Any cancer patient is a challenge for the anesthesiologist due to the increased cardiovascular risk factors resulting from the patient's condition, in addition to the increased toxicity from chemotherapy. Pre-anesthesia evaluation as part of the anesthetic activity is defined as the process of clinical evaluation before surgery and the administration of anesthesia per se. This review is intended to reassess the current approach to cardiovascular pre-anesthesia evaluation in cancer patients at referral centers. The search strategy used the major databases in the scientific literature, based on the keywords identified. The conclusion is that cardiovascular risk factors must be closely controlled and hence the approach to the management of the cancer patient should be in cooperation with clinical oncology and cardiology. The evaluation should include the ejection fraction as one of the most relevant predictors of the patient's prognosis.
El paciente con cáncer representa un desafío para el anestesiólogo debido a los factores de riesgo cardiovascular incrementados por su condición y a una mayor toxicidad por la quimioterapia que recibe. La evaluación preanestésica como parte del acto anestésico se define como el proceso de valoración clínica que precede al acto quirúrgico en el que se suministra anestesia. El objetivo de la presente revisión es replantear la manera como actualmente se está enfocando la evaluación pre-anestésica cardiovascular en los pacientes oncológicos en centros de referencia. La estrategia de búsqueda se realizó en las principales bases de datos con la búsqueda de literatura científica de acuerdo a palabras clave definidas. Como recomendaciones se concluye que los factores de riesgo cardiovascular se deben controlar en lo posible y por esto el enfoque y manejo oncológico del paciente debería hacerse en conjunto con las especialidades de oncología clínica y cardiología. Se debería incluir la fracción de eyección en esta valoración como uno de los predictores más importantes para el pronóstico de los pacientes.
Cancer per se is a pro-thrombotic condition, but it was only until 1865 that Armand Trousseau associated thrombosis and cancer for the first time. The risk of cancer-related thrombosis seems to be higher in patients with metastatic disease and in those with risk factors.
EpidemiologyCancer represents a group of pathologies with huge social, economic, and emotional impact. It has been estimated that there are over 11 million new cancer cases every year, of which around 80% present in developing countries. In Colombia cancer is a growing public health challenge, with an incidence of around 70,887 new cases per year, according to the estimates from 2000 to 2006 (32,316 cases in males and 38,571 cases in females). In men, the main cancer sites were in order of importance: prostate, stomach, lung, rectum and non-Hodgkin lymphomas. In women, the most common sites were: breast, cervix, thyroid, stomach, colon, rectum and anus.1
DefinitionPre-anesthesia evaluation as part of the anesthetic procedure is defined as the process of clinical evaluation that precedes surgery and the administration of anesthesia. Such evaluation comprises the information collected from multiple sources, including the patient's medical record, the interview, the physical examination, lab tests, and inter-consultation with other specialities.2,3
The main goal of pre-anesthesia evaluation is to assess any potential cardiovascular risks that may arise in the course of surgery, collecting all the information required on the extension and stability cardiovascular disease, if any, or any cardiovascular risk factors, understanding the scope of the procedure in order to adopt strategies that help to mitigate risks, as well as any short and long term adverse outcomes.4–7
The specific characteristics of the disease, the social conditions, and even the healthcare coverage have generated a new challenge for the cardiovascular management of cancer patients. The higher survival rates and access to healthcare are additional considerations in the management of patients. Aging and age-related physiological and pathological changes have led to new approaches to the cardiovascular management strategy in the elderly population, with emphasis on age as an independent risk factor for perioperative morbidity and mortality.8–11
In accordance with The National Confidential Enquiry Into Patient, Outcome and Death (NCPOD), most surgeries in cancer patients are a high priority and hence should not be delayed12; however, notwithstanding the availability of pre-anesthesia evaluation guidelines, these procedures are often delayed due to inadequate approaches or unnecessary pre-surgical testing.
The cancer patient is a challenge for the anesthesiologist due to all the factors involved, such as the exposure to chemotherapeutic agents or radiation therapy and the consequences thereof. Cardiotoxicity is one of the most relevant complications of cancer chemotherapy that affects patients and increases with age; cardiotoxicity may show gender differences. The oncological approach and the management of cancer patients should be a joint effort between clinical oncology and cardiology. Very often the cardiovascular risk is underestimated in cancer patients and these patients will usually undergo cancer surgery and then cardiovascular surgery.13–16
This paper was intended to review the literature and consider new approaches to pre-anesthesia cardiovascular evaluation of cancer patients, in order to reassess the current approaches used at referral centers.
