Environmental exposure to man-made electromagnetic fields has been steadily increasing with the growing demand for electronic items that are operational at various frequencies. Testicular function is particularly susceptible to radiation emitted by electromagnetic fields.
OBJECTIVES:This study aimed to examine the therapeutic effects of a pulsed electromagnetic field (100 Hz) on the reproductive systems of male Wistar rats (70 days old).
METHODS:The experiments were divided into five groups: microwave sham, microwave exposure (2.45 GHz), pulsed electromagnetic field sham, pulsed electromagnetic field (100 Hz) exposure, and microwave/pulsed electromagnetic field exposure. The animals were exposed for 2 hours/day for 60 days. After exposure, the animals were sacrificed, their sperm was used for creatine and caspase assays, and their serum was used for melatonin and testosterone assays.
RESULTS:The results showed significant increases in caspase and creatine kinase and significant decreases in testosterone and melatonin in the exposed groups. This finding emphasizes that reactive oxygen species (a potential inducer of cancer) are the primary cause of DNA damage. However, pulsed electromagnetic field exposure relieves the effect of microwave exposure by inducing Faraday currents.
CONCLUSIONS:Electromagnetic fields are recognized as hazards that affect testicular function by generating reactive oxygen species and reduce the bioavailability of androgen to maturing spermatozoa. Thus, microwave exposure adversely affects male fertility, whereas pulsed electromagnetic field therapy is a non-invasive, simple technique that can be used as a scavenger agent to combat oxidative stress.
The electromagnetic fields emitted from various sources (e.g., microwave ovens, mobile phones) have been reported to have causative effects on biological systems.1 Electromagnetic fields have many effects on biological systems, but the testes are more sensitive to a variety of stresses, such as hyperthermia, inflammation, radiation, and exposure, which lead to germ cell apoptosis.2,3 Exposure to various frequencies present in the environment can evoke a number of alterations at the cellular level, such as increased Ca2+ efflux,4 reduced melatonin secretion,5 disturbed antioxidant enzyme balance,6 and the induction of micronuclei.7 Microwave exposure disrupts the seminiferous tubules and reduces the Leydig cell population and testosterone concentration in rats while increasing the LH level.8 It is well established that testosterone is essential for spermatogenesis and the formation of spermatozoa.9
During spermatogenesis, apoptosis plays a critical role in adjusting the appropriate number of proliferating germ cells associated with the surrounding Sertoli cells. The regulation of apoptosis is based on the intracellular dominance of various proteins that induce or inhibit apoptosis, such as BAX, Bcl, caspase-3, and several key enzymes.10 Apoptosis is a cell death process that is characterized by blebbing, the loss of the cell membrane, asymmetry, cell detachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation, which are eliminated by phagocytes.11 Caspases are present as inactive precursors and are activated by caspase initiators through auto-active proteolysis.12 The caspase 8 and 9 initiators and the caspase 3 effector are considered to be the main executors of apoptosis.13 The caspase 3 effector acts on two pathways: the mitochondrial pathway (through the caspase 9 initiator) and the death receptor pathway (through the caspase 8 initiator).14
Creatine kinase is a marker of sperm maturity, and its elevated level is associated with an increased rate of functional abnormalities and increased cytoplasmic retention.15 Creatine kinase (E.C.2.7.3.2) catalyses the reversible phosphorylation of ADP to ATP or creatine to creatine phosphate, thus maintaining an immediately accessible energy reservoir in the cell.16 Creatine phosphokinase, which is a key enzyme in transport and energy synthesis, supplies ATP to the sperm.17 Sperm are frequently associated with elevated activities of certain key enzymes, including creatine kinase, which indicate male infertility.18–20
In most cases of infertility, high concentrations of reactive oxygen species (ROS) have been reported.21 Kumar et al7 also reported an increased level of ROS due to microwave exposure. Oxidative stress is a cellular condition of increased ROS concentrations that causes molecular damage and leads to various diseases.22,23 However, ROS are neutralized by melatonin and its stimulatory effects on antioxidants. Melatonin is synthesized from serotonin in the pineal gland, and its level increases during the dark period and decreases during the light period. It has been reported that melatonin synthesis is suppressed by low-frequency EMF.24 Brainard et al25 have shown that melatonin synthesis and secretion from the pineal gland are under the control of visible light.
