptyline (25, 50 and 100 µg per rat) produced a noticeable inhibitory effect on paw edema formation in a dose-dependent manner (P < 0.001) as compared to the control group.
Effect of intracerebroventricular injection of amitriptyline on carrageenan-induced paw edema in the rats. Amitriptyline or the vehicle was administrated 30 min prior to carrageenan (1%) injection, and the rats were evaluated for paw edema 4 h post-carrageenan injection. The values represent the mean variation in the paw volume ± S.D. (n = 8, ∗ P < 0.05, ∗∗∗ P < 0.001).
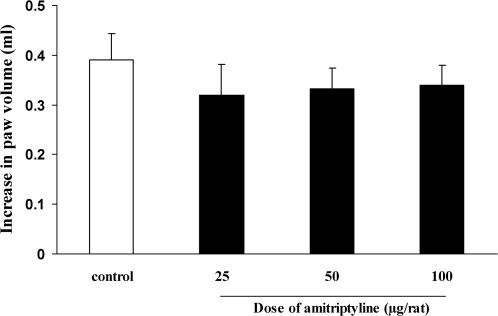
As illustrated in Figure 3, i.t. administration of amitriptyline through the i.c.v. route (25, 50 and 100 µg per rat) did not cause any change in the paw edema 4 h post-carrageenan as compared to the control group.
Lack of effect of intrathecal injection of amitriptyline on carrageenan-induced paw edema in the rats. Amitriptyline or the vehicle was administrated intrathecally 30 min prior to carrageenan (1%) injection, and the rats were evaluated for paw edema 4 h post-carrageenan. The values represent the mean variation in the paw volume ± S.D. (n = 6).
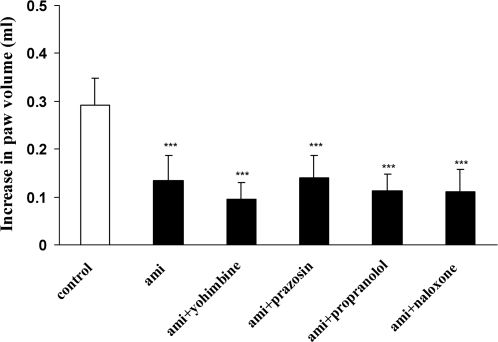
As shown in Figure 4, the pretreatment of animals with propranolol (10 mg kg-1, i.p.), yohimbine (10 mg kg-1, i.p.), and prazosin (4 mg kg-1, i.p.) 30 min prior to amitriptyline (40 mg kg -1, i.p.) did not alter the anti-inflammatory activity of amitriptyline. Additionally, coadministration of naloxane (4 mg kg-1, i.p.) with amitriptyline (40 mg kg -1, i.p.) did not modify the inhibitory effect of amitriptyline on the degree of paw swelling.
Effect of pretreatment with yohimbine (10 mg kg -1), prazosin (4 mg kg -1) and propranolol (10 mg kg -1) on the anti-inflammatory activity of amitriptyline (40 mg kg-1). Naloxone (4 mg kg -1) was coadministrated with amitriptyline (40 mg kg-1). The values represent the mean variation in the paw volume ± S.D. (n = 6, ∗∗∗ P < 0.001). ami: amitriptyline.
In the present study, we confirmed that i.p. injection of amitriptyline produces a noticeable anti-inflammatory effect on carrageenan-induced paw edema in rats, and this effect was found to be comparable to that observed with the reference drug indomethacin. Our results are in good agreement with the research of Abdel-Salam and co-workers,4 although, in our study, the anti-edematogenic effect of amitriptyline was observed at higher doses than in the earlier study, a fact that may be due to the different experimental conditions used.