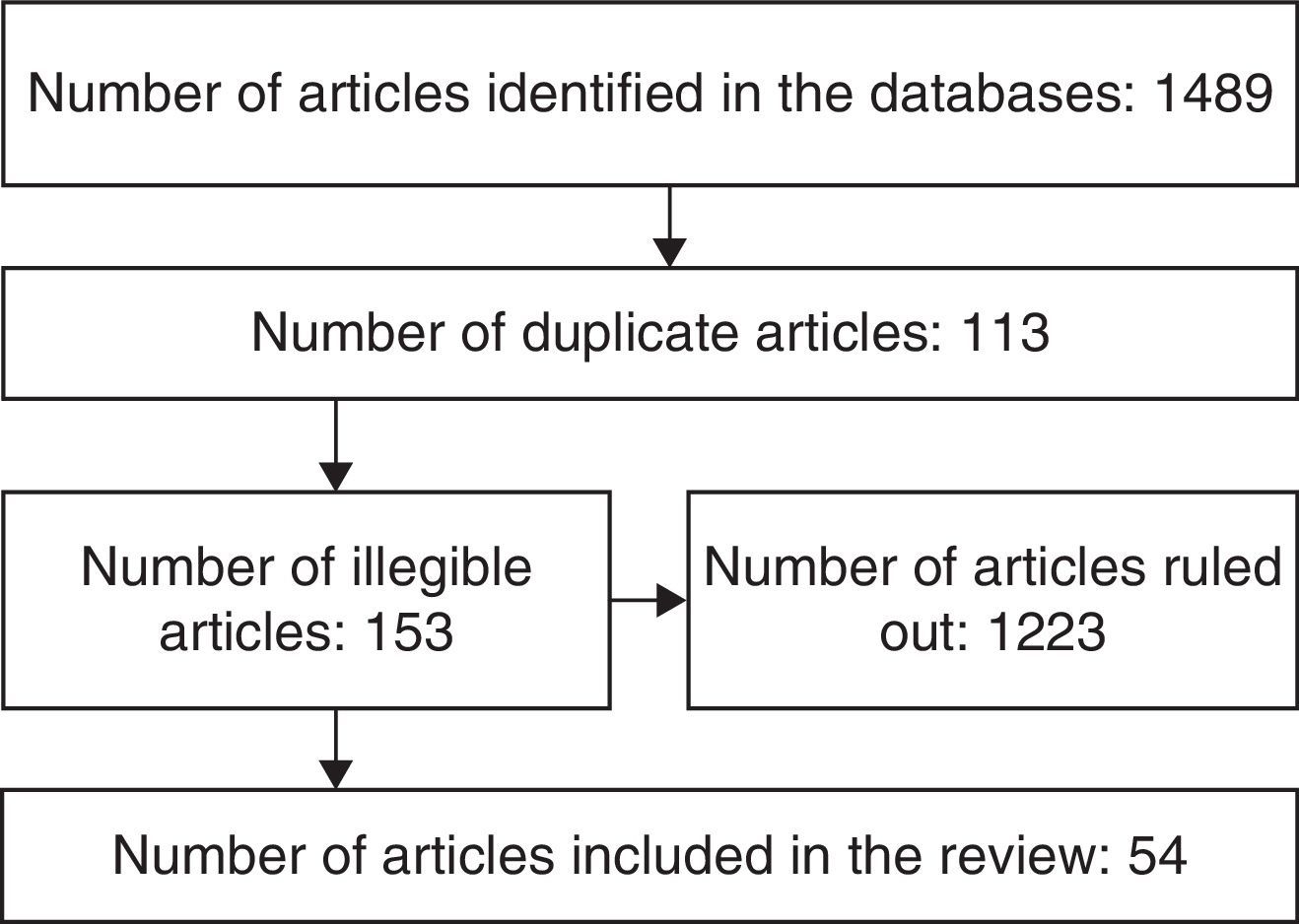
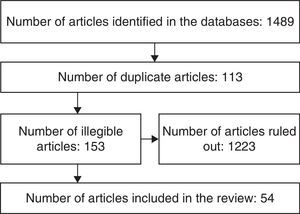
MethodologyA literature review was completed searching English and Spanish articles published since 1995, using the following keywords neoplasm, cancer centers, medical oncology, anesthesia, anesthesia recovery period, cardiovascular diseases with combinations including AND, OR, ADJ, NOT or NAND, XOR. The major databases accessed were: PubMed, OVID, MedLine, The Cochrane Library, Embase, Lilacs. Articles were selected on the basis of the title, the abstract, date of publication, language and the relationship to the subject being reviewed. (The selection process is illustrated in Fig. 1.)
Cardiovascular risk factors and cardiotoxicity of cancer patientsCardiovascular complications are becoming more evident as survival increases after the diagnosis and as a result of combined chemotherapy approaches. It is important to emphasize that the selection of the chemotherapeutic agent and the survival prognosis depend on a fine balance between cancer treatment and cardiovascular therapy; consequently, notwithstanding the fact that cardiac monitoring is time-consuming and expensive, it is nonetheless highly recommended.