In this study, a pulsed electromagnetic field (PEMF) was used to relieve the effects caused by microwaves. Following the experiments of DiCarlo et al26 on chick embryos exposed to radiation, the protection conferred by the magnetic fields appears to influence free radical scavenging. PEMF has a variety of biological effects, such as its effects on bone healing,27,28 pain relief,29 and the balance of the neuroendocrine system (including hormone production and melatonin levels).30 PEMF has frequencies at the lower end of the electromagnetic spectrum (from 6 Hz to 500 Hz). In this study, we demonstrate that PEMF can be used as healing agent that activates the normal physiology of the body. PEMF activates normal metabolic processes, indirectly acts through the endocrine system, and controls the main cause of stress in the exposed system. We treated rats that had previously been exposed to 2.45 GHz radiation with PEMF. As biomarkers of this treatment, we chose the following parameters: melatonin, creatine kinase activity, caspase assay, and testosterone.
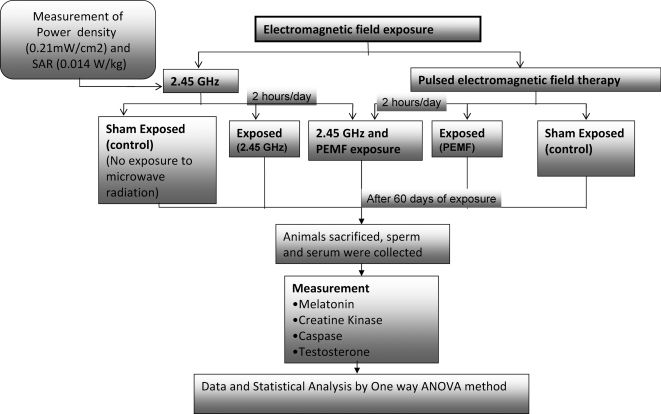
MATERIALS AND METHODSAnimalsSeventy-day-old male Wister rats (190±10 g) were used in this study. The animals were maintained in accordance with national guidelines and protocols. The study was approved by the Institutional Animal Ethics Committee (IAEC-JNU/83/675-687; code no. 12/2008). A flowchart of the entire methodology is shown in Figure 1. The animals were housed in clean polypropylene cages and maintained at a controlled temperature with a constant 12-h/12-h light/dark schedule. The animals were fed a standardized normal diet (Tetragon Cheime Private Limited, Bangalore) and water ad libitum.
Animal ExposureThe rats were divided into five groups (Figure 1): Group I, microwave (MW) sham; Group II, MW exposure (2.45 GHz); Group III, PEMF sham; Group IV, PEMF (100 Hz) exposure; Group V, MW/PEMF exposure. Each group contained three animals, and the experiments were repeated in triplicate. The rats were placed into a Plexiglas cage ventilated with one-cm-diameter holes; the exposure cage was fabricated such that the animals were comfortably housed. For the 2.45-GHz exposure, the chamber was lined with radar-absorbing material (attenuation, 40 dB) to minimize the reflection of the scattered beam. The exposure cage was placed vertically such that all of the animals were irradiated homogeneously at a single power level. The rats were exposed to a 2.45-GHz radiation source at a 50-Hz modulation frequency (input, 1080 W; output, 700 W). A microwave oven (Haier India Co. Ltd, made by PRC (China); model no. HR-18MS1) was used as the source of exposure. Exposure was performed through the horn antenna for 2 h per day for 60 days (Figure 2).