The well-known theory of the antidepressive effect of amitriptyline states that the drug modulates the synaptic concentrations of monoamine neurotransmitters such as norepinephrine and serotonin in the central nervous system (CNS).28,29 Therefore, an important aspect investigated herein was the possible involvement of central sites in the anti-inflammatory effect of amitriptyline. Our results revealed that i.c.v. application of amitriptyline is effective in reducing inflammation in a dose-dependent manner. Indeed, this is the first work to show that i.c.v injection of amitriptyline produces an anti-inflammatory effect in experimental models of inflammation. In this context, in our previous study, we found that i.c.v. injection of maprotiline, an atypical antidepressant acting as a selective norepinephrine reuptake inhibitor, reduces the development of paw edema in rats.30 Thus, it seems plausible that supraspinal sites have an important role in the anti-inflammatory properties of antidepressant medications. Furthermore, the anti-inflammatory effect observed after i.c.v. injection of amitriptyline could not be attributed to diffusion of the drug from the central sites into peripheral sites because the doses of amitriptyline that were effective via the i.c.v. route were not able to suppress paw swelling after i.p. injection (data not shown).
In several studies, it has been reported that the spinal cord is a site for modulation of neurogenic elements of peripheral inflammation,31,32 but in this study, the spinal pretreatment of animals with the doses of amitriptyline that were effective in reducing paw edema via i.c.v. application failed to alter the development of paw edema. Thus, it seems unlikely that the spinal levels participate in this effect of amitriptyline. Consistent with this finding, Sawynok and Reid reported that i.t. administration of amitriptyline does not influence the paw edema produced by injection of formalin into the hind paw in rats.33
The underlying mechanisms by which antidepressants reduce inflammation remain unknown, although it has been suggested that the suppression of some inflammatory mediators such as substance P (SP) and prostaglandin E2 might participate in the anti-inflammatory effects of clomipramine and fluoxetine.5 Abdel-Salam et al. found that the anti-inflammatory effect of fluoxetine, as a selective serotonin reuptake inhibitor (SSRI) antidepressant, is partially reduced by coadministration of naloxone.25 Because it has been well established that amitriptyline interacts with opiate receptors to generate some of its therapeutic effects, especially its analgesic activities, 34,35 we evaluated the role of opioid receptors in the anti-inflammatory activity of amitriptyline. In this regard, the inhibitory effect of amitriptyline on paw edema was not influenced by the coadministration of naloxone, indicating that opioid receptors are not involved in this effect of amitriptyline.
Moreover, there is evidence that the anti-inflammatory effect of TCA drugs can be attributed to their ability to potentiate adrenergic transmission,36 but in our experimental conditions, the pretreatment of animals with some adrenergic receptor antagonists, including propranolol, yohimbine and prazosin, did not modify the anti-inflammatory effect of amitriptyline. Thus, these observations did not provide a link between the anti-inflammatory action of amitriptyline and some important adrenergic receptors.
Indeed, in our experimental conditions, i.p. injection of amitriptyline at doses of 40 or 80 mg kg-1 produced a marked sedation in the animals. It has been established that sedation can suppress the function of the immune system in several ways.37 Therefore, the sedative effect of amitriptyline may contribute to its anti-inflammatory effect.
Finally, it is worth mentioning that carrageenan-induced paw edema is a well-known model of acute inflammation that includes biphasic phases and a number of mediators participate in the inflammatory response elicited by carrageenan.38,39 On the other hand, amitriptyline is a pleiotropic tricyclic antidepressant that interacts with histaminic, cholinergic, serotonin, and N-methyl-D-aspartate (NMDA) receptors, biogenic amines, and substance P in addition to inhibiting norepinephrine and serotonin reuptake.40,41 Therefore, based on these facts, there are a variety of putative sites at which amitriptyline may exert its anti-inflammatory action.
In summary, our results verify the findings of Abdel-Salam et al. regarding the anti-inflammatory effect of amitriptyline in an acute model of inflammation4 and demonstrate that the supraspinal sites have an important role in this effect of amitriptyline, while ruling out the possible participation of opioid and some important adrenergic receptors in this effect. Therefore, this study not only extends our knowledge about the anti-inflammatory effect of amitriptyline but also provides new insights into the central mechanisms involved in the anti-inflammatory effects of antidepressants.
This research was supported by the research council of the Isfahan University of Medical Sciences, Isfahan, Iran.