Physiological aging is an independent risk factor for perioperative morbidity and mortality, due to changes such as stiffness of the connective tissue that reduces the compliance of the veins, the arteries and the myocardial tissue, and leads to systolic hypertension, increased afterload and myocardial hypertrophy. Moreover, a decreased beta-receptor response reduces the heart rate and the contractile response to hypotension, to exertion and the exogenous administration of catecholamine. Not to mention the cell loss at the sinoatrial node and subsequent heart rate disorders. The ischemic pre-conditioning effect that protects against secondary infarction in extended – though not indefinite – ischemia, seems to be absent in elderly patients and makes them more vulnerable to ischemic heart attacks.8–10
In the long term, cancer survivors have a high incidence of hypertension, dyslipidemia, acute coronary syndromes and acute CVAs, in addition to the risk of chemotherapy exposure that is reported to be similar to smoking. On top of the subsequent and concurrent exposure to a number of cardiovascular noxae, the patient experiences lifestyle changes. Usually, upon learning about his/her diagnosis, the patient quits exercising, tends to gain weight and to become depressed. Depression is acknowledged as a new risk factor for coronary disease.
Pre-existing cardiovascular risk factors are strong predictors for later chemotherapy-mediated cardiotoxicity.
Thyroxin kinase inhibitors have been associated with hypertension and cardiotoxicity, while the anti-vascular endothelial growth factor bevacizumab has been associated with pulmonary embolism, pulmonary bleeding, pulmonary edema and GI tract bleeding. Other symptoms like hypotension or hypertension, arrhythmias, cardiac failure, left ventricular dysfunction are seen in patients treated with monoclonal antibodies, interleukins and alpha-interferon.
Other agents that seem to have an effect on the vascular system are the selective estrogen receptors modulators like tamoxifen that induces changes in the high and low-density lipoproteins.13–16
Some drugs such as anthracycline medications may lead to congestive heart failure and left ventricular dysfunction and is more frequently observed in women with heart disease. Other antimetabolite agents such as capecitabine or citarabine may cause ischemia, pericarditis, heart failure, and cardiogenic shock.19–21
Cardiotoxicity from fluoropiridines such as 5-fluorouracil is expressed as myocardial ischemia that is evidenced in the electrocardiographic tracing. Antimicrotubular molecules such as paclitaxel or the vinca alkaloids are involved in sinus bradycardia, AV block, ventricular tachycardia, hypotension, heart failure and ischemia.22–25
Sub-endocardial fibrosis secondary to carcinoid heart or tumor infiltration that typically causes thickening of the tricuspid and pulmonary valves is found in up to 60% of the patients with carcinoid heart. Although valve regurgitation is the usual outcome, stenosis may also occur. Valve disease on its own, associated with endocardial fibrosis, finally results in right ventricular failure and death. Extension of the disease is less common to the left side of the heart, though the echocardiographic detection of a drop in the left ventricular ejection fraction is suggestive of endocardic fibrosis extending into the left heart.17,18
Radiation therapy to the mediastinum has been associated with myocardial fibrosis, left heart valve disease, and endothelial cell damage.26,27
Preoperative evaluationMorbidity during the perioperative period is directly related to underlying surgical pathology, the patient's comorbidities, the extent of surgery, and the age-related physiological reserve, as established under the American Heart Association (AHA) or the European Guidelines.
Cardiac death and non-fatal MI are associated with major surgeries such as surgery over the aorta, major vascular surgery, and peripheral vascular surgery with a mortality that exceeds 5%. Intermediate risk surgeries exhibit mortality rates ranging from 1% to 5%. These include intraperitoneal and intrathoracic surgery, carotid endarterectomy, head and neck surgery, orthopedic surgery and prostate surgery. Finally, the low risk surgeries with a risk of death below 1% include endoscopic procedures, cataract surgery, breast surgery, superficial and outpatient procedures.
The functional status is another item in the pre-anesthesia cardiovascular evaluation. It is easily measured through the performance of everyday activities in metabolic equivalents that are extrapolated according to the physical activity that the patient is able to perform, based on pre-established targets under the American and European Guidelines.5,6
A number of mortality predictors have been developed throughout the years for the coronary patient undergoing non-coronary surgery that are constantly used for pre-anesthesia evaluations such as the Goldman index, the revised Lee cardiac index, Detsky score or modified Goldman, and the Boersma, among others. These indexes summarize the patient's comorbidities and the level of compensation, age as an independent risk factor and the functional class.28–30
Despite the identification of risk factors in the cancer patient, including any pre-existing risk factors before the onset of cancer – i.e., hypertension, dislipidemia, diabetes, etc. – and those derived from cancer as a systemic disease and from cancer treatment, there are few convincing data to predict which patients will develop chemotherapy-related cardiotoxicity. Systolic dysfunction seems to be the most common manifestation, though diastolic dysfunction may also be found. Once the dysfunction occurs, its progression is almost unavoidable and mortality is high. So a lot of emphasis is placed on early detection of systolic dysfunction in patients undergoing chemotherapy.