Specific Absorption Rate (SAR) CalculationThe emitted power of the microwaves was measured by a power meter, which is a peak-sensitive device (RF power sensor (6900 series) and IFR 6960 B RF power meter; made by Aeroflex, Inc., Wichita, Kansas, USA). Every day, the cage was placed in the same position below the horn antenna, and the same numbers of rat positions were reshuffled. A similar experiment was performed with the MW sham but without energizing the system. A similar experimental setup was adopted previously by Paulraj and Behari31,32 and Kesari and Behari.33 A full description of the exposure setup has been discussed by Kesari et al.34 The SAR was calculated for the average size of the small animals by following the work of Durney et al.35 For a plane-wave exposure with a random polarization and a power density of 0.21 mW/cm2, the SAR is 0.014 W/kg.
MaterialsThe caspase 3 assay kit (colorimetric; cat. no. CASP-3-C) was purchased from Sigma, USA. The creatine assay kit (cat no. K635-100) was purchased from BioVision Research Products (Mountain View, CA, USA), the ELISA melatonin kit (cat. no. E90908Ra) was purchased from Uscn Life Science Inc. (Wuhan, China), and the testosterone EIA kit (cat. no. 582701) was purchased from the Cayman Chemical Company (Ann Arbor, MI, USA). The remaining chemicals were purchased from Merck Chemicals, India.
Melatonin AssayThe melatonin level in the serum was estimated using an ELISA kit developed by Uscn Life Science Inc. Briefly, 50 μl of dilutions of the standards, the blanks and each sample were added to wells that were pre-coated with a polyclonal antibody specific for rat melatonin followed by the addition of 50 μl of detection reagent A to each tube. After incubation for one hour at 37°C, the wells were washed with wash solution three times. A total of 100 μl of detection reagent B was added to each well and incubated for 30 min at 37°C. The plate was washed again, which was followed by addition of the substrate solution to each well and incubation for 15 min at 37°C. The color development was stopped by adding stop solution, and the intensity of the yellow color was measured using a spectrophotometer (450 nm). The concentrations of the unknown samples were calculated by comparison with a standard curve. The minimum detectable dose of rat melatonin is typically less than 1.02 pg/mL.
Creatine Kinase AssayA CK kit was used. The seminal plasma was removed by washing the sperm sample with ice-cold imidazole buffer (0.15 M NaCl and 0.03 M imidazole (pH 7.0) at a ratio of 1:15). The supernatant was decanted after centrifugation at 500 g, and the pellet was re-suspended in a 0.1% Triton X-100 detergent solution by vortexing for 20 seconds. The sample was centrifuged again at 500 g, and the CK activity of the supernatant was analyzed. In this assay, the creatine is enzymatically converted into sarcosine, which is then specifically oxidized to generate a product that converts a colorless probe to an intensely red product. This final product is detected colorimetrically (λmax = 570 nm). The CK activity is expressed in international units/108 spermatozoa.
Measurement of Caspase 3 ActivityThe activity of caspase 3 was also measured using an assay kit. The spermatozoa were centrifuged at 300 g for 10 min at 4°C. The pellet was then re-suspended in lysis buffer for 20 min and centrifuged at 20,000 g for 20 min at 4°C, and the supernatant was collected. The assays were conducted in 96-well plates. The colorimetric caspase 3 assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA), which results in the release of the p-nitroaniline (pNA) moiety. To assess the specific contribution of caspase 3 activity, the Ac-DEVD-pNA substrate (2 mM) was added to each well according to the instructions of the manufacturer. The plates were incubated overnight at 37°C to measure the caspase 3 activity. The absorbance was measured using a microplate reader (Spectromax M2) at 405 nm. The caspase 3 activity is expressed in μmol of released pNA per min per ml of cell lysate at 37°C.
Testosterone AssayA serum testosterone assay was performed using the testosterone EIA kit (Cayman, USA). The procedures for the assay were followed as described in the manual of the manufacturer. In brief, 50 μl of the testosterone standard or serum, 50 μl of the testosterone AChE tracer, and 50 μl of the testosterone antiserum were added to the wells of an ELISA plate containing 100 μl of EIA buffer. The sensitivity of the assay was 6 pg/ml. The optical density was read using a spectrophotometer (SpectroMax M2) that was sensitive at a wavelength range of 405-420 nm.
Pulsed electromagnetic field therapyImmediately after exposure to the 2.45-GHz radiation, the Group-V animals (MW + PEMF) were exposed to PEMF for two hours daily. Three animals were exposed at a time. The rats were placed into a cage (286×172×115 mm) ventilated by one-cm-diameter holes. The animals were exposed in a mu-metal box at 100 Hz (Pulsed Magnetic Field Inventors Groups for Lawson Health Research, London, Ontario). The Mu box (386×333×206 mm) was lined with opaque black plastic and fitted with acrylic rods to transmit light into the enclosure (Figure 3). The box was made of 1.6-mm-thick mu-metal (Magnetic Shield, Bensenville, IL), with four rectangular Merritt-like configurations in the box. The coils were 0.32 mm in diameter, had a thin layer of enamel insulation (Belden, St. Louis, MO), and were framed on rectangular plastic formers (30 cm×17.8 cm) with 150 turns. The four coils were spaced 9.7 cm apart from one another. The coils were connected in parallel pairs. Each pair was connected to one channel of a two-channel amplifier, and the connection leads were connected to a distribution unit that permitted individual coil-phase selection. The coils were electrically shielded with conductive silver paint and copper foil to minimize the introduction of an electric field. An in-house-built arbitrary function signal generator was configured to produce a 100 Hz (64-step) step. Prato et al36 also used the same setup. The wave shape of the PEMF in the time domain is shown in Figure 4.
Diagram of the mu-metal box with a pulsed electromagnetic field generator at 100 Hz. Four vertical light pipes were mounted within the enclosure (one in each corner port). These acrylic rods are removable. Light was supplied by four LED sources that fit under the lower ports. The four rectangular coils were mounted inside the mu-metal box in a Merritt-like configuration.
All of the experimental results were compared with those of the sham exposure group and are expressed as means±SEMs. The analyses were performed using GraphPad Prism software and a one-way analysis of variance. p<0.05 was considered to be significant. To test the effectiveness of the exposure, a multiple-range test was performed by using Fisher's LSD test. The mean differences between the various experimental groups were found to be greater than the LSD values at a significance level of 0.05, which reveals that the experimental groups were significantly different from their controls and from one another.
RESULTSMelatoninThe average concentration of melatonin in the serum of the MW exposure group (78.82±6.28 pg/ml) was significantly less (p<0.003) than the average concentration of melatonin in the MW sham group (124±7.29 pg/ml, Figure 5). In contrast, the concentration of melatonin (108.29±3.67) in the MW + PEMF group was significantly (p<0.01) decreased. The melatonin level in PEMF exposure group (114±3.76) was significantly (p<0.05) less than that of the PEMF sham group (123.22±8.63).
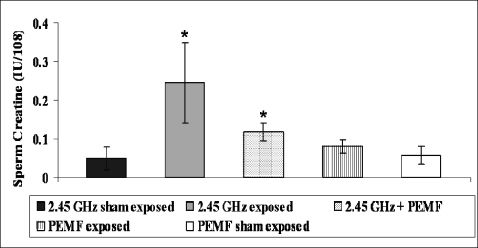
Creatine KinaseThe critical role for CK in sperm energy transport was examined by measuring the ATP concentrations or ATP/ADP ratios. The mean value of the CK level was higher (p<0.001) in the sperm from the MW exposure group (0.24±0.10) than in the sperm from the MW sham group (0.04±0.03). A significant (p<0.001) decline was also observed in the MW + PEMF group (0.11±0.02). The PEMF exposure group (0.08±0.01) showed an insignificant increase (p>0.06) in the CK level compared with the PEMF sham group (0.05±0.02, Figure 6).
Caspase 3 ActivityA statistically significant activation of caspase-3 was observed in the MW-induced animals (Figure 7). The sperm caspase activity showed a significant increase (p<0.003) in the MW exposure group (34.62±1.98) compared with the MW sham group (13.72±0.81). After exposure to 2.45-GHz radiation, the animals were subjected to PEMF treatment (22.72±1.74, p<0.001). A significant (p<0.049) decrease was found in the PEMF exposure group (15.62±1.31) compared with the PEMF sham (13.81±1.48).