Cancer treatment causes endothelial dysfunction and thus accelerates the atherosclerotic process, leading to an increased risk of developing cardiovascular events later in life. Early identification of cancer patients at high risk of developing cardiovascular events continues to be the key strategy to reduce morbidity and mortality; however, there are no recommendations at present in that regard.
Ejection fraction is one of the most important predictors in the prognosis of patients undergoing chemotherapy and radiotherapy at high risk of developing cardiotoxicity. However, the ejection fraction is a low sensitivity tool during the early stages of cardiotoxicity; moreover, a normal left ventricular ejection fraction does not rule out the potential for later decline. Magnetic resonance is the gold standard for evaluating cardiac volume, cardiac mass, and systolic and diastolic function; however, the cost or MRI is high and its availability is limited. Troponins have been used as biomarkers for acute cardiotoxicity, in combination with natriuretic peptides, but there is no evidence as to the role they play, except when the heart damage is already established. Troponins are highly sensitive to identify minor necrosis. In every clinical context, troponins do not only allow for the identification of myocardial damage, but they are also used as a tool to stratify cardiac risk. In fact, high sensitivity troponins may be particularly useful in cardiotoxicity from chemotherapeutic agents, but further studies are needed to determine their accuracy. In terms of endothelial damage, endothelial damage markers such as endogenous nitric oxide inhibitors and symmetric/asymmetric dimethyl arginines have been measured and identified after several years.
Other measurements such as the thickening of the intima of the carotid have also been used as markers of endothelial damage. Early detection of decreased left ventricle contractility using dobutamine stress echocardiography is another option for the early detection of a decline in ejection fraction following high doses of chemotherapy, even at early stages before being identified with conventional echocardiography l.31–38
ConclusionThese patients represent a new challenge for referral cancer centers and for the multidisciplinary teams managing these patients. Anesthesiologists have to deal with a top priority surgery, in patients with multiple cardiovascular risk factors since a large percentage of these patients are 65 or older. The risk of the procedure alone is at a 5% level (intermediate risk) in terms of perioperative morbidity and mortality, in addition to the exposure to chemotherapeutic cardiotoxic agents or radiation therapy in a large proportion of these patients.
To what extent shall we stratify these patients in terms of their cardiovascular risk?It is clear that cancer behaves as a systemic disease that affects all kinds of people and requires a multidisciplinary approach. It is highly recommended that cancer centers become aware of the need to involve the cardiologist during the treatment of cancer and integrate cardio-oncology teams to manage and monitor these patients.
The pre-anesthesia evaluation should consider cardiovascular evaluation a priority. Extrapolating the guidelines for coronary patients to non-cardiac surgery is difficult because of the nature of the disease, the cytoreduction process involved and the rate at which the disease stage may progress. Naturally, every coronary risk factor should be controlled, but to what extent should a patient undergo cancer surgery when uncontrolled risk factors for coronary disease are present?
Past studies have shown that prophylactic myocardial revascularization does not improve survival and is only an option for patients with unstable coronary syndromes and advanced coronary disease that do benefit from the procedure.39–45
Surgical procedures such as Sugar Baker's, inter alia, with the multiple physiological variables that have to be controlled, entail more than an intermediate risk and may even be considered major surgeries with adverse cardiac outcomes that deserve complementary studies and specific stratification to rule out coronary disease.46–54
Cardiovascular risk factors should be closely controlled, particularly in patients that will later be transferred to the ICU. We should then include exposure to chemotherapy or radiation therapy in the list of risk factors for this population, together with strategies for prevention, detection, and treatment for cardiotoxicity resulting from both chemo and radiation therapy. Consequently, patients with predictors of intermediate risk that have been exposed to cycles of mediastinal chemotherapy or radiotherapy and will undergo intermediate risk cancer surgery should at least present with a left ventricle ejection fraction evaluation combined with biomarkers. Is it necessary to evaluate the myocardial reserve in these patients?
There are still several unanswered questions about the perioperative cardiovascular evaluation of the patient undergoing cancer surgery. Though the answers may seem obvious, this is not usually the case in everyday practice. Further studies are needed to be able to address these concerns and provide strong guidance for the pre-anesthesia evaluation and perioperative cardiovascular care of the surgical cancer patient.
FundingThe authors did not receive sponsorship to undertake this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gómez-Henao PA, Dueñas JAC. Evaluación preanestésica cardiovascular en cirugía oncológica. Rev Colomb Anestesiol. 2016;44:17–22.