The responses of the sperm caspase activities to an electromagnetic field. Caspase 3 activity was measured after overnight incubation (expressed as μmol of released pNA per min per ml of sperm lysate). The values are means±SEMs. *, significantly (p<0.05, Fisher's test) different from its respective sham group.
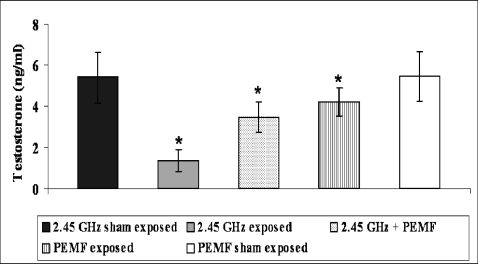
The serum testosterone level decreased significantly (p<0.002) in the MW exposure group (1.36±0.54) compared with the MW sham group (5.41±1.25). PEMF exposure produced a significant recovery effect (p<0.008) on the serum testosterone level in the MW + PEMF exposure group (3.46±0.74). However, significant (p = 0.05) changes were observed in the PEMF exposure group (4.20±0.69) compared with the PEMF sham group (5.46±1.21, Figure 8).
DISCUSSIONThe interaction between EMF and biosystems is a wide-ranging phenomenon that is not essentially confined to emission from any particular source. In general, such exposure has already been identified as a stressor whose receptor mechanism at the cellular level is still unknown. The thermal energy inside the body at room temperature is random, whereas the thermal energy from an external source is coherent (over a period of time exceeding 10 sec). It may be possible that a coherent signal interferes with the rhythmicity of the physiological process inside the body.
ROS generation in the testes was responsible for the possible toxic effects on the physiology of reproduction. However, cells have a defense mechanism [i.e., antioxidants (reduced glutathione, catalase, superoxide dismutase)] to fight against increased ROS production. Furthermore, melatonin stimulates the activity of several important endogenous antioxidants (GSH-Px, SOD) to combat the effect of reactive oxygen species.37 Melatonin is exclusively synthesized and secreted at night and is an efficient endogenous free radical scavenger.
Decreased serum testosterone levels and increased creatine kinase activity were associated with reduced male fertility. Our data reflect an appreciable increase in sperm creatine kinase activity and a decline in the activities of antioxidants and melatonin. In spermatozoa, CK-BB is localized to the mitochondria of the midpiece region.38 Creatine phosphate serves as a donor for the re-phosphorylation of adenosine diphosphate (ADP) into ATP, which supports flagellar dynein/adenosine triphosphate and sperm quality. The CK activity differences were reflected in the sperm ATP concentrations and ATP/ADP ratios.39 CK was associated with increased ROS and served as an indicator for oxidative stress.
Genotoxic stresses induced by irradiation trigger an arrest or delay in the G2 phase of the cell cycle to permit the repair of damaged DNA.40 Cell cycle analysis by flow cytometry with propidium iodide has confirmed these results because EMF exposure induces the appearance of a sub-G1 apoptotic peak, which is characteristic of DNA fragmentation in spermatozoa.7 Activated caspases commit the cells with defective repair machinery to an apoptotic death by cleaving a number of substrates. The results of the caspase assay suggest that the apoptotic cell counts are significantly increased. Therefore, apoptosis is considered to be involved in the impairment of spermatogenesis and the seminiferous tubules.
It is apparent from our data that a 2.45-GHz frequency increases caspase activity and decreases melatonin levels. These effects are indications of increased apoptosis and, hence, cancer promotion, which affects fertility. These effects were controlled by the administration of PEMF. Previously, our laboratory reported increased levels of PKC and ODC, changes in the levels of antioxidant enzymes (GPx, CAT, and SOD), micronucleus formation, and many other related changes.32,33 The present work supports these findings and others.41 Von Wilmsdorff et al42 hypothesized that brain behavioral modifications due to kainic acid treatment cause sex-specific responses via the hypothalamic-pituitary-adrenal-axis. Such effects may be achieved through either a chemical or radiation-based treatment, which suggests that the effects are mediated by the higher brain centers and are transferred to the reproductive system, thereby affecting the testosterone and sperm counts through the endocrine system.
The exposure of a biological system to a microwave causes a weakly induced signal near the cellular boundary. Energy from a coherent signal penetrates the body, with amplification derived from noise via stochastic phenomena. Thus, the signal can circumvent the barrier height of the plasma membrane (∼105 V/m), which may cause DNA damage (strand breaks). This signal may also provide sufficient energy to exacerbate silent mutations existing in biological systems. ROS overproduction is inhibited by the application of a pulsed electromagnetic field (100 Hz, magnetic component), which provides a free electron and thus eliminates the effect of the stressor. Triplet states can be transformed into singlet states via spin-lattice relaxation, which originates in fluctuating local magnetic fields around the electron (because of its random motion).43
In fact, many medical devices used in medical therapies generate circulating currents that trigger cellular, hormonal, and behavioral responses. Wolsko et al44 demonstrated that PEMF relieves or eliminates pain through a mechanism mediated by Ca2+ ion channels. Luo et al45 found that metal ions are necessary for enzymatic activity, and, thus, pulsed electromagnetic field are able to influence the catalytic activities of enzymes. Pulsed electromagnetic therapy is believed to have beneficial effects on tissue growth and repair, probably via alterations in the cellular microenvironment.46 A low-level alternating electromagnetic field (∼50 Hz) has also been reported to have a healing effect on spinal cord injury.47
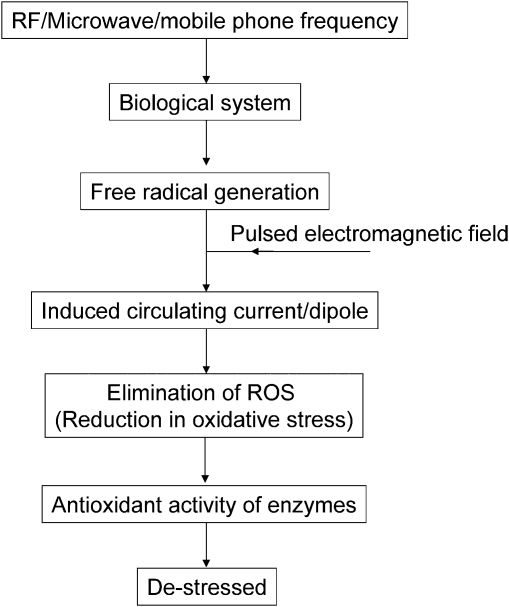
When a microwave field penetrates a biological body, it induces endogenous physiological processes. The major difference between the EMF generated from a 2.45-GHz source and that of the pulsed electromagnetic fields (100 Hz) is that the latter induces a circulating electric current in the tissue because of its constantly changing magnetic flux,29 thereby scavenging the free radical. Free radical formation also occurs at other microwave frequencies, which suggests that this phenomenon occurs generally. The destressor effect is derived from the antioxidant role of the electromagnetic field of the applied pulsed field. The pulsed field contains a set of frequencies that may provide accumulative benefits. The biomarkers that were adopted in this study are indicative of such processes. A schematic representation of such a process is shown in Figure 9.
CONCLUSIONSOur results suggest that a 2.45-GHz exposure causes apoptosis during spermiogenesis or sperm maturation, and sperm caspase-3 activity seems to affect the physiology of reproduction. Several other parameters are affected by electromagnetic field exposure. All of these studies reveal that oxidative stress is a major mechanism affecting health, and microwave fields cause chronic stress via ROS overproduction. Pulsed electromagnetic field therapy provides significant protection by controlling ROS production.
The authors would like to thank the Indian Council of Medical Research (ICMR) for its financial support.